Abstract
Background
Ciprofol, a novel intravenous general anesthetic with a chemical structure similar to propofol, exhibits significantly enhanced potency. It offers a rapid onset, reduced incidence of injection pain, and has comparable effects on heart rate and blood pressure to propofol. However, clinical data on its use for anesthesia induction in cardiac surgery remain limited.
Methods
Seventy-eight patients undergoing coronary artery bypass grafting or valve replacement surgery were randomly assigned to receive either ciprofol (N = 40) or propofol (N = 38) for anesthesia induction. Variables recorded included changes in mean arterial pressure and heart rate during anesthesia, alterations in the oxygenation index and lactic acid concentration before and 10 min after anesthesia induction, and the incidence of adverse events such as bradycardia, hypotension, and injection pain.
Results
The incidence of anesthesia-induced injection pain was significantly lower in the ciprofol group compared to the propofol group (3% vs. 18%, P < 0.05). The incidence of other adverse events was similar between the groups. No significant differences in hemodynamics or oxygenation index were observed during anesthesia induction between ciprofol and propofol.
Conclusions
Ciprofol demonstrated a significantly lower incidence of injection pain compared to propofol, potentially improving patient comfort during anesthesia induction. Additionally, ciprofol showed comparable circulatory stability to propofol during anesthesia induction in cardiac surgery, suggesting it may be a suitable alternative to propofol for this application.
Trial registration
The trial was registered at the ClinicalTrials.gov on 03/10/2024 (NCT06312345).
Keywords: Ciprofol, Propofol, Coronary artery bypass graft, Heart valve replacement, Anesthesia induction
Introduction
The prevalence of heart diseases, such as coronary heart disease and heart valve disease, continues to rise due to demographic shifts and an aging population [1–3]. Patients unresponsive to medical treatment often necessitate cardiac surgery, which presents unique challenges due to significant hemodynamic fluctuations during anesthesia induction and an elevated incidence of adverse events [4]. Propofol is a common choice for anesthesia induction in various surgical procedures, including cardiac surgery [5, 6]. However, it exhibits significant cardiovascular and respiratory depression effects, and propofol infusion syndrome, a rare yet life-threatening adverse effect [7–9].
Ciprofol (HSK3486) is a recently developed short-acting sedative with greater potency than propofol and a chiral structure that enhances its affinity for GABAA receptors [10–12]. A phase 3 clinical study of ciprofol demonstrated a BIS change pattern similar to propofol, stability during anesthesia maintenance, comparable safety, and a lower incidence of injection pain [13]. Ciprofol's rapid onset, swift recovery, reduced injection pain, and stable cardiopulmonary function position it as a promising alternative to propofol [14]. However, no existing literature explores the use of ciprofol for anesthesia induction in cardiac surgery. As such, we conducted a randomized, double-blind study to investigate the safety and hemodynamic effects of ciprofol in inducing anesthesia for cardiac surgery.
Methods
Study design and participants
Prior to participation, written informed consent was obtained from all patients. A member of the research team explained the study procedures, potential risks, and benefits to the participants. Patients were assured that their participation was voluntary and that they could withdraw from the study at any time without consequences. The study protocol was approved by the Ethics Committee of East Hospital (2022/No.171), Tongji University, Shanghai, China, and was registered on ClinicalTrials.gov (NCT06312345). The trial enrolled patients who underwent cardiac surgery at Shanghai East Hospital between March 2024 and May 2024. The study focused on elective surgery patients aged 55 to 75 with New York Heart Association class II or III cardiac function who were scheduled for coronary artery bypass grafting or heart valve replacement via median sternotomy. Exclusion criteria included a history of benzodiazepine allergy, significant liver or kidney insufficiency, coagulation disorders, neurological or psychiatric conditions, or major surgery within the preceding three months.Patients were randomly allocated to one of two groups, receiving either propofol or ciprofol for anesthesia induction. A nurse anesthetist conducted the randomization process utilizing computerized software prior to the patients entering the operating room. The nurse prepared the anesthetic medications according to the group assignments but did not partake in the anesthesia induction. The anesthesiologist administered anesthesia using the prepared medications and documented the relevant data. Subsequently, the nurse anesthetist transferred the experimental data to an independent statistician for analysis. Consequently, patients, anesthesiologists, and nurses remained blinded to the group allocations by having identical preparation and labeling of the treatment (ciprofol and propofol), so the treatments are indistinguishable.
Anesthesia procedures
Before surgery, patients were visited, informed consent was obtained, and they were instructed to fast for 8 h and abstain from water. In the operating room, patients' ECG, pulse oximetry, and electroencephalographic bispectral index (BIS) were monitored. Under local anesthesia using lidocaine, a radial artery catheter was inserted to enable continuous monitoring of arterial blood pressure. Arterial blood samples were obtained for blood gas analysis, while venous blood was collected to assess activated clotting time (ACT). Upon establishing peripheral venous access, anesthesia was induced, followed by the placement of a triple-lumen catheter through puncture of the right internal jugular vein.
Induction of anesthesia
Prior to anesthesia induction, patients underwent pre-oxygenation for 3 min. Midazolam and sufentanil were administered intravenously at doses of 0.03 mg/kg and 0.5 μg/kg, respectively. Patients were subsequently administered either 0.3 mg/kg of ciprofol or 1.5 mg/kg of propofol over a period of 30 s while asking the patient if they felt pain at the injection site VAS > 5. Upon loss of corneal reflex, rocuronium bromide 1.0 mg/kg was administered. Tracheal intubation was performed 1 min later. Hypotension, defined as a mean arterial pressure below 60 mmHg, was treated by administering a single intravenous injection of 40 μg phenylephrine. Severe bradycardia, defined as a heart rate below 45 beats per minute, was treated with a single 0.25 mg dose of atropine. Hypoxia was identified when oxygen saturation fell below 90%, and tachycardia was recognized when heart rate exceeded 120 beats per minute. The administration frequency of phenylephrine and atropine was recorded.
Anesthesia maintenance
Both groups received propofol (3–8 mg/kg/h), sufentanil (0.8 μg/kg/h), dexmedetomidine (0.5 μg/kg/h), and rocuronium bromide (0.6 mg/kg/h) for the maintenance of anesthesia. During the maintenance of anesthesia, the mechanical ventilation settings were standardized for all patients. The respiratory rate was adjusted to maintain an end-tidal carbon dioxide tension (EtCO2) between 35 and 40 mmHg, as measured by capnography. The fraction of inspired oxygen (FiO2) was set at 0.6 to maintain arterial oxygen saturation (SpO2) between 95 and 99%, as monitored by pulse oximetry. Tidal volume was set at 7 ml/kg, and the positive end-expiratory pressure (PEEP) was maintained at 5 cmH2O to prevent atelectasis. These settings were adjusted as necessary to ensure optimal oxygenation and ventilation for each patient. The BIS value was sustained within a range of 40–60.
Outcome measures
The primary outcome measure was to evaluate hemodynamic fluctuations during anesthesia induction, specifically the changes in mean arterial pressure (MAP) and heart rate (HR). MAP was recorded at four critical time points: before induction of anesthesia (T1), before tracheal intubation (T2), 1 min after tracheal intubation (T3), and five minutes after tracheal intubation (T4). The incidence of hypotension and bradycardia during induction and the number of doses of phenylephrine and atropine were also recorded. The secondary outcomes were the incidence of injection pain, latency to loss of corneal reflex (LOC), oxygen saturation, and oxygenation index before and ten minutes after induction of anesthesia.
Statistical analysis
We determined the sample sizes required based on the difference in mean arterial pressure (∆MAP) at the T1 time point. In a preliminary study, we observed a ∆MAP of -15.1 ± 7.3 mmHg and -19.8 ± 6.7 mmHg in the propofol and ciprofol groups, respectively. With an assumed alpha level of 0.05 and a power of 0.80, we calculated that approximately 40 patients would be necessary in each group. The statistical analysis was performed using SPSS version 26.0. The normally distributed measurement data were presented as mean ± standard deviation, and the t-test was used to compare the groups. The χ2 test was used to compare the enumeration data. Statistical significance was considered at P < 0.05.
Results
The study initially screened 87 patients who met the inclusion criteria. Seven patients declined to participate, and two patients were excluded because they did not meet the inclusion criteria after changing their surgical method. Therefore, a total of 78 patients were enrolled in the study and randomized into two groups: the ciprofol group (N = 40) and the propofol group (N = 38). All 78 patients successfully completed the anesthesia procedure (Fig. 1).
Fig. 1.
Participants' flowchart
In terms of baseline characteristics, there were no significant differences observed between the ciprofol group and the propofol group, indicating that the baseline characteristics of the two groups did not affect the results (Table 1).
Table 1.
Characteristics of the participants
| Ciprofol group (n = 40) | Propofol group (n = 38) | P | |
|---|---|---|---|
| Biological sex (n) | 0.622 | ||
| Male | 22(55%) | 23(61%) | |
| Female | 18(45%) | 15(39%) | |
| Age (years) | 64.71 ± 6.32 | 63.18 ± 4.71 | 0.231 |
| BMI (kg/m2) | 23.27 ± 2.65 | 23.89 ± 2.82 | 0.320 |
| ASA class III | 27(67%) | 29(76%) | 0.387 |
| ASA class IV | 13(33%) | 9(24%) | |
| NYHA class II | 35(87%) | 32(84%) | 0.677 |
| NYHA class III | 5(13%) | 6(16%) | |
| Merger disease (n) | |||
| Hypertension | 31(78%) | 27(71%) | 0.515 |
| Diabetes | 2358%) | 19(50%) | 0.507 |
| Stroke | 3(8%) | 3(8%) | 0.948 |
| Type of surgery (n) | |||
| CABG | 29(73%) | 31(82%) | 0.342 |
| Heart valve replacement | 11(27%) | 7(18%) | |
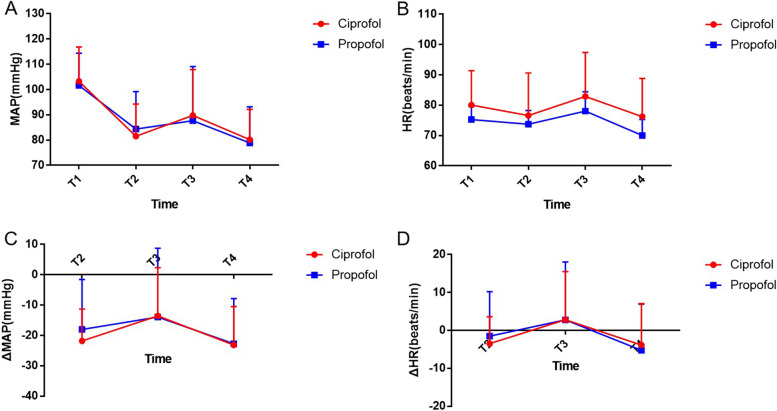
The results showed that there were no significant differences between the ciprofol and propofol groups in terms of mean arterial pressure and heart rate at four time points during induction of anesthesia (Fig. 2A and ). The mean arterial pressure decreased in both groups during induction, but there was no significant difference in the magnitude of the decrease between the two groups (T2: − 21.81 ± 10.50 mmHg vs. − 18.08 ± 16.47 mmHg, P = 0.23) (Fig. 2C). Additionally, both groups experienced a decrease in heart rate during induction, followed by an increase after tracheal intubation, but there was no significant difference in the magnitude of these changes (Fig. 2D). These findings suggest that there were no significant differences in haemodynamic fluctuations between the two groups during induction of anesthesia.
Fig. 2.
Variations in heart rate and blood pressure during anesthesia induction. Error bars represent the standard error of the mean (SEM). T1: before induction of anesthesia; T2: before tracheal intubation; T3: 1 min after tracheal intubation; T4: 5 min after tracheal intubation. ΔMAP: the difference in mean arterial pressure (MAP) calculated as the pressure at each time point minus the baseline pressure (T1). A negative ΔMAP indicates a drop in pressure, while a positive ΔMAP indicates an increase. ΔHR: the difference between the heart rate at each time point and the baseline heart rate. A Mean arterial pressure (MAP) at different time points during anesthesia induction. B Heart rate at different time points during anesthesia induction. C ΔMAP at different time points during anesthesia induction. D ΔHR at different time points during anesthesia induction
In the ciprofol group, the incidence of anesthesia-induced injection pain was significantly lower compared to the propofol group (3% vs. 18%, P < 0.05). However, no significant differences were observed in hypotension, bradycardia, hypersensitivity, and the use of phenylephrine and atropine between the two groups (Table 2). While our primary focus was on hemodynamic outcomes, specifically the changes in mean arterial pressure (MAP) and heart rate during anesthesia induction, we also closely monitored the incidence of hypotension and bradycardia. Although there were no significant differences between the Ciprofol and Propofol groups in these critical hemodynamic parameters, we acknowledge that these findings require a more thorough discussion.
Table 2.
Adverse reactions during anesthesia induction
| Ciprofol group (n = 40) | Propofol group (n = 38) | P | |
|---|---|---|---|
| Time to LOC (min) | 0.89 ± 0.35 | 0.81 ± 0.26 | 0.26 |
| Injection pain | 1(3%) | 7(18%) | 0.02* |
| Hypotension | 13(33%) | 15(39%) | 0.82 |
| Bradycardia | 5(13%) | 3(8%) | 0.50 |
| Tachycardia | 7(18%) | 10(26%) | 0.35 |
| Rash | 0 | 1(3%) | 0.31 |
| Hypoxia | 1(3%) | 1(3%) | 0.97 |
| Patients given phenylephrine | 13(33%) | 15(39%) | 0.82 |
| Patients given atropine | 5(13%) | 3(8%) | 0.50 |
There were no significant differences in oxygen saturation, arterial partial pressure of oxygen, arterial partial pressure of carbon dioxide, or lactate concentration between the ciprofol and propofol groups at baseline and after 10 min of anesthesia induction. (Table 3).
Table 3.
Effect of induction of anesthesia on oxygenation function
| Ciprofol group (n = 40) | Propofol group (n = 38) | P | |
|---|---|---|---|
| SaO2 (%) | |||
| Before anesthesia | 95.9 ± 1.3 | 96.1 ± 1.2 | 0.48 |
| 10 min after anesthesia | 99.2 ± 0.3 | 99.1 ± 0.3 | 0.15 |
| PaO2 (mmHg) | |||
| Before anesthesia | 78.9 ± 6.3 | 81.2 ± 7.1 | 0.13 |
| 10 min after anesthesia | 243.8 ± 25.5 | 221.5 ± 37.5 | 0.32 |
| PCO2 (mmHg) | |||
| Before anesthesia | 32.3 ± 3.8 | 34.5 ± 5.2 | 0.21 |
| 10 min after anesthesia | 35.2 ± 2.2 | 34.2 ± 3.1 | 0.10 |
| Lac (mmol/L) | |||
| Before anesthesia | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.15 |
| 10 min after anesthesia | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.15 |
Discussion
Induction of anesthesia for cardiac surgery carries a higher risk than for other types of surgery, with potential complications such as hemodynamic instability, myocardial depression, arrhythmia, and postoperative cognitive dysfunction [15–17]. Among the sedatives used for anesthesia induction in cardiac surgery, propofol, etomidate, and sevoflurane are commonly utilized [18]. Propofol offers several advantages compared to etomidate, such as a faster onset of action, shorter duration of action, and smoother induction process, which facilitates control over the depth of anesthesia. When compared to sevoflurane, propofol is more straightforward to administer. However, propofol also presents some drawbacks, including myocardial inhibition, a predisposition to hypotension, and notable pain upon injection [19, 20].
Ciprofol, has emerged as an alternative to propofol due to its unique similarity in chemical structure, yet less side effects in terms of pain on injection (Table 4). An intravenous anesthetic, functions as a GABAA receptor agonist. It influences the chloride channel mediated by GABAA receptors, enhancing current conduction and causing neuronal hyperpolarization. This hyperpolarization leads to consistent neural signal transmission, decreasing the likelihood of action potential generation and subsequently inhibiting the central nervous system, ultimately resulting in anesthesia. Ciprofol exhibits favourable safety and tolerability, with pharmacodynamic activity approximately five times that of propofol. Its onset of action is rapid and stable, and it allows for swift and complete recovery upon cessation. Furthermore, the incidence of injection pain associated with ciprofol is exceedingly low. Its respiratory impact is superior to that of propofol, while its effects on heart rate and blood pressure are noninferior. Additionally, ciprofol requires less lipid infusion compared to propofol [14, 21].
Table 4.
Characteristics of ciprofol and propofol
| Ciprofol | Propofol | |
|---|---|---|
| Chemical Formula |
C14H20O (R)-2-(1-cyclopropylethyl)-6-isopropylphenol |
C12H18O 2,6-diisopropylphenol |
| pKa Value | 11.8 | 11.1 |
| Potency Difference | 5 more potent than propofol | - |
| Solution Type | Lipid-in-water solution | Lipid-based solution |
| Complications | Hypotension, bradycardia, apnea, respiratory depression, hypoxia | Propofol infusion syndrome |
| Dosage (Adults) | 0.4 mg/kg | Typically 1.5–2.5 mg/kg for induction, followed by maintenance infusion rates |
| Dosage (Children) | - | Typically 1–3 mg/kg for induction, followed by maintenance infusion rates |
| Cost Difference | 1–2 times higher than propofol | Varies depending on the region and brand |
This randomized, double-blind, controlled clinical trial aimed to evaluate the safety and hemodynamic effects of ciprofol for anesthesia induction in cardiac surgery. The results demonstrated that the use of ciprofol for anesthesia induction in cardiac surgery patients provided similar hemodynamic stability compared to propofol. Moreover, the incidence of injection pain was significantly lower in the ciprofol group than in the propofol group.
Hemodynamic stability is of paramount importance during anesthesia induction in cardiac surgery, as it helps to minimize the risk of myocardial ischemia and other complications. Our study found no significant differences in mean arterial pressure and heart rate changes between the ciprofol and propofol groups during anesthesia induction, suggesting that ciprofol is as effective as propofol in maintaining hemodynamic stability in cardiac surgery patients. In light of our primary focus on hemodynamic outcomes, it is important to discuss the implications of our findings on hypotension and bradycardia (Fig. 2).
The hemodynamic profiles of ciprofol and propofol were found to be similar, which is crucial for high-risk cardiac surgery patients where maintaining circulatory stability is paramount. Both agents demonstrated comparable effects on mean arterial pressure and heart rate during anesthesia induction, suggesting that ciprofol may be a viable alternative to propofol for maintaining hemodynamic stability in cardiac surgery patients. This similarity is particularly relevant in high-risk patients, where stable hemodynamic parameters are critical to minimizing the risk of complications such as myocardial ischemia. Although no significant differences in the incidence of bradycardia and hypotension were observed between the groups, ciprofol's comparable safety profile makes it a viable alternative to propofol. Further investigation is required to explore whether ciprofol offers additional benefits in specific patient populations. The lower incidence of injection pain observed in the ciprofol group is a notable advantage over propofol. Injection pain can cause discomfort and anxiety in patients during anesthesia induction, potentially affecting the overall patient experience [22]. The significantly lower incidence of injection pain in the ciprofol group could contribute to improved patient comfort during anesthesia induction. While the reduced incidence of injection pain with ciprofol is noteworthy, its clinical impact may be secondary to the need for maintaining hemodynamic control. Thus, ciprofol's ability to provide a smoother patient experience through less injection pain, combined with its similar hemodynamic profile, may enhance patient comfort without compromising safety in this vulnerable patient population. Further research could explore whether ciprofol offers advantages in specific subgroups of high-risk patients, potentially guiding its use in practice. Regarding oxygenation parameters and lactate concentration, no significant differences were found between the ciprofol and propofol groups, indicating comparable respiratory and metabolic effects for both anesthetics. This further supports the potential use of ciprofol as an alternative to propofol for anesthesia induction in cardiac surgery. While the lower incidence of injection pain with ciprofol is a noteworthy finding, we acknowledge the critical importance of hemodynamic stability and respiratory function in cardiac surgery. Bradycardia and hypotension can have significant consequences, including the potential for myocardial ischemia, increased morbidity, and prolonged hospital stays. In our study, although there were no significant differences between the ciprofol and propofol groups in the incidence of bradycardia (13% vs. 8%) or hypotension (33% vs. 39%), these outcomes remain a primary concern. Management of these adverse events typically involves the use of medications such as atropine for bradycardia and phenylephrine for hypotension, as was done in our study. However, the prevention of these events is ideal, and further research is needed to identify strategies to minimize their occurrence. Respiratory depression, another critical concern in anesthesia, can lead to hypoxia and increased risk of postoperative complications. Both ciprofol and propofol have been shown to have minimal effects on respiratory function compared to other anesthetic agents, which is an advantage in cardiac surgery. However, close monitoring and prompt management remain essential. The clinical significance of these adverse events underscores the need for ongoing evaluation and comparison of new anesthetic agents like ciprofol with established agents such as propofol. Future studies should continue to focus on these critical outcomes to ensure the safety and efficacy of new agents in the cardiac surgery population."
Limitations
A significant limitation of this study is the sample size, which may be underpowered to detect clinically meaningful differences in adverse outcomes such as bradycardia and hypotension fully capture the safety profile. Secondly, the study only included patients with NYHA class II or III cardiac function; therefore, the results may not be applicable to patients with more severe cardiac dysfunction. The present study provides valuable insights into the potential use of ciprofol as a suitable alternative to propofol for anesthesia induction in cardiac surgery. While our results suggest that ciprofol offers similar hemodynamic stability and a lower incidence of injection pain compared to propofol, further investigation is warranted to better understand the clinical implications and potential advantages of ciprofol in various settings. While our study focused on the intraoperative hemodynamics and immediate safety profile of ciprofol, we acknowledge the importance of assessing long-term outcomes to fully evaluate the safety and efficacy of new anesthetic agents. The lack of long-term outcome data represents a limitation of this study, particularly given that anesthesia induction is known to have potential longer-term effects, especially in cardiac surgery patients. Future research will aim to address this gap by including extended follow-up to assess key outcomes such as postoperative cognitive dysfunction, respiratory complications, and myocardial injury. These efforts will provide a more comprehensive evaluation of ciprofol's long-term safety and efficacy, building on the current findings.
Conclusions
In summary, this study suggests that ciprofol anesthesia results in a lower incidence of injection pain compared to propofol, potentially enhancing patient comfort during anesthesia induction. Additionally, ciprofol demonstrates circulatory stability comparable to propofol during anesthesia induction in cardiac surgery, indicating that it could be a viable alternative to propofol for this specific application.
Acknowledgements
The authors would like to thank all patients who participated in this study and the surgeons and nurses who helped complete this study.
Abbreviations
- NYHA
New York Heart Association
- BIS
Bispectral index
- ACT
Activated clotting time
- LOC
Loss of corneal reflex
- MAP
Mean arterial pressure
Authors’ contributions
Le Yu: Conceptualization, Methodology, Investigation process, Formal analysis, Original draft preparation. Xiang Liu: Methodology, Investigation process, Revising manuscript, Original draft preparation. Xiang Zhao: Interpretation of data, Revising manuscript. Xiu Shan: Investigation process. Evelyne Bischof: Conceptualization, Revising manuscript, Project administration, Revising manuscript. Hui-hong Lu: Conceptualization, Methodology, Resources, Project administration, Revising manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The trial was authorized by the Ethics Committee of East Hospital(2022/No.171), Tongji University, Shanghai, China, and was registered on ClinicalTrials.gov (NCT06312345). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Evelyne Bischof, Email: bischofevelyne@gmail.com.
Hui-hong Lu, Email: luhuihongdfyy@163.com.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 4.Diaz Milian R. Anesthesia for cardiac surgery. N Engl J Med. 2019;381(1):97. [DOI] [PubMed] [Google Scholar]
- 5.Walsh CT. Propofol: milk of amnesia. Cell. 2018;175(1):10–3. [DOI] [PubMed] [Google Scholar]
- 6.Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemphill S, McMenamin L, Bellamy MC, Hopkins PM. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth. 2019;122(4):448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62(7):690–701. [DOI] [PubMed] [Google Scholar]
- 10.Chen BZ, Yin XY, Jiang LH, Liu JH, Shi YY, Yuan BY. The efficacy and safety of ciprofol use for the induction of general anesthesia in patients undergoing gynecological surgery: a prospective randomized controlled study. BMC Anesthesiol. 2022;22(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin L, Ren L, Wan S, Liu G, Luo X, Liu Z, Li F, Yu Y, Liu J, Wei Y. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606–17. [DOI] [PubMed] [Google Scholar]
- 12.Wei Y, Qiu G, Lei B, Qin L, Chu H, Lu Y, Zhu G, Gao Q, Huang Q, Qian G, et al. Oral delivery of propofol with methoxymethylphosphonic acid as the delivery vehicle. J Med Chem. 2017;60(20):8580–90. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Wang X, Liu J, Zuo YX, Zhu QM, Wei XC, Zou XH, Luo AL, Zhang FX, Li YL, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26(5):1607–17. [DOI] [PubMed] [Google Scholar]
- 14.Ding YY, Long YQ, Yang HT, Zhuang K, Ji FH, Peng K. Efficacy and safety of ciprofol for general anaesthesia induction in elderly patients undergoing major noncardiac surgery: a randomised controlled pilot trial. Eur J Anaesthesiol. 2022;39(12):960–3. 10.1097/EJA.0000000000001759. [DOI] [PubMed] [Google Scholar]
- 15.Habibi MR, Baradari AG, Soleimani A, Emami Zeydi A, Nia HS, Habibi A, Onagh N. Hemodynamic responses to etomidate versus ketamine-thiopental sodium combination for anesthetic induction in coronary artery bypass graft surgery patients with low ejection fraction: a double-blind, randomized, clinical trial. J Clin Diagn Res: JCDR. 2014;8(10):Gc01-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedge J, Balajibabu PR, Sivaraman T. The patient with ischaemic heart disease undergoing non cardiac surgery. Indian J Anaesth. 2017;61(9):705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman MF, Mathew JP, Grocott HP, Mackensen GB, Monk T, Welsh-Bohmer KA, Blumenthal JA, Laskowitz DT, Mark DB. Central nervous system injury associated with cardiac surgery. Lancet (London, England). 2006;368(9536):694–703. [DOI] [PubMed] [Google Scholar]
- 18.Sury MR, Palmer JH, Cook TM, Pandit JJ. The state of UK anaesthesia: a survey of National Health Service activity in 2013. Br J Anaesth. 2014;113(4):575–84. [DOI] [PubMed] [Google Scholar]
- 19.Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ (Clinical research ed). 2011;342:d1110. [DOI] [PubMed] [Google Scholar]
- 20.Grounds RM, Twigley AJ, Carli F, Whitwam JG, Morgan M. The haemodynamic effects of intravenous induction. Comparison of the effects of thiopentone and propofol. Anaesthesia. 1985;40(8):735–40. [DOI] [PubMed] [Google Scholar]
- 21.Lu M, Liu J, Wu X, Zhang Z. Ciprofol: a novel alternative to propofol in clinical intravenous anesthesia? Biomed Res Int. 2023;2023:7443226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Guo P, Yang L, Liu Z, Yu D. Comparison and Clinical Value of Ciprofol and Propofol in Intraoperative Adverse Reactions, Operation, Resuscitation, and Satisfaction of Patients under Painless Gastroenteroscopy Anesthesia. Contrast Media Mol Imaging. 2022;2022:9541060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.