Abstract
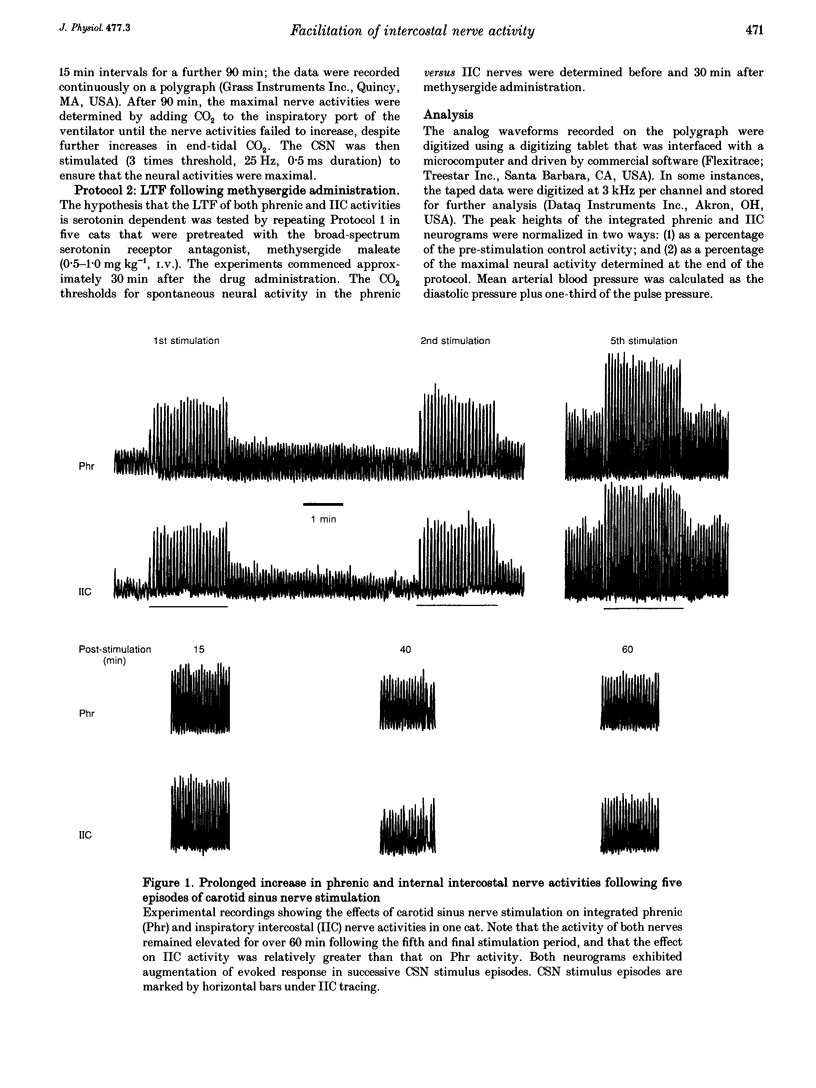
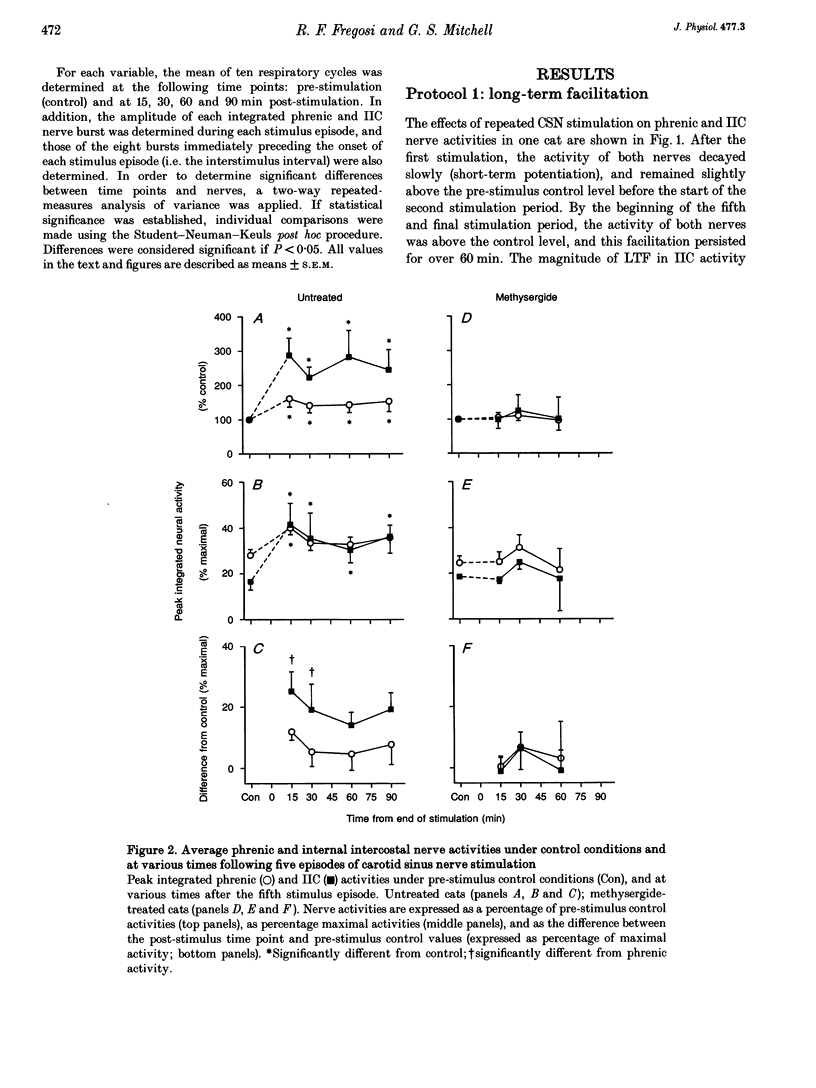
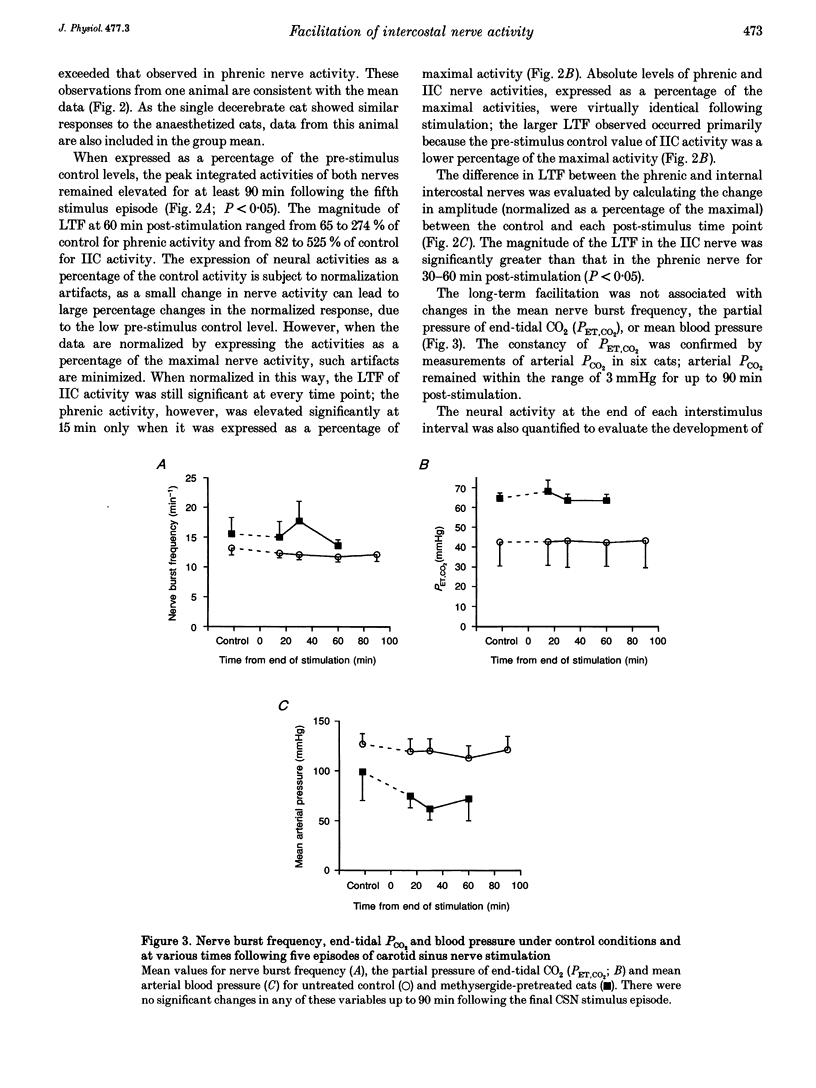
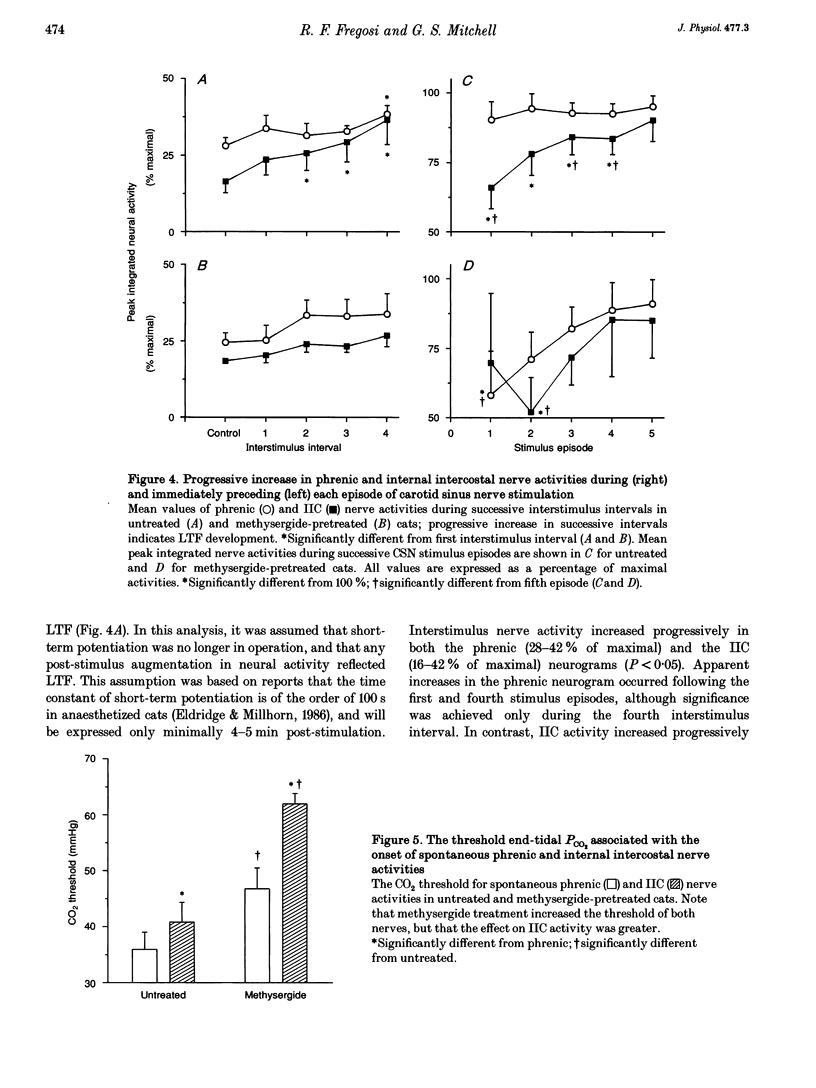
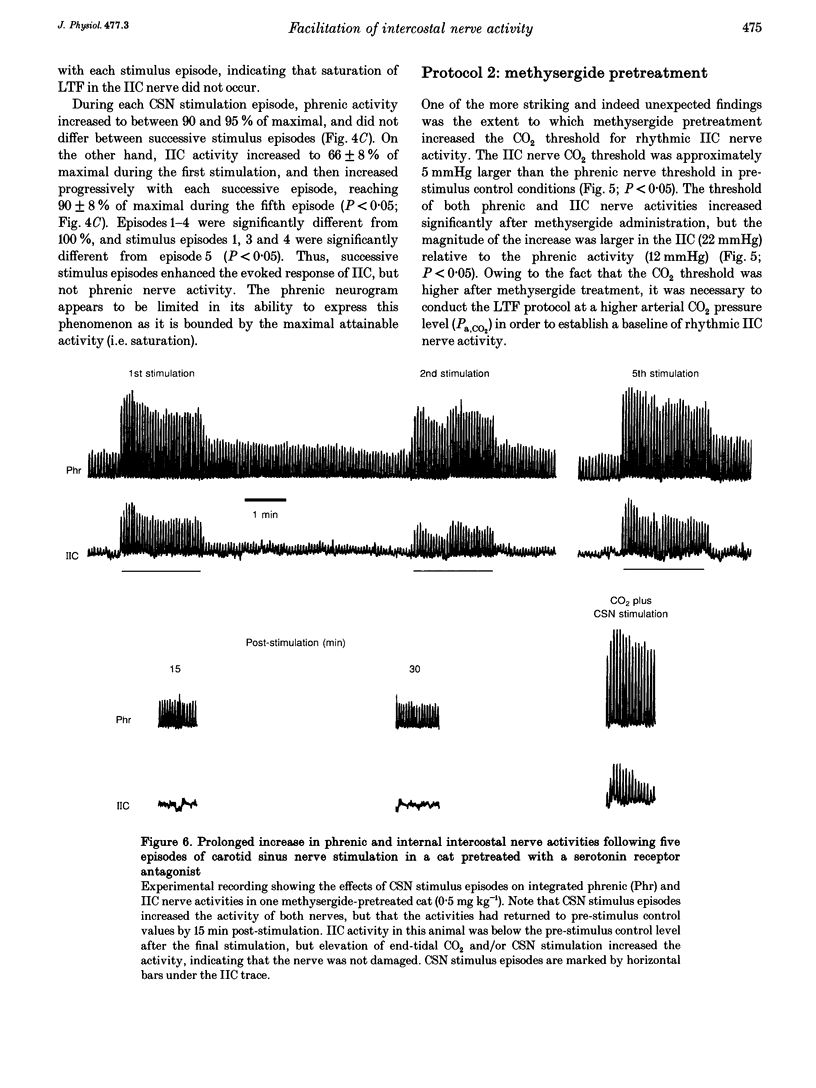
1. Repeated carotid sinus nerve (CSN) stimulation evokes a serotonin-dependent long-term facilitation (LTF) of phrenic nerve activity in cats. To determine whether CSN stimulation-evoked LTF is a general property of spinal inspiratory motoneurones, phrenic and inspiratory internal intercostal (IIC) nerve activities were recorded in nine cats (eight anaesthetized; one decerebrate), which were vagotomized, paralysed, thoracotomized and ventilated with O2; airway CO2 was controlled by means of of a servo-respirator. Baseline conditions were established by setting the arterial CO2 pressure (Pa,CO2) at approximately 2 mmHg above the threshold for IIC activity. One CSN was stimulated (3 times threshold, 25 Hz, 0.5 ms duration) with five (2 min) trains, each separated by 5 min. 2. The peak integrated phrenic activity was elevated by 33% whereas IIC activity was elevated by 226% above baseline, 90 min post-stimulation (P < 0.05). The results were similar when expressed as a percentage of the maximal neural activities (elicited by combined hypercapnia and CSN stimulation), although differences between the nerves were less pronounced. The burst frequency was not change following stimulation. 3. In five additional cats that were pretreated with the serotonin receptor antagonist, methysergide maleate (0.5-1 mg kg-1, I.V.), the CO2 thresholds of the phrenic (12 mmHg) and IIC nerves (22 mmHg) were increased (P < 0.05), and LTF could not be elicited in either neurogram. 4. Successive CSN stimulation episodes evoked a previously undescribed phenomenon. Although the peak integrated phrenic activity was unchanged (90-95% of maximal), IIC activity increased progressively during successive stimulus episodes (66-90% of maximal; P < 0.05). However, after methysergide treatment, the initial stimulus-evoked phrenic response decreased to 58% of maximal and both neurograms exhibited progressive augmentation of the stimulus-evoked response. As stimulus-evoked augmentation does not require serotonin, it is independent of LTF. 5. We conclude that CSN stimulation-evoked LTF of IIC activity exceeds that of phrenic activity. Since LTF requires the neuromodulator serotonin and is expressed predominantly by changes in burst pattern formation versus rhythm generation, serotonin may exert a greater influence on IIC relative to phrenic respiratory motor output. A unique mechanism is described whereby successive CSN stimulus episodes cause progressively increasing responses in both neurograms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arita H., Ochiishi M. Opposing effects of 5-hydroxytryptamine on two types of medullary inspiratory neurons with distinct firing patterns. J Neurophysiol. 1991 Jul;66(1):285–292. doi: 10.1152/jn.1991.66.1.285. [DOI] [PubMed] [Google Scholar]
- Berger A. J., Bayliss D. A., Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992 Aug 31;143(1-2):164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Cao K. Y., Zwillich C. W., Berthon-Jones M., Sullivan C. E. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol (1985) 1992 Nov;73(5):2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Ellenberger H. H., Feldman J. L. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci Lett. 1989 Oct 23;105(1-2):34–40. doi: 10.1016/0304-3940(89)90007-4. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwall M. J., Daristotle L., Niu W. Z., Dempsey J. A., Bisgard G. E. Ventilatory afterdischarge in the awake goat. J Appl Physiol (1985) 1991 Oct;71(4):1511–1517. doi: 10.1152/jappl.1991.71.4.1511. [DOI] [PubMed] [Google Scholar]
- Erickson J. T., Millhorn D. E. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991 Dec 13;567(1):11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Fregosi R. F., Bartlett D., Jr Internal intercostal nerve discharges in the cat: influence of chemical stimuli. J Appl Physiol (1985) 1989 Feb;66(2):687–694. doi: 10.1152/jappl.1989.66.2.687. [DOI] [PubMed] [Google Scholar]
- Fregosi R. F. Short-term potentiation of breathing in humans. J Appl Physiol (1985) 1991 Sep;71(3):892–899. doi: 10.1152/jappl.1991.71.3.892. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Coles S. K., Bach K. B., Mitchell G. S., McCrimmon D. R. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993 Oct;265(4 Pt 2):R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988 Jul;26(1):169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Norman W. P., Skirboll L., Dretchen K. L., Cuello C., Visser T. J., Hökfelt T., Gillis R. A. Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci. 1984 Apr;4(4):1064–1071. doi: 10.1523/JNEUROSCI.04-04-01064.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley G. D., Martin-Body R. L., Dawson N. J., Sinclair J. D. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience. 1987 Jul;22(1):237–250. doi: 10.1016/0306-4522(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Jacobs B. L., Azmitia E. C. Structure and function of the brain serotonin system. Physiol Rev. 1992 Jan;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jiang C., Mitchell G. S., Lipski J. Prolonged augmentation of respiratory discharge in hypoglossal motoneurons following superior laryngeal nerve stimulation. Brain Res. 1991 Jan 11;538(2):215–225. doi: 10.1016/0006-8993(91)90433-v. [DOI] [PubMed] [Google Scholar]
- Jiang Z. H., Shen E. [Synaptic connection between the monoaminergic terminals and intercostal respiratory motoneurons in cats]. Sheng Li Xue Bao. 1985 Oct;37(5):479–485. [PubMed] [Google Scholar]
- Kubin L., Tojima H., Davies R. O., Pack A. I. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992 May 25;139(2):243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lindsay A. D., Feldman J. L. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993 Feb;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol. 1992 Apr;67(4):890–904. doi: 10.1152/jn.1992.67.4.890. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980 Jul;41(1):87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980 Dec;42(3):171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hennequin S., Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990 Mar 26;111(1-2):127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Morin D., Monteau R., Hilaire G. 5-Hydroxytryptamine modulates central respiratory activity in the newborn rat: an in vitro study. Eur J Pharmacol. 1991 Jan 3;192(1):89–95. doi: 10.1016/0014-2999(91)90073-y. [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., de Castro D., Llewellyn-Smith I., Lipski J., Voss M. D. Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci. 1990 Apr;10(4):1091–1098. doi: 10.1523/JNEUROSCI.10-04-01091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Böhmer G., Merkelbach S. Serotonergic control of phrenic motoneuronal activity at the level of the spinal cord of the rabbit. Neurosci Lett. 1990 Aug 14;116(1-2):204–209. doi: 10.1016/0304-3940(90)90411-2. [DOI] [PubMed] [Google Scholar]
- Sessle B. J., Henry J. L. Effects of enkephalin and 5-hydroxytryptamine on solitary tract neurones involved in respiration and respiratory reflexes. Brain Res. 1985 Feb 18;327(1-2):221–230. doi: 10.1016/0006-8993(85)91515-x. [DOI] [PubMed] [Google Scholar]
- Skagerberg G., Björklund A. Topographic principles in the spinal projections of serotonergic and non-serotonergic brainstem neurons in the rat. Neuroscience. 1985 Jun;15(2):445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Voss M. D., De Castro D., Lipski J., Pilowsky P. M., Jiang C. Serotonin immunoreactive boutons form close appositions with respiratory neurons of the dorsal respiratory group in the cat. J Comp Neurol. 1990 May 8;295(2):208–218. doi: 10.1002/cne.902950205. [DOI] [PubMed] [Google Scholar]
- Wagner P. G., Eldridge F. L. Development of short-term potentiation of respiration. Respir Physiol. 1991 Jan;83(1):129–139. doi: 10.1016/0034-5687(91)90098-4. [DOI] [PubMed] [Google Scholar]
- Zhan W. Z., Ellenberger H. H., Feldman J. L. Monoaminergic and GABAergic terminations in phrenic nucleus of rat identified by immunohistochemical labeling. Neuroscience. 1989;31(1):105–113. doi: 10.1016/0306-4522(89)90033-x. [DOI] [PubMed] [Google Scholar]


