Abstract
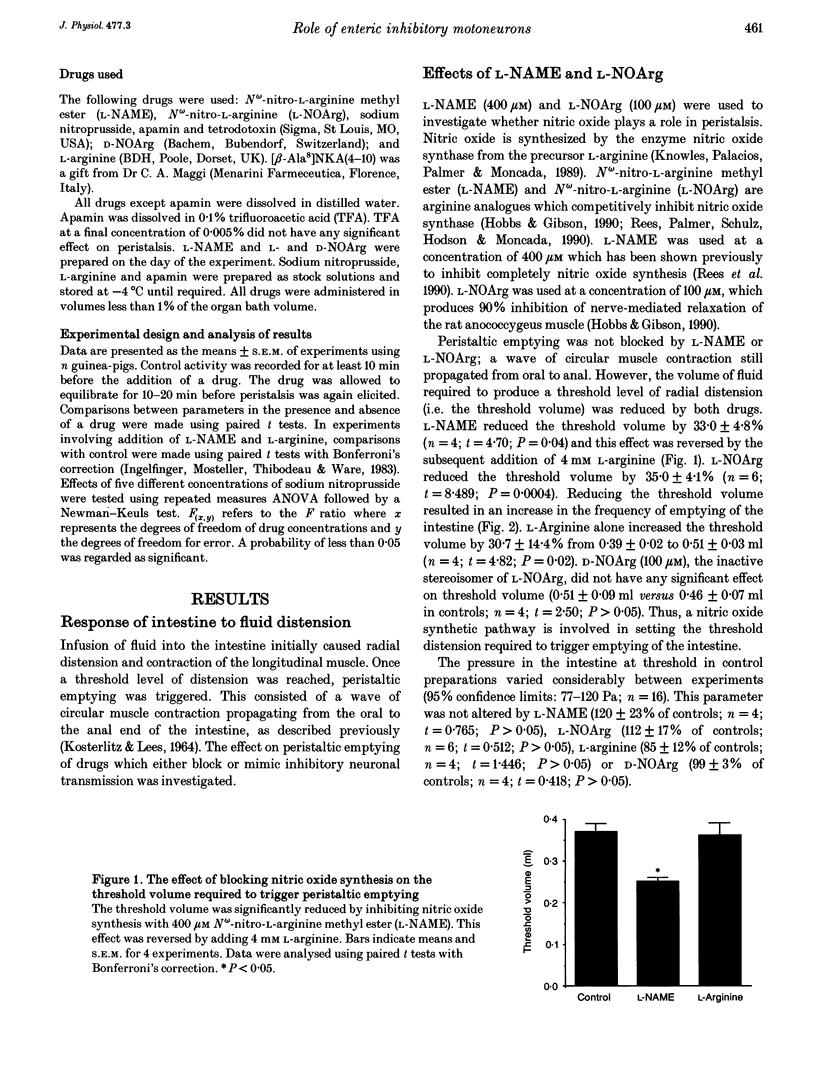
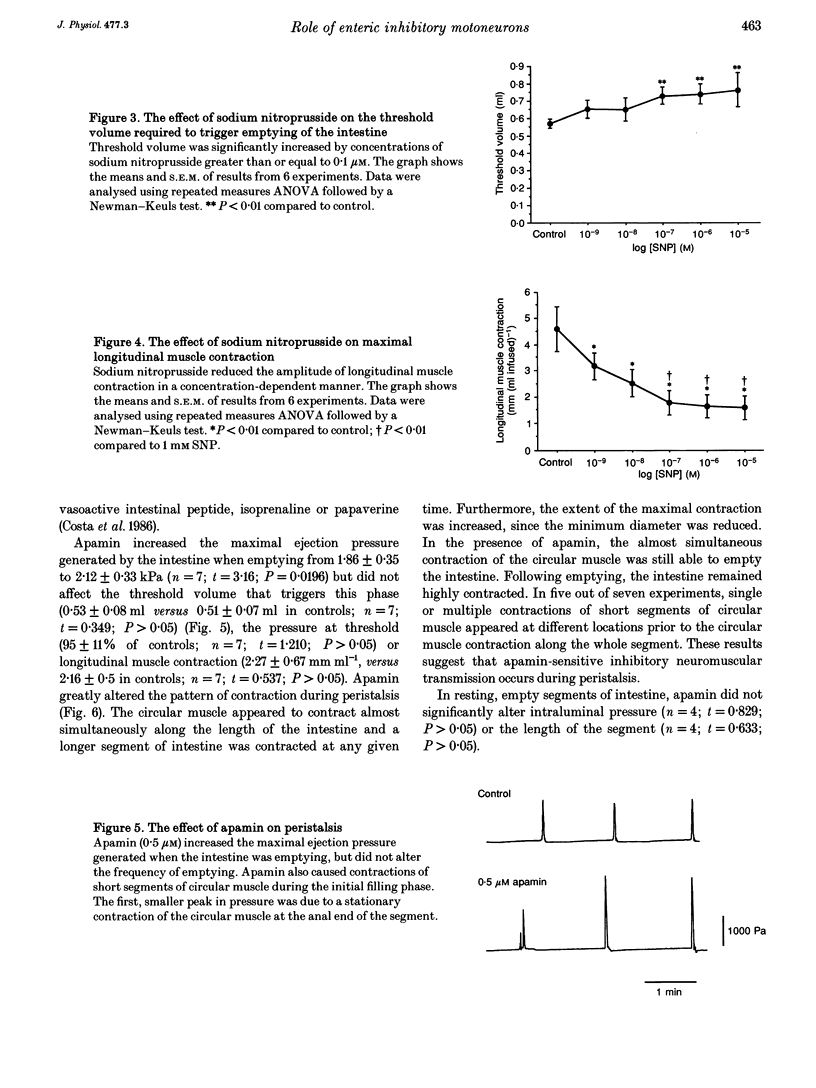
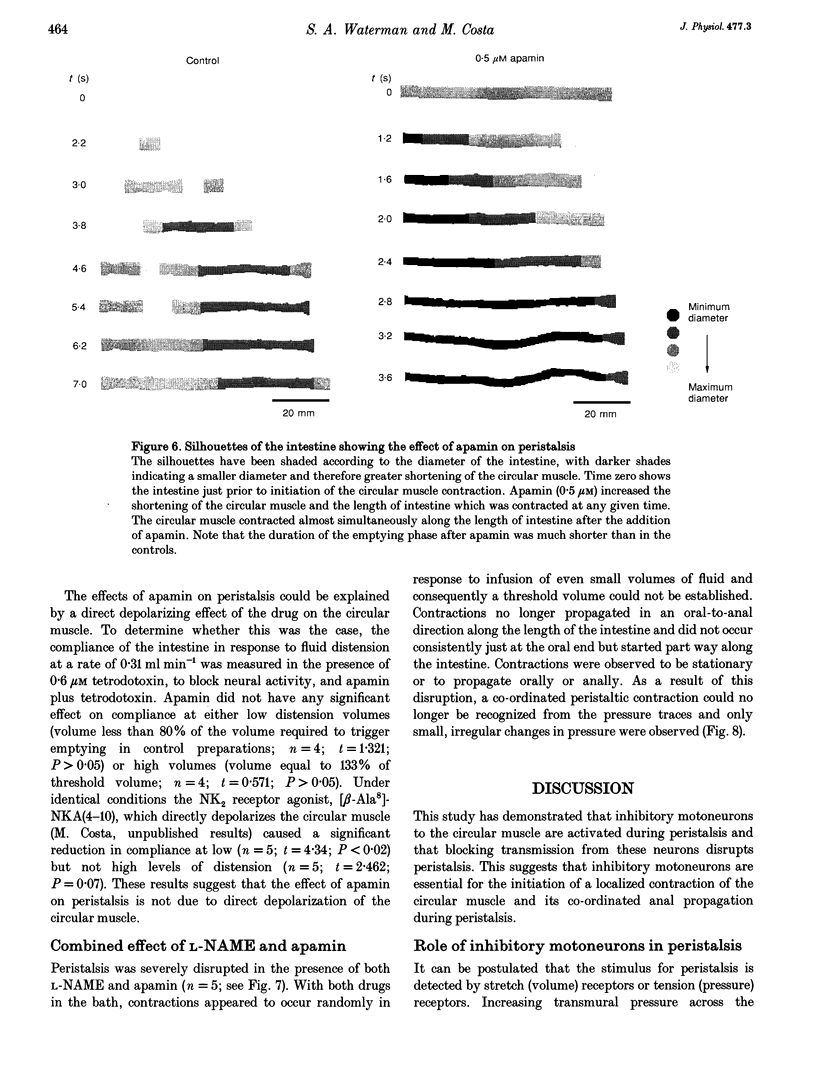
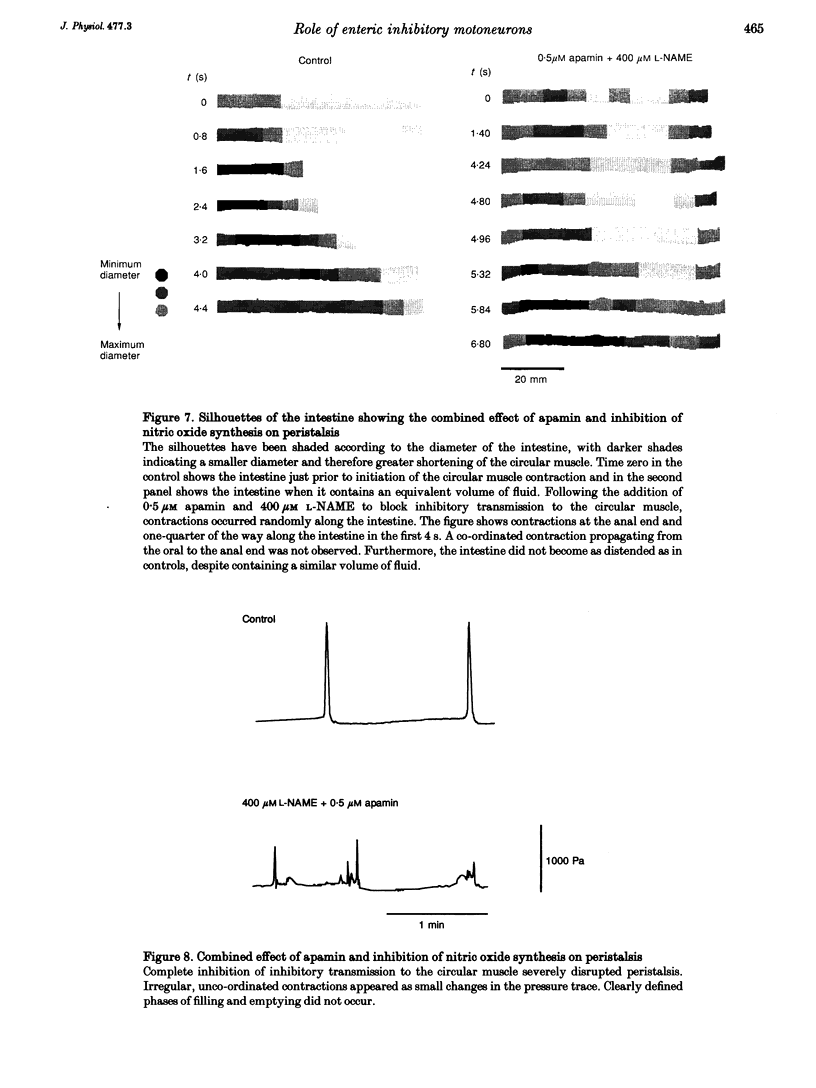
1. Peristalsis is a co-ordinated motor behaviour in which an anally propagated contraction of the circular muscle propels intraluminal contents. The role of excitatory motoneurons in peristalsis is well established; however the role of enteric inhibitory motoneurons is unknown. 2. A combination of a nitric oxide synthase inhibitor and apamin, which blocks relaxation of the circular muscle of guinea-pig small intestine mediated by enteric inhibitory motoneurons, was used to investigate the role of inhibitory motoneurons in peristalsis in isolated segments of guinea-pig small intestine. 3. N omega-nitro-L-arginine methyl ester (L-NAME, 400 microM) and N omega-nitro-L-arginine (L-NOArg, 100 microM) significantly reduced the threshold volume required to trigger emptying of the intestine. This effect was reversed by L-arginine (4 mM) and L-arginine alone increased the threshold volume for initiation of peristalsis. Sodium nitroprusside (0.1-10 microM), which generates nitric oxide, also increased the threshold volume. L-NAME, L-NOArg, L-arginine and sodium nitroprusside did not alter the maximal intraluminal pressure generated during emptying. Contraction of the longitudinal muscle during the initial phase of fluid infusion was significantly increased by L-NAME and L-NOArg and reduced by sodium nitroprusside (1 nM to 10 microM). 4. Apamin (0.5 microM) did not significantly alter the threshold volume necessary to initiate peristalsis or contraction of the longitudinal muscle. However, the maximal pressure generated when the intestine was emptying was significantly increased. Furthermore, short segments of circular muscle contracted apparently randomly, before peristaltic emptying was triggered. 5. A combination of L-NAME and apamin completely disrupted peristalsis. Contractions of the circular muscle did not always start at the oral end. Stationary contractions as well as contractions propagating orally and anally were observed. 6. It is concluded that enteric inhibitory motoneurons are crucial for peristalsis to occur. They are important in setting the threshold at which peristaltic emptying is triggered, via nitric oxide. They are essential for the propagation of the circular muscle contraction, via an apamin-sensitive mechanism of transmission. Contraction of the longitudinal muscle during peristalsis is partly inhibited by a nitric oxide-mediated mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. J Physiol. 1899 May 11;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C., Costa M., Furness J. B., Lang R. J. Electrophysiological analysis of projections of enteric inhibitory motoneurones in the guinea-pig small intestine. J Physiol. 1986 Jan;370:61–74. doi: 10.1113/jphysiol.1986.sp015922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater R. A., Taylor G. S. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea-pig ileum. J Physiol. 1986 May;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Humphreys C. M. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Pompolo S., Brookes S. J., Bornstein J. C., Bredt D. S., Snyder S. H. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neurosci Lett. 1992 Dec 14;148(1-2):121–125. doi: 10.1016/0304-3940(92)90819-s. [DOI] [PubMed] [Google Scholar]
- Crist J. R., He X. D., Goyal R. K. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992 Feb;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Mechanisms of peristalsis. Br Med Bull. 1979 Sep;35(3):263–268. doi: 10.1093/oxfordjournals.bmb.a071587. [DOI] [PubMed] [Google Scholar]
- Hobbs A. J., Gibson A. L-NG-nitro-arginine and its methyl ester are potent inhibitors of non-adrenergic, non-cholinergic transmission in the rat anococcygeus. Br J Pharmacol. 1990 Aug;100(4):749–752. doi: 10.1111/j.1476-5381.1990.tb14086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTERLITZ H. W., LEES G. M. PHARMACOLOGICAL ANALYSIS OF INTRINSIC INTESTINAL REFLEXES. Pharmacol Rev. 1964 Sep;16:301–339. [PubMed] [Google Scholar]
- KOSTERLITZ H. W., ROBINSON J. A. Reflex contractions of the longitudinal muscle coat of the isolated guinea-pig ileum. J Physiol. 1959 May 19;146(2):369–379. doi: 10.1113/jphysiol.1959.sp006198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyster D. J., Bywater R. A., Taylor G. S., Watson M. J. Effects of a nitric oxide synthase inhibitor on non-cholinergic junction potentials in the circular muscle of the guinea pig ileum. J Auton Nerv Syst. 1992 Dec;41(3):187–196. doi: 10.1016/0165-1838(92)90058-o. [DOI] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A., Ras R., Van den Akker J. The action of apamin on guinea-pig taenia caeci. Eur J Pharmacol. 1980 Oct 17;67(2-3):265–274. doi: 10.1016/0014-2999(80)90507-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Niel J. P., Bywater R. A., Taylor G. S. Apamin-resistant post-stimulus hyperpolarization in the circular muscle of the guinea-pig ileum. J Auton Nerv Syst. 1983 Nov;9(2-3):565–569. doi: 10.1016/0165-1838(83)90014-0. [DOI] [PubMed] [Google Scholar]
- Osthaus L. E., Galligan J. J. Antagonists of nitric oxide synthesis inhibit nerve-mediated relaxations of longitudinal muscle in guinea pig ileum. J Pharmacol Exp Ther. 1992 Jan;260(1):140–145. [PubMed] [Google Scholar]
- Ozaki H., Blondfield D. P., Hori M., Publicover N. G., Kato I., Sanders K. M. Spontaneous release of nitric oxide inhibits electrical, Ca2+ and mechanical transients in canine gastric smooth muscle. J Physiol. 1992 Jan;445:231–247. doi: 10.1113/jphysiol.1992.sp018921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol. 1992 Mar;262(3 Pt 1):G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- Smith T. K., Bornstein J. C., Furness J. B. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J Auton Nerv Syst. 1990 Mar;29(3):203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Sugisawa K., Komori S., Takewaki T., Ohashi H. Stimulative effect of sodium nitroprusside on peristaltic reflex in isolated guinea pig ileal segments. Jpn J Pharmacol. 1991 Nov;57(3):279–289. doi: 10.1254/jjp.57.279. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Bywater R. A. Intrinsic control of the gut. Baillieres Clin Gastroenterol. 1988 Jan;2(1):1–22. doi: 10.1016/0950-3528(88)90018-8. [DOI] [PubMed] [Google Scholar]
- Tonini M., Frigo G., Lecchini S., D'Angelo L., Crema A. Hyoscine-resistant peristalsis in guinea-pig ileum. Eur J Pharmacol. 1981 May 22;71(4):375–381. doi: 10.1016/0014-2999(81)90181-3. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hope B. T. Neurons that say NO. Trends Neurosci. 1992 Mar;15(3):108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- Waterman S. A., Costa M., Tonini M. Accommodation mediated by enteric inhibitory reflexes in the isolated guinea-pig small intestine. J Physiol. 1994 Feb 1;474(3):539–546. doi: 10.1113/jphysiol.1994.sp020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman S. A., Costa M., Tonini M. Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. Br J Pharmacol. 1992 Aug;106(4):1004–1010. doi: 10.1111/j.1476-5381.1992.tb14448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




