Abstract
Background
Recent studies have identified a correlation between insulin resistance (IR) and depression. This study aims to explore the correlation between estimated glucose disposal rate (eGDR), a practical and noninvasive measure for assessing IR, and depression in the general population.
Methods
In this population-based cross-sectional study, data from 28,444 adults aged 18 years old or older in the NHANES during the period from 1999 to 2018 were analyzed. The correlation between eGDR and depression was examined through multivariate logistic regression analyses, subgroup analyses, restricted cubic spline, and interaction tests. Furthermore, a mediation analysis was conducted to elucidate the role of the atherogenic index of plasma (AIP) in mediating the effect of eGDR on depression.
Results
Multivariate logistic regression analysis and restricted cubic splines analysis indicated that eGDR can exhibit a linearly correlation with depression (OR = 0.913; 95% CI: 0.875, 0.953). Subjects in eGDR6-8 and eGDR > 8 groups had a decrease risk of depression as 25.4% and 41.5% than those in the eGDR < 4 group. This negative correlation was more pronounced in those with obesity. Mediation analysis indicated that AIP mediated 9.6% of the correlation between eGDR and depression.
Conclusions
eGDR was linear negatively correlated with depression, with AIP playing a mediating role. This study provides a novel perspective on the mechanism connecting IR to depression. Managing IR and monitoring AIP may contribute to alleviating depression.
Keywords: Estimated glucose disposal rate, Depression, Insulin resistance, Waist circumference
Introduction
Depression is a widely acknowledged mental illness having a substantial correlation with suicide. Approximately 40% of individuals who died by suicide have been diagnosed as depression [1]. The World Health Organization (WHO) identified depression as the third largest contributor to the global disease burden as early as 2008 and projected that it could become the leading cause of the diseased burden by 2030 [2]. In the United States, an estimated 17.3 million adults, representing 6.7% of the population, experienced at least one major depressive episode in 2017, being positioned as the foremost cause of disability among individuals aged 15 to 44 [3]. Furthermore, depression is correlated with an elevated risk of developing a range of physical illnesses, including cardiovascular disease, metabolic disorders, dementia, and cancers [4–7]. Given the frequent comorbidity and high prevalence of depression with other diseases, preventing depression has become a critical public health concern.
Numerous population-centric observational studies have illuminated the correlation between IR-induced metabolic diseases and depression [8–10]. The underlying etiology and the pathophysiological link between depressive symptoms and metabolic disorder such as T2DM have to be elucidated yet. However, some studies suggested that IR play a key role in its pathophysiology [11, 12], which may be attributed to peripheral IR leading to central IR. Central insulin is instrumental in multiple neural circuits. It can modulate dopamine release, participate in signal transduction in various glial cells in the brain, regulate the production, structure, and function of mitochondria, thereby influencing emotional cognition and behaviors [13]. The conventional assessment on IR has traditionally been a protracted and laborious process, predominantly depending on the hyperinsulinemiceuglycemic clamp (HIEC) as the paramount standard.
eGDR, combined with easily obtainable clinical parameters such as hypertension, waist circumference, and hemoglobin A1c (HbA1C), has been proposed as a straightforward surrogate marker for IR in patients with T2DM [14]. Previous studies have demonstrated that this method exhibits high accuracy compared with the gold standard [14, 15]. Additionally, earlier studies have expanded the application of eGDR to patients with T2DM, acute ischemic stroking and non-diabetes mellitus, revealing a significant correlation between eGDR and outcomes such as the mortality of cardiovascular disease (CVD), all-cause mortality, and diabetic complications [16–19].
However, the correlation between eGDR and depression remains unclear. Consequently, this study aims to explore the correlation between eGDR and depression in a large, nationally-representative sample of American adults. The dataset from National Health and Nutrition Examination Survey (NHANES) was collected from 1999 to 2018.
Methods
Research subjects and design
NHANES, conducted by National Center for Health Statistics (NCHS) [20], is a comprehensive study designed to assess the correlation between nutrition, health promotion, and disease prevention. The survey shall be conducted every two years by taking physical examinations, interviews, and various sections covering dietary, demographic, examinations, and laboratory data.
For the present study, data were collected from ten 2-year cycles of NHANES from 1999 to 2018. Subjects aged 18 years old and older were included (n = 59204). Those with incomplete data on Patient Health Questionnaire-9 (PHQ-9) (n = 28013) and eGDR (n = 2182) were excluded. Furthermore, 565 pregnant individuals were excluded due to potential alterations in waist circumference, blood lipid profiles, and depression status (as shown in Fig. 1). Ultimately, the study comprised a total of 28,444 subjects.
Fig. 1.
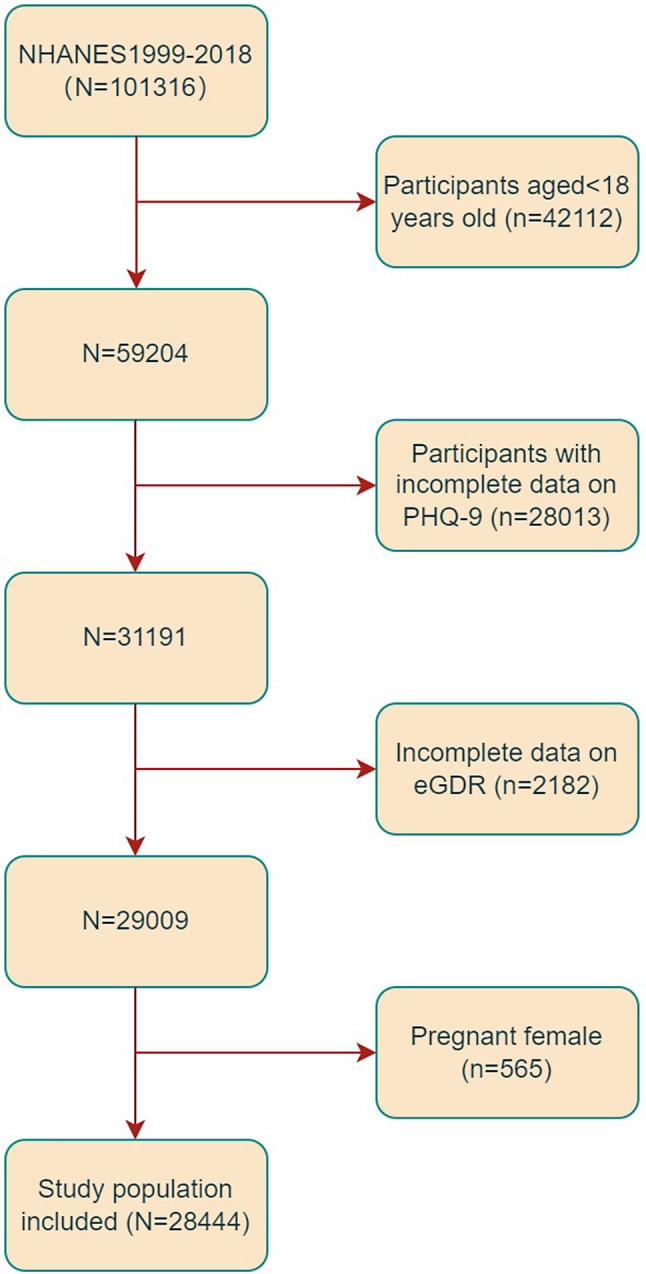
Flowchart of the sample selection from the 1999–2018 NHANES
Ascertainment of depression
PHQ-9 patient health questionnaire scale, a self-administered scale, was for screening and assessing the severity of depression. PHQ-9 score ranges from 0 to 27, with each of the nine items scoring from 0 to 3 (0 = never; 1 = many days; 2 = half the days; 3 = almost daily) [21]. A cumulative score of 10 or higher is indicative of the presence of depression [21]. This threshold is frequently employed in epidemiological studies to identify patients with depression and has been validated through clinical evaluation [21].
Measurement of eGDR
eGDR was calculated as a previously established formula: eGDR (mg/kg/min) = 21.158 - (hypertension × 3.407) - (0.551 × HbA1C [%]) - (0.09 × waist circumference [cm]), where, hypertension was coded as either 0 (absence of hypertension) or 1 (presence of hypertension) [22]. Hypertension was characterized by a physician’s diagnosis, the current use of antihypertensive medications, or a systolic and/or diastolic blood pressure measurement ≥ 140/90 mmHg [23]. According to previous studies [24], subjects were classified into four groups based on their baseline eGDR:< 4, 4–5.99, 6–7.99, and ≥ 8 mg/kg/min, with the lowest eGDR category (< 4 mg/kg/min) as the control group.
Ascertainment of atherogenic index of plasma (AIP)
The determination of AIP was predicated upon the measurement on high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) concentrations in the bloodstream. The precise mathematical formula utilized for calculating AIP was expressed as follows: log10 [TG (mmol/L)/HDL-C (mmol/L)] [25].
Covariates
The analysis incorporated various covariates, including socioeconomic and demographic factors (age, poverty-income ratio (PIR), gender, marital status, race, education level, height, weight and waist circumference), lifestyle variables (smoking, physical activities, and alcohol consumption, sleep disorder, dietary intake factors, encompassing energy and fiber intake), medical history (coronary heart disease (CHD), stroking, diabetes mellitus, hypertension and cancers), as well as laboratory test results (serum TG, urine albumin-to-creatinine ratio (UACR), total cholesterol, creatinine, HDL-C, HbA1c and low density lipoprotein cholesterol (LDL-C)). Self-reported race was categorized into the following four races: non-Hispanic Black, non-Hispanic White, other Hispanic, Mexican Americans, and other races. Educational level was divided into two levels: high school or above, less than high school. Marital status was sorted into three groups: married or living with a partner, separated, divorced or widowed and never married. Smoking status was categorized into the following two groups: former (smoked more than 100 cigarettes in life and do not smoke at all now), or current (smoked more than 100 cigarettes in life and smoke some days or every day), and never (smoke less than 100 cigarettes in life). Alcohol consumption was assessed by using a question: “In one year, have you had at least 12 drinks of any type of alcoholic beverage?” Participants who answered ‘yes’ were identified as alcohol drinkers. Participants having diabetes mellitus were identified by having any of the following conditions: Have been told by a doctor or health professional having diabetes mellitus, HbA1c ≥ 6.5%, fasting plasma glucose ≥ 7.0 mmol/l, two-hour OGTT blood glucose ≥ 11.1 mmol/l, and use of diabetes mellitus medication or insulin. Hypertension in participants was defined based on any of the following: ever been told by a doctor or a health professional that had hypertension, mean systolic blood pressure ≥ 140 mmHg, and mean diastolic blood pressure ≥ 90 mmHg. The diagnoses of CHD, stroking, cancers and sleep disorder were based on self-report by participants. All participants completed 24-hour dietary recalls, and the mean consumption rates derived from these recalls were utilizes. eGFR was calculated as the CKD-EPI formula [26]. CKD was defined in accordance with current clinical guidelines as UACR exceeding 30 mg/g, eGFR of less than 60 mL/min/1.73 m², or both conditions [27, 28]. Body mass index (BMI), calculated as weight in kilograms divided by the height in meters. Additional information regarding the NHANES database can be found at http://www.cdc.gov/nhanes.
Statistical analyses
In accordance with PHQ-9 scores, subjects in this study were categorized into two distinct groups: those with depression and those without depression [21]. Continuous variables were expressed as mean ± standard deviation (SD) and compared with Student’s t-test between groups. Categorical data were presented as counts and percentages [n (%)] and analyzed with chi-square test. Multivariate logistic regression models were adopted to describe the correlation between eGDR and depression. Significant variables identified in the univariate analysis were included in the multivariate analysis. Three models were employed in the analyses: Model 1 was unadjusted, and Model 2 was adjusted for gender and age. Model 3, the final multivariable model, included additional adjustments for race, total energy and fiber intake, AIP, drinking, BMI, smoking, moderate physical activities, CHD, stroking, diabetes mellitus, CKD, sleep disorder, PIR, education level, marital status, albumin, and cancers. Additionally, restricted cubic spline (RCS) analysis was conducted to determine whether the correlation between eGDR and depression is linear. Subgroup analyses were also conducted to assess the influence of eGDR on depression concerning several stratified covariates, including age, BMI, education level, gender, and disease status (including CHD, diabetes mellitus, cancers, CKD and stroking). Subsequently, the mediation package was employed for mediation analysis, and the confidence interval of the mediation effect was evaluated with the Bootstrap method to quantify the proportion of AIP in the mediation effect. Data analyses were executed by using R software and Free Statistics software, with statistical significance established at a two-sided P value of less than 0.05.
Results
Baseline characteristics
Table 1 shows a comprehensive summary of the subjects’ characteristics. Of the 28,444 subjects included in the study, 14,116 (49.6%) were females and 14,328 (50.4%) were males, with a mean age of 47.7 ± 18.6 years old. Among these subjects, 2,417 (8.5%) exhibited depression. The majority of patients with depression were females, who had a higher prevalence of current or former smoking, lived alone, sleep disorder, and had lower HDL-C, total energy and fiber intake and family PIR levels. Additionally, they were more likely to lack physical activities and had higher BMI, waist circumference, HbA1c, TC, and TG. Furthermore, these individuals were more frequently correlated with underlying medical conditions such as hypertension, CHD, diabetes mellitus, CKD, stroking, and cancers. eGDR in the depression group was 7.24 ± 2.92, which was lower than 8.07 ± 2.67 observed in the non-depression group.
Table 1.
Characteristics of the study population based on depression
| Characteristic | Total (n = 28444) | PHQ-9 < 10 (n = 26027) | PHQ-9 ≥ 10 (n = 2417) | P value |
|---|---|---|---|---|
| Age | 47.7 ± 18.6 | 47.8 ± 18.7 | 47.4 ± 16.8 | 0.365 |
| Gender, % | < 0.001 | |||
| Male | 14,328 (50.4) | 13,437 (51.6) | 891 (36.9) | |
| Female | 14,116 (49.6) | 12,590 (48.4) | 1526 (63.1) | |
| Race, % | < 0.001 | |||
| Mexican American | 4731 (16.6) | 4338 (16.7) | 393 (16.3) | |
| Other Hispanic | 2761 (9.7) | 2431 (9.3) | 330 (13.7) | |
| Non-Hispanic White | 12,393 (43.6) | 11,382 (43.7) | 1011 (41.8) | |
| Non-Hispanic Black | 5934 (20.9) | 5410 (20.8) | 524 (21.7) | |
| Other Race | 2625 (9.2) | 2466 (9.5) | 159 (6.6) | |
| Education level, % | < 0.001 | |||
| Less than high school | 6636 (24.8) | 5786 (23.7) | 850 (36.9) | |
| High school or above | 20,110 (75.2) | 18,655 (76.3) | 1455 (63.1) | |
| Marital, % | < 0.001 | |||
| Married/living with partner | 17,026 (62.6) | 15,800 (63.5) | 1226 (52.6) | |
| Separated/divorced/widowed | 4989 (18.3) | 4378 (17.6) | 611 (26.2) | |
| Never married | 5194 (19.1) | 4701 (18.9) | 493 (21.2) | |
| Moderate physical activity, % | < 0.001 | |||
| Yes | 9935 (41.0) | 9369 (42.4) | 566 (26.1) | |
| No | 14,322 (59.0) | 12,721 (57.6) | 1601 (73.9) | |
| Alcohol status, n% | 0.727 | |||
| Current or ever, % | 19,639 (71.4) | 17,951 (71.4) | 1688 (71.7) | |
| Never | 7856 (28.6) | 7191 (28.6) | 665 (28.3) | |
| Smoking status, n% | < 0.001 | |||
| Current or ever, % | 12,321 (45.2) | 10,912 (43.8) | 1409 (60.2) | |
| Never | 14,921 (54.8) | 13,989 (56.2) | 932 (39.8) | |
| Sleep disorder | < 0.001 | |||
| Yes | 1885 (7.9) | 1420 (6.5) | 465 (22.8) | |
| No | 21,867 (92.1) | 20,295 (93.5) | 1572 (77.2) | |
| Hypertension, % | < 0.001 | |||
| Yes | 9635 (33.9) | 8548 (32.8) | 1087 (45.0) | |
| No | 18,809 (66.1) | 17,479 (67.2) | 1330 (55.0) | |
| Diabetes, % | < 0.001 | |||
| Yes | 3882 (13.7) | 3387 (13.0) | 495 (20.5) | |
| No | 24,542 (86.3) | 22,625 (87.0) | 1917 (79.5) | |
| CHD, % | < 0.001 | |||
| Yes | 1054 (4.0) | 912 (3.7) | 142 (6.2) | |
| No | 25,614 (96.0) | 23,465 (96.3) | 2149 (93.8) | |
| Stroke | < 0.001 | |||
| Yes | 914 (3.4) | 750 (3.1) | 164 (7.1) | |
| No | 25,820 (96.6) | 23,684 (96.9) | 2136 (92.9) | |
| CKD, % | < 0.001 | |||
| Yes | 5130 (18.0) | 4600 (17.7) | 530 (21.9) | |
| No | 23,309 (82.0) | 21,422 (82.3) | 1887 (78.1) | |
| Cancer, % | 0.002 | |||
| Yes | 2507 (9.4) | 2250 (9.2) | 257 (11.2) | |
| No | 24,233 (90.6) | 22,187 (90.8) | 2046 (88.8) | |
| Body mass index, kg/m2 | 28.8 ± 6.7 | 28.7 ± 6.5 | 30.4 ± 7.8 | < 0.001 |
| Waist circumference, cm | 98.5 ± 16.3 | 98.2 ± 16.1 | 101.8 ± 18.0 | < 0.001 |
| HbA1c, % | 5.71 ± 1.04 | 5.69 ± 1.02 | 5.85 ± 1.24 | < 0.001 |
| Albumin, g/dl | 42.72 ± 3.29 | 42.80 ± 3.26 | 41.92 ± 3.56 | < 0.001 |
| Total cholesterol, mmol/L | 4.89(4.22,5.61) | 4.86(4.22, 5.61) | 4.97(4.24, 5.72) | 0.003 |
| Triglycerides, mmol/L | 1.15(0.80,1.71) | 1.14(0.79, 1.68) | 1.31(0.88, 1.95) | < 0.001 |
| HDL-cholesterol, mmol/L | 1.29(1.06,1.58) | 1.29(1.09, 1.60) | 1.27(1.03, 1.55) | < 0.001 |
| LDL-cholesterol, mmol/L | 2.85(2.28,3.46) | 2.85(2.28, 3.46) | 2.90(2.28, 3.54) | 0.303 |
| Creatinine, umol/L | 0.86(0.72, 1.00) | 0.86(0.72, 1.01) | 0.82(0.70, 0.98) | < 0.001 |
| AIP | -0.05 ± 0.33 | -0.06 ± 0.33 | 0.01 ± 0.35 | < 0.001 |
| PIR | 2.51 ± 1.63 | 2.58 ± 1.63 | 1.70 ± 1.38 | < 0.001 |
| Energy intake, kcal/d | 2045.4 ± 825.2 | 2053.8 ± 824.5 | 1953.7 ± 827.9 | < 0.001 |
| Dietary fiber intake, g | 16.7 ± 9.0 | 16.9 ± 9.0 | 14.3 ± 8.1 | < 0.001 |
| eGDR | 8.00 ± 2.70 | 8.07 ± 2.67 | 7.24 ± 2.92 | < 0.001 |
Values are mean±SD or number (%). P < 0.05 was deemed significant. TC, Total cholesterol; TG, Triglyceride; HDL-c, High density lipoprotein cholesterol; LDL-c, Low density lipoprotein cholesterol; AIP, Atherogenic index of plasma; PIR, Poverty-income ratio; eGDR, Estimated glucose disposal rate; CHD, Coronary heart disease
Correlation between eGDR and depression
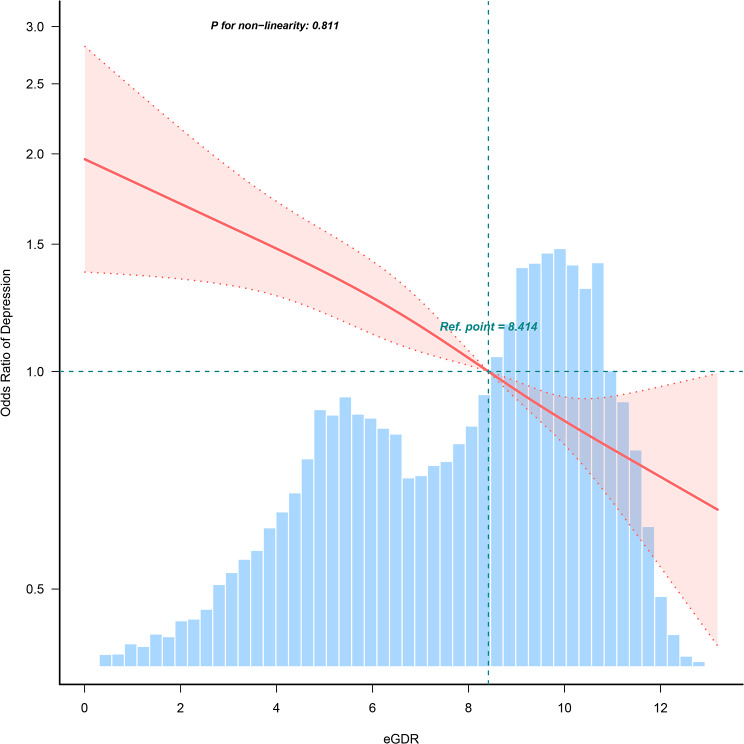
In the multiple logistic regression analysis, a significant inverse correlation between eGDR and depression was identified after adjusting for confounders in Model 3 (OR = 0.913, 95% CI: 0.875, 0.953). To further explore eGDR, the subjects were categorized into four groups. The comparison between the eGDR < 4 group and the eGDR4-6 group under Model 3 adjusted did not show a significant difference (OR = 0.849, 95% CI: 0.658, 1.096), with the eGDR < 4 group as the control group. However, a substantial reduction in the prevalence of depression within eGDR6-8 and eGDR > 8 groups were observed compared to the eGDR < 4 group, with decreases of 25.4% (OR = 0.746, 95% CI: 0.562, 0.990) and 41.5% (OR = 0.585, 95% CI: 0.431, 0.795), respectively, as detailed in Table 2. RCS analyses demonstrated a linear correlation between eGDR and depression (as shown in Fig. 2).
Table 2.
Associations between eGDR and depression
| Subgroups | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value | |
| eGDR | 0.897 (0.884, 0.911) | < 0.001 | 0.864 (0.850, 0.878) | < 0.001 | 0.913 (0.875, 0.953) | < 0.001 |
| eGDR (category) | ||||||
| < 4 | 1(Ref) | 1(Ref) | 1(Ref) | |||
| 4–6 | 0.669 (0.58 ~ 0.772) | < 0.001 | 0.671 (0.581 ~ 0.775) | < 0.001 | 0.849 (0.658 ~ 1.096) | 0.210 |
| 6–8 | 0.622 (0.537 ~ 0.72) | < 0.001 | 0.57 (0.491 ~ 0.661) | < 0.001 | 0.746 (0.562 ~ 0.990) | 0.043 |
| > 8 | 0.42 (0.37 ~ 0.476) | < 0.001 | 0.345 (0.302 ~ 0.395) | < 0.001 | 0.585 (0.431 ~ 0.795) | < 0.001 |
| P for trend | 0.764 (0.735 ~ 0.793) | < 0.001 | 0.709 (0.68 ~ 0.739) | < 0.001 | 0.836 (0.761 ~ 0.918) | < 0.001 |
Model 1: None covariates were adjusted; Model 2: Gender and age were adjusted; Model 3: Gender, age, race, AIP, drinking, BMI, smoking, moderate physical activities, CHD, stroke, diabetes, sleep disorder, CKD, PIR, education level, marital status, albumin, cancer, total energy and fiber intake were adjusted
Fig. 2.
Restricted cubic spline fitting for the association between eGDR and depression
Subgroup analysis
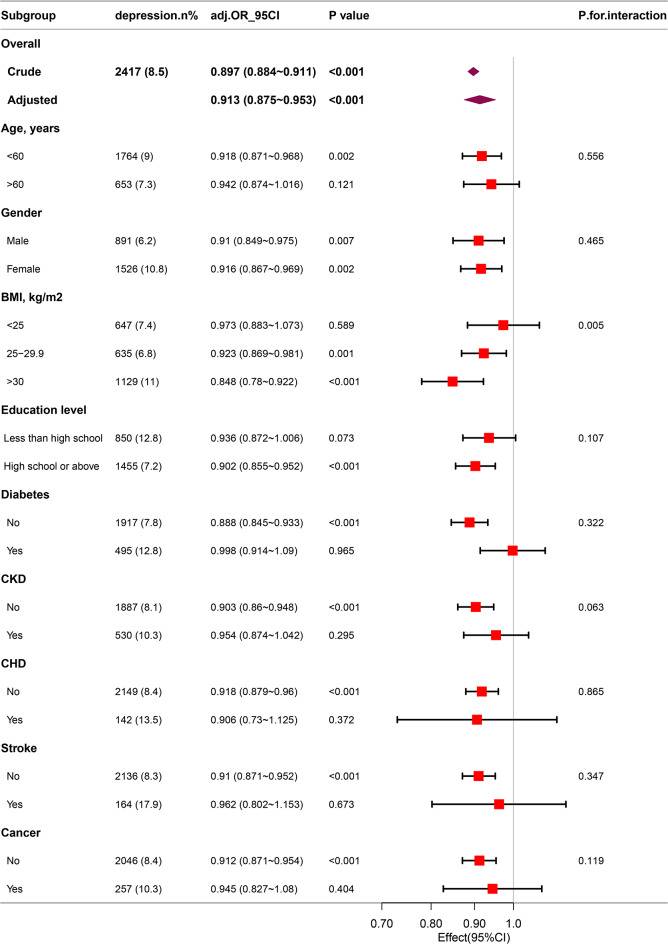
To further explore the correlation between eGDR and depression across various populations stratified by age, BMI, education level, gender, and disease status (including CHD, diabetes mellitus, cancers, CKD and stroking), subgroup analyses were conducted. This study identified a significant interaction effect of BMI on the correlation between eGDR and depression, with an interaction p < 0.05. Specifically, among patients with obesity, each one-unit increase in eGDR was correlated with a 15.2% reduction in the incidence of depression (OR: 0.848; 95%CI: 0.780–0.922). Notably, other covariates, including age and gender, did not demonstrate interactive effects on the correlation between eGDR and depression (as shown in Fig. 3).
Fig. 3.
Association between eGDR and the risk of depression in various subgroups
Mediation analysis
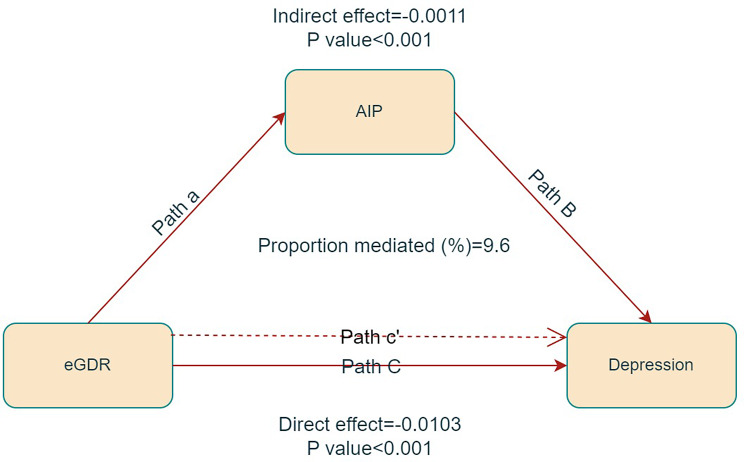
In the mediation analysis, eGDR was treated as the independent variable, AIP as the mediator, and depression as the dependent variable. The mediation model and corresponding pathways are illustrated in Fig. 4. The findings revealed a significant correlation between eGDR and AIP (β = -0.025, p < 0.001), as well as between AIP and depression (β = 0.393, p < 0.001). Further analysis indicated a significant indirect effect of eGDR on depression mediated by AIP, with an effect of -0.0103 (p < 0.001), suggesting that AIP partially mediates the correlation between eGDR and depression, accounting for approximately 9.6% of the total effect (as shown in Table 3).
Fig. 4.
Mediated analysis model path diagram. Notes: eGDR was defined as the independent variable; depression as the dependent variable; and AIP as the mediating variable. Path a represents the regression coefficient of the association between eGDR and AIP. Path b represents the regression coefficient of the association between AIP and depression. Path c represents the simple total effect of eGDR on depression. Path c’ represents the direct effect of eGDR on depression when controlling for AIP
Table 3.
Mediation analysis of AIP in the association between eGDR and depression
| Independent variable | Mediator | Total effect | Indirect effect | Direct effect | Proportion mediated, % |
|||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |||
| eGDR | AIP | -0.0114 (-0.0192, -0.0055) | < 0.001 | -0.0011 (-0.0019, -0.0004) | < 0.001 | -0.0103 (-0.0178, -0.0045) | < 0.001 | 9.6 |
Discussion
This study identified a negative correlation between eGDR and depression, with a significantly stronger correlation among patients with obesity. Furthermore, mediation analysis indicated that AIP partially mediated the correlation between eGDR and depression.
Multiple previous studies have documented a correlation between IR and depression. A specific exploration, drawn upon data from the Netherlands Study of Depression and Anxiety, revealed a link between IR and ongoing, persistent major depression. Additionally, it was proposed in this study that IR could serve as a characteristic feature of depressive conditions [29]. Adriaanse et al. examined a sample of 541 individuals aged 55–75 years old in the Netherlands and found a weak correlation between depression and IR (assessed by HOMA-IR) [30].
eGDR, combining with easily obtainable clinical parameters such as hypertension, waist circumference, and HbA1C, has been proposed as a straightforward surrogate marker for IR in patients with T2DM [14]. Previous studies have demonstrated that this method exhibits high accuracy compared with the gold standard [14, 15]. Previous studies have demonstrated that eGDR serves as an independent predictor of all-cause mortality [31], coronary artery disease [32] and peripheral vascular disease [33] in patients with T2DM. More recently, several studies have explored the applicability of eGDR in non-diabetic individuals [34], individuals with T2DM [15, 35], and those with acute ischemic stroking [15, 36]. Given that many of these conditions involve vascular sclerosis and damage, which are critical risk factors for depression, a strong correlation between eGDR and depression was hypothesized. To the best of our knowledge, this is the first study to explore the correlation between eGDR and depression.
The precise mechanisms underlying the correlation between depression and IR has not been fully elucidated. However, several potential pathways have been proposed. Emerging evidence indicates that patients with depression exhibit dysregulation in homeostatic systems, particularly in the inflammatory responses and hypothalamic-pituitary-adrenal (HPA) axis, both of which have been implicated in the pathogenesis of MetS and IR. Disorders of the HPA axis have been implicated in the pathogenesis of depression [37, 38], with alterations in glucocorticoid sensitivity and systemic cortisol effects documented in patients with stress-related disorders [39]. Hyperactivation of the HPA axis is correlated with increased lipid storage, accumulation of visceral adiposity, and enhanced lipogenesis. Moreover, hypercortisolism induces the synthesis of very low-density lipoproteins and lipolysis, ultimately resulting in hypertriglyceridemia [40]. Elevated serum triglyceride levels enhance the release of free fatty acids from adipose tissues, thereby contributing to IR in non-adipose tissues [41, 42]. Additionally, in instances of severe obesity, abdominal adipose tissues function as an endocrine organ, secreting hormones and inflammatory cytokines [43, 44]. These inflammatory cytokines can cross the blood-brain barrier, disrupting neurotransmission and causing neurological dysfunction, as well as reducing neurogenesis in brain structures involved in mood regulation [45]. Chronic inflammation and activation of the HPA axis can also affect insulin sensitivity, resulting in metabolic disturbances. The components of eGDR, such as waist circumference, hypertension, and HbA1c, are integral to these mechanisms. Therefore, eGDR can reflect IR in the body, and its use in predicting the risk of having depression provides a certain basis for health promotion and prevention of depression.
The findings suggest that AIP partially mediates the correlation between IR (as determined by the eGDR) and depression, which underscores the importance of monitoring AIP in patients with low eGDR. Previous studies have demonstrated a significant correlation between depression and lipid metabolism disorders [46, 47]. Therefore, regulating AIP, particularly by reducing TG levels and increasing HDL-C levels, may mitigate the risk of having depression in individuals with low eGDR.
A significant interaction effect was identified between eGDR and BMI, indicating that the correlation between eGDR levels and depression is particularly pronounced among patients with obesity. Elevated fasting glucose levels are critical risk factors for obesity. Furthermore, patients with obesity were predisposed to comorbidities such as hypertension and diabetes mellitus. As indicated in the research, obese individuals were 3.88 times more likely to have comorbid diabetes mellitus compared to individuals with normal weight [48]. The metabolic disorders correlated with obesity are also significant contributors to the exacerbation of depression.
A significant strength of this study lies in the utilization of a nationally representative sample from the NHANES, enabling a thorough evaluation on diverse population characteristics. This methodological approach enhances the generalizability of findings to a broader spectrum of American adults. Furthermore, the study was meticulously controlled for numerous confounding variables, yielding robust estimates of the independent correlation between eGDR and the prevalence of depression. Finally, this study performed an intermediary analysis to explore the correlation between IR, AIP, and depression.
It is essential to recognize several significant limitations inherent in this study. Firstly, the cross-sectional study design constrains the capacity to infer causality between eGDR and depression. To address this limitation, future research should incorporate experimental methodologies and longitudinal surveys to elucidate temporal correlation and underlying mechanisms. Secondly, the PHQ-9 scale used for diagnosing depression relies on self-reported data and lacks validation by clinical practitioners. It is important to acknowledge that PHQ-9 is extensively employed in both epidemiological and clinical contexts and has been rigorously validated, demonstrating high specificity and sensitivity [49]. Lastly, the findings are specific to Americans, thereby constraining the generalizability of the results to other demographic groups.
Conclusion
eGDR was negatively correlated with depression, with AIP playing a mediating role. This study provides a novel perspective on the mechanism connecting IR to depression. Managing IR and monitoring AIP may contribute to alleviating depression. Given the clinical significance of the correlation with depression, reducing IR and AIP may represent a potential treatment approach.
Acknowledgements
We would like to thank the NHANES database for providing the data source for this study.
Author contributions
XHZ designed the study; HL, JX collected biochemical data; YYC drafted the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
The datasets generated and analysis during the current study are available in the NHANES, www.cdc.gov/nchs/NHANEs/.
Declarations
Ethics approval and consent to participate
The National Center for Health Statistics Ethics Review Board has approved the implementation of NHANES, and every participant signed informed consent. This study also was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University (No. LCKY2023-21, date: Jan 2023).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanyuan Chen and Hao Lin contributed equally to this work.
References
- 1.Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(8):868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiller JW. Depression and anxiety. Med J Aust. 2013;199(S6):S28–31. [DOI] [PubMed] [Google Scholar]
- 4.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE. Depression and coronary heart disease. Nat Rev Cardiol. 2017;14(3):145–55. [DOI] [PubMed] [Google Scholar]
- 6.Hidese S, Asano S, Saito K, Sasayama D, Kunugi H. Association of depression with body mass index classification, metabolic disease, and lifestyle: a web-based survey involving 11,876 Japanese people. J Psychiatr Res. 2018;102:23–8. [DOI] [PubMed] [Google Scholar]
- 7.Wakefield CE, Butow PN, Aaronson NA, Hack TF, Hulbert-Williams NJ, Jacobsen PB. International Psycho-Oncology Society Research C: patient-reported depression measures in cancer: a meta-review. Lancet Psychiatry. 2015;2(7):635–47. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Zhu X, Liu Y, Jia F, Yuan H, Wang Q, Zhang X, Li Z, Du X, Zhang X. Association between triglyceride glucose index and suicide attempts in patients with first-episode drug-naive major depressive disorder. Front Psychiatry. 2023;14:1231524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L, Wu Q, Wang S. Cardiometabolic index is associated with increased depression: a population-based study. J Affect Disord. 2024;348:259–64. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, Wang D, Chen S, Tang L, Ma C. Association of METS-IR index with depressive symptoms in US adults: a cross-sectional study. J Affect Disord. 2024;355:355–62. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Zhang Q, Dai G, Hu J, Zhu C, Su L, Wu X. Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: a meta-analysis. Endocrine. 2016;53(1):35–46. [DOI] [PubMed] [Google Scholar]
- 12.Wahlqvist ML, Lee MS, Chuang SY, Hsu CC, Tsai HN, Yu SH, Chang HY. Increased risk of affective disorders in type 2 diabetes is minimized by sulfonylurea and metformin combination: a population-based cohort study. BMC Med. 2012;10:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Cai W, Hoover B, Kahn CR. Insulin action in the brain: cell types, circuits, and diseases. Trends Neurosci. 2022;45(5):384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabala A, Darsalia V, Lind M, Svensson AM, Franzen S, Eliasson B, Patrone C, Jonsson M, Nystrom T. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: a nationwide cohort study. Cardiovasc Diabetol. 2021;20(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng C, Xing Y, Huo L, Ma H. Relationship between estimated glucose disposal rate and type 2 Diabetic Retinopathy. Diabetes Metab Syndr Obes. 2023;16:807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X, Jiang M, Han L, Zheng X. Estimated glucose disposal rate and risk of cardiovascular disease: evidence from the China Health and Retirement Longitudinal Study. BMC Geriatr. 2022;22(1):968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong X, Wang W. Estimated glucose disposal rate and risk of cardiovascular disease and mortality in U.S. adults with prediabetes: a nationwide cross-sectional and prospective cohort study. Acta Diabetol. 2024;61(1):93-102. [DOI] [PubMed]
- 19.Lu Z, Xiong Y, Feng X, Yang K, Gu H, Zhao X, Meng X, Wang Y. Insulin resistance estimated by estimated glucose disposal rate predicts outcomes in acute ischemic stroke patients. Cardiovasc Diabetol. 2023;22(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56):1–37. [PubMed]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–32. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138(17):e426-e483. [DOI] [PubMed]
- 24.Linn W, Persson M, Rathsman B, Ludvigsson J, Lind M, Andersson Franko M, Nystrom T. Estimated glucose disposal rate is associated with retinopathy and kidney disease in young people with type 1 diabetes: a nationwide observational study. Cardiovasc Diabetol. 2023;22(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apob-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34(7):583–8. [DOI] [PubMed] [Google Scholar]
- 26.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–35. [DOI] [PubMed] [Google Scholar]
- 29.Watson KT, Simard JF, Henderson VW, Nutkiewicz L, Lamers F, Rasgon N, Penninx B. Association of insulin resistance with Depression Severity and Remission Status: defining a metabolic endophenotype of Depression. JAMA Psychiatry. 2021;78(4):439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adriaanse MC, Dekker JM, Nijpels G, Heine RJ, Snoek FJ, Pouwer F. Associations between depressive symptoms and insulin resistance: the Hoorn Study. Diabetologia. 2006;49(12):2874–7. [DOI] [PubMed] [Google Scholar]
- 31.Nystrom T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63. [DOI] [PubMed] [Google Scholar]
- 32.Pane A, Conget I, Boswell L, Ruiz S, Vinals C, Perea V, Gimenez M, Cofan M, Blanco J, Vinagre I, et al. Insulin resistance is associated with preclinical carotid atherosclerosis in patients with type 1 diabetes. Diabetes Metab Res Rev. 2020;36(7):e3323. [DOI] [PubMed] [Google Scholar]
- 33.Olson JC, Erbey JR, Forrest KY, Williams K, Becker DJ, Orchard TJ. Glycemia (or, in women, estimated glucose disposal rate) predict lower extremity arterial disease events in type 1 diabetes. Metabolism. 2002;51(2):248–54. [DOI] [PubMed] [Google Scholar]
- 34.Liu C, Liu X, Ma X, Cheng Y, Sun Y, Zhang D, Zhao Q, Zhou Y. Predictive worth of estimated glucose disposal rate: evaluation in patients with non-ST-segment elevation acute coronary syndrome and non-diabetic patients after percutaneous coronary intervention. Diabetol Metab Syndr. 2022;14(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, Vedovato M, Cavalot F, Zerbini G, Lamacchia O, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xuan J, Juan D, Yuyu N, Anjing J. Impact of estimated glucose disposal rate for identifying prevalent ischemic heart disease: findings from a cross-sectional study. BMC Cardiovasc Disord. 2022;22(1):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–29. [DOI] [PubMed] [Google Scholar]
- 38.Penninx B, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. [DOI] [PubMed] [Google Scholar]
- 40.van Rossum EF, Koper JW, Huizenga NA, Uitterlinden AG, Janssen JA, Brinkmann AO, Grobbee DE, de Jong FH, van Duyn CM, Pols HA, et al. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes. 2002;51(10):3128–34. [DOI] [PubMed] [Google Scholar]
- 41.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(SupplSuppl):S74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parhofer KG. Interaction between glucose and lipid metabolism: more than Diabetic Dyslipidemia. Diabetes Metab J. 2015;39(5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314(1):1–16. [DOI] [PubMed] [Google Scholar]
- 44.McGown C, Birerdinc A, Younossi ZM. Adipose tissue as an endocrine organ. Clin Liver Dis. 2014;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 45.Shelton RC, Miller AH. Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol. 2010;91(4):275–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang TL, Chen JF. Cholesterol and lipids in depression: stress, hypothalamo-pituitary-adrenocortical axis, and inflammation/immunity. Adv Clin Chem. 2005;39:81–105. [PubMed] [Google Scholar]
- 47.Guillemot-Legris O, Muccioli GG. Obesity-Induced Neuroinflammation: beyond the Hypothalamus. Trends Neurosci. 2017;40(4):237–53. [DOI] [PubMed] [Google Scholar]
- 48.Aggarwal R, Yeh RW, Joynt Maddox KE, Wadhera RK. Cardiovascular Risk factor prevalence, treatment, and control in US adults aged 20 to 44 years, 2009 to March 2020. JAMA. 2023;329(11):899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levis B, Benedetti A, Thombs BD, Collaboration DESD. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysis during the current study are available in the NHANES, www.cdc.gov/nchs/NHANEs/.