Abstract
Objective
The cross-sectional study was designed to evaluate the association of ocular surface inflammation with systemic conditions in patients with systemic lupus erythematosus (SLE).
Methods
The study enrolled 30 SLE patients and 30 controls. Ocular symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire. Tear samples from all participants were collected for tear multi-cytokine and chemokine concentration analysis. All participants were assessed for dry eye disease (DED), including Schirmer I test, tear break-up time (TBUT), corneal fluorescein staining (CFS), meibomian gland secretion (MGS), lid-parallel conjunctival folds (LIPCOF), corneal clarity, and symblepharon. Besides, all participants were also examined for conjunctival impression cytology to measure the density of conjunctival goblet cells (CGCs). The peripheral blood indicators from SLE patients were also collected to measure the SLE-associated autoantibody specificities and systemic inflammatory indicators. Pearson and Spearman’s analysis were uesd to examine the correlation between tear cytokines, CGCs, DED-related indicators, and systemic conditions.
Results
The two groups were matched for age and gender in this study. 36.67% of eyes (11 in 30) of SLE patients and 13.33% of eyes (4 in 30) of controls were diagnosed with DED. OSDI scores, abnormal TBUT percentages, CFS percentages, and DED grading were all higher in SLE patients than in control group, while density of CGCs was lower. There were no significant differences in Schirmer I test, MGS, LIPCOF, corneal clarity, and symblepharon between SLE patients and controls. The levels of tear chemokine (C-X-C motif) ligand 11 (CXCL11) and cytokine interleukin-7 (IL-7) in patients with SLE were significantly higher than those in control group. Moreover, among SLE patients, the severity of DED and the level of tear chemokine CXCL11 were significantly positively correlated with SLE-associated autoantibody specificities.
Conclusion
Dry eye and tear cytokines and chemokines-mediated ocular surface inflammation persist in SLE patients and are associated with systemic conditions. Therefore, it is necessary for patients with SLE to combine systemic and ocular assessments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03760-8.
Keywords: Dry eye disease, Systemic lupus erythematosus, Ocular surface inflammation, Autoantibody
Key points
SLE patients have higher OSDI scores, abnormal TBUT percentages, CFS percentages, DED grading, and lower density of CGCs compared to controls. SLE patients have higher levels of tear chemokine CXCL11 and cytokine IL-7 compared to controls.
Among SLE patients, the severity of DED and the level of tear chemokine CXCL11 are significantly positively correlated with SLE-associated autoantibody specificities.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03760-8.
Introduction
Systemic lupus erythematosus (SLE), a complex autoimmune multisystem disease, has significant clinical heterogeneity and considerable potential for morbidity and mortality [1–3]. It is characterized by aberrant immune system activity, leading to an expansion of SLE-associated autoantibody specificities and elevated levels of serum cytokines and chemokines [4–6]. SLE manifests in various ways, including lupus nephritis, arthritis, hematological disease, pulmonary hypertension, and skin erythema [7]. Additionally, SLE may initially present with ocular manifestations [8]. Reports indicate that ocular involvement occurs in 31% of SLE patients and may be associated with systemic disease activity [9]. Autoantibody formation, immune complex deposition, and complement fixation in SLE patients lead to inflammation and damage to ocular structures, such as the eyelid, conjunctiva, cornea, uvea, retina, even optic nerve [9, 10]. Dry eye disease (DED) is the most common manifestation of ocular surface inflammation in SLE patients, with 36–85% reporting dry eye symptoms [10–12].
DED, as a multifactorial ocular surface disease, is characterized by a loss of tear film homeostasis and multiple ocular symptoms. Underlying causes include ocular surface damage and inflammation, tear film hyperosmolarity and instability, and neurosensory abnormalities [13]. Recent studies have found that tear inflammatory cytokines and conjunctival goblet cells (CGCs) are closely related to the pathogenesis of DED. Elevated levels of tear pro-inflammatory cytokines and chemokines are observed in DED, such as chemokine (C-X-C motif) ligand 8 (CXCL8), interleukin (IL)-1β, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) [14–16]. The loss of CGCs has been linked to the severity of dry eye [17–21]. Some studies have shown that the levels of pro-inflammatory cytokines and chemokines in tears, including CXCL8, IL-6, IL-17, IL-21, and TNF-α, are increased in patients with SLE-related DED [22, 23]. However, few studies focus on the correlation between ocular surface inflammation and systemic conditions in SLE patients. Moreover, our study found that many SLE patients have ocular surface pathological changes before the diagnosis of dry eye. Therefore, we believe that the ocular surface damage and the severity of DED may be underestimated in SLE patients. Identifying the correlation between ocular surface pathological changes, DED-related indicators, and systemic conditions in SLE patients, as well as determining if these are distinct from normal controls, could allow for more sensitive identification of dry eye conditions, improve long-term quality of life, and reduce vision loss.
The study aims to collect data on tear cytokines, CGCs, DED-related indicators, and SLE-related systemic indicators in SLE patients and compare them with healthy controls. By exploring the association between tear cytokines, CGCs, DED-related indicators, and SLE-related blood indexes through correlation analysis, we aim to better understand the mechanisms involved. This could contribute to the early diagnosis and treatment of SLE-related DED, ultimately reducing the pain and burden of living with the disease.
Methods
Research population
The study recruited patients sequentially, aged 18–80 years with SLE (excluding secondary Sjogren's syndrome) who were admitted to the Rheumatology Department of Xiangya Second Hospital, Central South University from February 2021 to August 2022. The diagnostic criteria for SLE are based on the 2019 EULAR/ACR Classification Criteria [24, 25]. Details were shown in Supplementary Table 1. The control group included normal volunteers matched for gender and age. Exclusion criteria for all participants was a history of eye drops therapy in the past month, ocular trauma, ocular surgery, other ocular diseases, other autoimmune rheumatic diseases. All participants volunteered for the study and signed an informed consent form. This study has been approved by the Clinical Research Ethics Committee of the Second Xiangya Hospital of Central South University (Ethics No.: LYF2021028).
Assessment for ocular surface disorders
Ocular Surface Disease Index (OSDI)
OSDI is a widely used questionnaire to evaluate the frequency of ocular symptoms and impact among DED patients [26]. It consists of 12 items divided into 3 parts: ocular symptoms, daily activity restrictions, and the influence of environmental factors on the eyes [27–29]. Higher OSDI scores indicate more severe ocular symptoms. The calculation and grade of OSDI are described previously [30]. OSDI score ranges from 0 to 100, with a score ≥ 13 considered DED.
Schirmer I test
The test is performed without anesthesia. In the middle and outer 1/3 of the conjunctival sac in the lower eyelids, tear secretion test papers are placed for five minutes. Record the wetted length as reflex tear secretion. A secretion length of < 10 mm/5 min is considered abnormal [29, 31].
Tear break-up time (TBUT)
Conjunctival sac of the lower eyelid is dropped with sodium fluorescein. Under cobalt blue light, the time when the first spot appeared on the cornea is recorded. TBUT < 10 s indicates abnormal tear stability [31].
Corneal fluorescein staining (CFS)
Cornea is stained with sodium fluorescein, and observed under the cobalt blue light of a slit lamp. OXFORD scale (range from 0 to 4) is used to grade: 0, normal; 1, 2, 3, and 4, abnormal [32].
Meibomian gland secretion (MGS)
The eyelids are gently squeezed with the thumb. The secretions of the meibomian gland are observed under slit lamp. The secretions are scored as follows: 0, clear and transparent liquid (normal); 1, cloudy liquid (abnormal); 2, cloudy granular secretions (abnormal); 3, toothpaste (abnormal) [33].
Lid-parallel conjunctival folds (LIPCOF)
LIPCOF is evaluated on the bulbar conjunctiva above the lower eyelid in an area perpendicular to the temporal and nasal margins. The number of folds is counted and classified: 0 = no conjunctival folds, 1 = 1 permanent and clear parallel fold, 2 = 2 permanent and clear parallel folds, and 3 = more than 2 permanent and clear parallel folds). 0 is considered normal, and 1, 2, and 3 are considered abnormal [34].
Corneal clarity and symblepharon
The slit lamp is used to observe corneal clarity and symblepharon presence. Corneal clarity is scored using a scale of 0–4 (0 = completely clear, 1 = slightly hazy, iris and pupils easily visible, 2 = slightly opaque, iris and pupils still detectable, 3 = opaque, pupils hardly detectable, and 4 = completely opaque with no view of the pupils). 0 is considered normal, and 1, 2, 3, and 4 are considered abnormal [35, 36].
Symblepharon is scored using a scale of 0–3 (0 = no symblepharon, 1 = symblepharon formation involving only the conjunctival surface, 2 = symblepharon formation on < 50% of the corneal surface, 3 = symblepharon formation on > 50% of the corneal surface. 0 is considered normal, and 1, 2, and 3 are considered abnormal [37].
Diagnosis, severity, and classification for DED
DED can be diagnosed in patients with signs (TBUT < 10 s, or CFS ≥ Level 1) and symptoms (OSDI score ≥ 13) according to DEWS II [29]. Given the separation of symptoms and signs of DED, patients with mild or no symptomscan can also be diagnosed if they exhibit severe tear function impairment or ocular surface damage. For sub-classification: Schirmer I test < 10 mm/5 min and TBUT < 10 s indicates mixed dry eye (MDE), Schirmer I test < 10 mm/5 min and TBUT ≥ 10 s indicates aqueous deficient dry eye (ADDE), Schirmer I test ≥ 10 mm/5 min and TBUT < 10 s indicates evaporative dry eye (EDE) [38]. DED severity is assessed according to DEWS I [31, 39], and divided into four levels (Supplementary Table 2).
Tear sample collection and cytokines concentration analysis
Using a sterile pipette tip, add 10 μL saline to the lower conjunctival sac, without topical anesthetics or any other eye drops. The Disposable Micro-Capillary Fluid Collector (Seinda, China) was placed in the middle 1/3 of lower eyelid margin, allowing tears to enter the collector through siphon action. Each tube collected 2.2 μL of tears per collection, and each eye of each patient was sampled three times with an interval of 10 s. 100 μL sterile Eppendorf tubes were used to store tears and stored at -80 °C until detection.
For cytokine detection, tears from the right eyes of all patients were selected and processed according to the instructions for the Milliplex Map Human High Sensitivity T Cell Panel-Immunology Multiplex Assay (Millipore, Billerica, MA, USA). Briefly, dilute each tear sample 2 μL with saline to 25 μL, and add the diluted tear into the corresponding sample well. The positive control group was given the standard of the concentration gradient, and the negative control group was given the same amount of solvent. After incubation and washing with beads and antibodies in all test wells, the fluorescence intensity of cytokines was measured using the liquid chip detector MAGPIX (Luminex, USA) and the software xPONENT® (Luminex, USA). The tear cytokine concentrations were calculated by fitting the standard curve generated from the standard samples.
Conjunctival impression cytology and CGCs density assessment
After administering surface anesthesia to the patient's eyes using Oxybuprocaine Hydrochloride eye drops, a semi-circular sterile cellulose acetate membrane with a diameter of 10 mm (Advantec, Tokyo, Japan) was used to collect cast-off cells of the supramporal conjunctiva.
For periodic acid-Schiff (PAS), the Glycogen Stain Kit (G1360, Solarbio) was used. Samples were fixed by 95% ethanol, washed with water, and treated with periodic acid. Schiff reagent was then added. Ethanol was used for gradient dehydration of the samples, followed by making the cellulose acetate membrane transparent with xylene, then sealing by neutral resin.
The microscopic imaging system Invitrogen™ EVOS™ M7000 (Thermo Scientific, U.S.) were used to observe samples. At 20 × magnification, 5 fields of view were randomly captured for each sample. Image-Pro Plus 6.0 (Media Cybernetics, U.S.) were used to processe all images, and the density of CGCs (CGC/mm2) was calculated.
All the above tests were carried out on the right eye of the participants, and all tests were carried out in the following order: information collection and OSDI questionnaire filling, tear collection, Schimer I test, TBUT, CFS, MGS, LIPCOF, corneal clarity, symblepharon, and finally conjunctival impression cytology.
Typical blood indicators of SLE patients
Collect peripheral blood indicatiors related to disease activity for all patients at the time of admission. These indicators include SLE-related autoantibodies, such as anti-histone antibody (AHA), anti-ribonucleoproteins antibody (anti-RNP), anti-Smith antibody (anti-Sm), anti-dsDNA antibody (anti-dsDNA), anti-Ro-52 antibody (anti-Ro-52), anti-Sjogren's syndrome A antibody (anti-SSA), anti-Sjogren's syndrome B antibody (anti-SSB), and anti-nucleosome antibody (ANuA). Additionally, systemic inflammatory indicators, including complement C4, erythrocyte sedimentation rate (ESR), and lymphocytes and their subsets were collected [40, 41].
Statistics
Samples with incomplete data were excluded. Independent samples t-test was used to compare continuous variables. Categorical variables were tested using chi-square test. Wilcoxon rank sum test was used for rank variables. Correlations between ocular surface disorders and systemic conditions among SLE patients were evaluated by Pearson and Spearman’s correlation analyses. P < 0.05 was considered statistically significant.
Results
Characteristics of study participants
The study enrolled a total of 60 participants, including 30 patients with SLE (30 eyes; 2 males and 28 females) with an average age of 38.90 ± 14.07 years (range 20–63), and 30 normal volunteers (30 eyes; 7 males and 23 females) with an average age of 36.43 ± 17.08 years (range 22–67). There was no significant difference in age and gender between the two groups (Table 1). All participants did not receive any therapeutic treatment, including systemic or topical medications, during the testing period.
Table 1.
The clinical information and ocular surface disorders of the study participants
| Characteristics | SLE, n = 30 | Control, n = 30 | P Value |
|---|---|---|---|
| Age (years) | 38.90 ± 14.07 | 36.43 ± 17.08 | 0.54 |
| Gender-Female, n (%) | 28 (93.33) | 23 (76.67) | 0.15 |
| OSDI score | 12.99 ± 13.71 | 6.05 ± 5.82 | 0.02* |
| OSDI level, n (%) | 0.14 | ||
| 0 (0–12) | 20 (66.67) | 25 (83.33) | |
| 1 (13–22) | 5 (16.67) | 5 (16.67) | |
| 2 (23–32) | 1 (3.33) | 0 | |
| 3 (≥ 33) | 4 (13.33) | 0 | |
| Schirmer I test (mm/5 min) | 12.47 ± 9.85 | 14.63 ± 10.86 | 0.42 |
| Schirmer I test level, n (%) | 0.78 | ||
| Abnormal (< 10 mm/5 min) | 10 (33.33) | 9 (30.00) | |
| Normal (≥ 10 mm/5 min) | 20 (66.67) | 21 (70.00) | |
| TBUT (s) | 5.97 ± 3.07 | 7.37 ± 3.85 | 0.13 |
| TBUT level, n (%) | 0.04* | ||
| Abnormal (< 10 s) | 25 (83.33) | 18 (60.00) | |
| Normal (≥ 10 s) | 5 (16.67) | 12 (40.00) | |
| CFS, n (%) | 0.02* | ||
| Abnormal (≥ Grade 1) | 5 (16.67) | 0 | |
| Normal (Grade 0) | 25 (83.33) | 30 (100.00) | |
| MGS, n (%) | 0.78 | ||
| Abnormal (≥ Grade 1) | 10 (33.33) | 11 (36.67) | |
| Normal (Grade 0) | 20 (66.67) | 19 (63.33) | |
| LIPCOF, n (%) | 0.45 | ||
| Abnormal (≥ Grade 1) | 3 (10.00) | 5 (16.67) | |
| Normal (Grade 0) | 27 (90.00) | 25 (83.33) | |
| Symblepharon presence, n (%) | 1 | ||
| Abnormal | 0 | 0 | |
| Normal | 30 (100) | 30 (100) | |
| Corneal clarity, n (%) | 1 | ||
| Abnormal | 0 | 0 | |
| Normal | 30 (100) | 30 (100) | |
| DED, n (%) | 11 (36.67) | 4 (13.33) | 0.04* |
| Severity of DED, n (%) | 0.01* | ||
| Grade I | 2 (18.18) | 4 (100) | |
| Grade II | 6 (54.55) | 0 | |
| Grade III | 3 (27.27) | 0 | |
| Grade IV | 0 | 0 | |
| Classification of DED, n (%) | 0.02* | ||
| Aqueous deficiency dry eye | 2 (18.18) | 2 (50.00) | |
| Evaporative dry eye | 8 (72.73) | 0 | |
| Mixed dry eye | 1 (9.09) | 2 (50.00) | |
| CGCs (CGC/mm2) | 98.24 ± 48.12 | 154.62 ± 81.83 | 0.03* |
OSDI Ocular Surface Disease Index, TBUT Tear film break-up time, CFS corneal fluorescein staining, MGS meibomian gland secretion, LIPCOF Lid-parallel conjunctival folds, DED dry eye disease, CGCs Conjunctival goblet cells, SLE Systemic lupus erythematosus, n number, mm millimeter, min minute, s second
P value means autoimmune rheumatic patients compared to control group, * and bold mean P < 0.05
Evaluation of ocular surface disorders in study participants
Among SLE patients, 33.33% reported ocular symptoms with an average OSDI score of 12.99 ± 13.71. Most were classified as OSDI level 1 (16.67%) and level 3 (13.33%), with 3.33% at level 2. For ocular signs, 33.33% had reduced tear secretion measured by the Schirmer I test (12.47 ± 9.85 mm/5 min), 83.33% had shortened TBUT (5.97 ± 3.07 s), 16.67% had CFS, 33.33% had abnormal MGS, 10% had abnormal LIPCOF, and no one had symblepharon or corneal opacity. Among these patients, 36.67% had DED, with 18.18% classified as ADDE, 72.73% as EDE, and 9.09% as MDE. For DED severity, 54.55% were in grade II, 27.27% in grade III, and 18.18% in grade I.
Among controls, 16.67% reported ocular symptoms with an average OSDI score of 6.05 ± 5.82, all at OSDI level 1. Additionally, 30% had reduced tear secretion (14.63 ± 10.86 mm/5 min), 60% had shortened TBUT (7.37 ± 3.85 s), 36.67% had abnormal MGS, 16.67% had abnormal LIPCOF, no one had CFS, symblepharon or corneal opacity. Among controls, 13.33% had DED, with 50% classified as ADDE and 50% as MDE. For DED severity, all were in grade II.
The OSDI score, percentage of abnormal TBUT and CFS were significantly higher in SLE patients than in control group (Table 1, P = 0.02; P = 0.04; P = 0.02). Higher prevalence and severity of dry eye were also shwon in SLE patients (Table 1, P = 0.04; P = 0.01). Lower density of CGCs was in SLE patients compared to controls (Table 1 and Fig. 1, 98.24 ± 48.12 and 154.62 ± 81.83, P = 0.03). No significant differences were shown in other ocular surface disorders between the two groups.
Fig. 1.
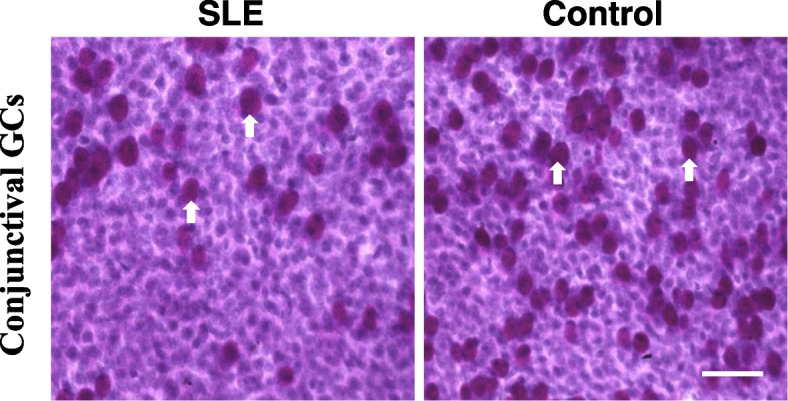
Representative images of conjunctival impression cytology in SLE patientsand control group (× 200, PAS staining). The dark purple cells indicated by the white arrow represent goblet cells. Scale bar = 50 μm
Levels of tear cytokines among study participants
The levels of tear cytokine IL-7 and chemokine CXCL11 in SLE patients were higher than in control group (Table 2, 729.24 ± 612.96 and 414.63 ± 185.86, P = 0.01; 5595.47 ± 5492.18 and 1489.94 ± 2040.85, P = 0.01). No significant differences were shwon in tear chemokine (C–C motif) ligand 3 (CCL3), CCL4, CCL20, CXCL8, chemokine (C-X3-C motif) ligand 1 (CX3CL1), granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, IL-21, IL-23, IFN-γ, and TNF-α between the two groups (Table 2).
Table 2.
Levels of tear cytokines among the study participants
| Cytokines (pg/mL) | SLE, n = 30 | Control, n = 14 | P Value |
|---|---|---|---|
| CCL3 | 30.36 ± 110.91 | 33.38 ± 124.89 | 0.94 |
| CCL4 | 88.76 ± 103.91 | 215.19 ± 523.71 | 0.37 |
| CCL20 | 613.10 ± 555.44 | 1045.12 ± 1235.91 | 0.12 |
| CXCL8 | 830.20 ± 901.42 | 1569.12 ± 3016.83 | 0.38 |
| CXCL11 | 5595.47 ± 5492.18 | 1489.94 ± 2040.85 | 0.001* |
| CX3CL1 | 2478.14 ± 3515.21 | 1373.59 ± 846.58 | 0.26 |
| GM-CSF | 23.73 ± 48.50 | 1.58 ± 10.17 | 0.45 |
| IFN-γ | 149.16 ± 358.94 | 42.17 ± 42.32 | 0.28 |
| IL-1β | 11.50 ± 19.52 | 8.88 ± 8.10 | 0.63 |
| IL-2 | 11.70 ± 41.36 | 2.83 ± 3.43 | 0.43 |
| IL-4 | 386.08 ± 947.84 | 152.11 ± 180.99 | 0.37 |
| IL-5 | 23.31 ± 54.73 | 11.32 ± 6.44 | 0.42 |
| IL-6 | 89.28 ± 94.80 | 160.79 ± 214.90 | 0.25 |
| IL-7 | 729.24 ± 612.96 | 414.63 ± 185.86 | 0.01* |
| IL-10 | 67.29 ± 146.77 | 42.32 ± 32.91 | 0.54 |
| IL-12 | 29.55 ± 67.12 | 13.52 ± 8.75 | 0.38 |
| IL-13 | 92.65 ± 129.96 | 50.90 ± 48.46 | 0.25 |
| IL-17 | 75.84 ± 169.35 | 33.71 ± 23.79 | 0.36 |
| IL-21 | 24.03 ± 79.45 | 2.05 ± 2.38 | 0.14 |
| IL-23 | 1159.65 ± 2726.29 | 485.96 ± 380.65 | 0.35 |
| TNF-α | 25.91 ± 44.88 | 13.99 ± 10.15 | 0.34 |
CCL3 chemokine (C–C motif) ligand 3, CCL4 chemokine (C–C motif) ligand 4, CCL20 chemokine (C–C motif) ligand 20, CXCL8 chemokine (C-X-C motif) ligand 8, CXCL11 chemokine (C-X-C motif) ligand 11, CX3CL1 chemokine (C-X3-C motif) ligand 1, GM-CSF granulocyte–macrophage colony-stimulating factor, IFN-γ interferon-gamma, IL interleukin, TNF-α Tumour Necrosis Factor alpha, SLE Systemic lupus erythematosus, pg picogram, mL milliliter, n number
P value means autoimmune rheumatic patients compared to control group, * and bold mean P < 0.05
Typical peripheral blood indicators among SLE patients
For SLE-associated autoantibodies (Table 3), the highest percentage of positive patients was for anti-RNP antibodies (70%), followed by anti-SSA (66.67%). Nearly half of the SLE patients were positive for ANuA, AHA, anti-dsDNA, anti-Ro-52, and anti-Sm antibodies (63.33%, 63.33%, 60%, 60%, and 46.67%, respectively). 20% of the patients were positive for anti-SSB antibodies.
Table 3.
Autoantibodies of SLE patients
| Autoantibodies | SLE, n = 30 |
|---|---|
| Anti-dsDNA (n, %) | |
| - | 12 (40.00) |
| + | 5 (16.67) |
| + + | 9 (30.00) |
| + + + | 4 (13.33) |
| Anti-Sm (n, %) | |
| - | 16 (53.33) |
| + | 3 (10.00) |
| + + | 5 (16.67) |
| + + + | 6 (20.00) |
| Anti-SSA (n, %) | |
| - | 10 (33.33) |
| + | 1 (3.33) |
| + + | 3 (10.00) |
| + + + | 16 (53.33) |
| Anti-SSB (n, %) | |
| - | 24 (80.00) |
| + | 2 (6.67) |
| + + | 0 |
| + + + | 4 (13.33) |
| Anti-Ro-52 (n, %) | |
| - | 12 (40.00) |
| + | 2 (6.67) |
| + + | 1 (3.33) |
| + + + | 15 (50.00) |
| Anti-RNP (n, %) | |
| - | 9 (30.00) |
| + | 5 (16.67) |
| + + | 4 (13.33) |
| + + + | 12 (40.00) |
| ANuA (n, %) | |
| - | 11 (36.67) |
| + | 5 (16.67) |
| + + | 8 (26.67) |
| + + + | 6 (20.00) |
| AHA (n, %) | |
| - | 11 (36.67) |
| + | 6 (20.00) |
| + + | 10 (33.33) |
| + + + | 3 (10.00) |
Anti-dsDNA anti-double stranded, DNA antibody, Anti-Sm anti-Smith antibody, Anti-SSA anti-Sjogren's syndrome A antibody, Anti-SSB anti-Sjogren's syndrome B antibody, Anti-Ro-52 anti Ro-52 antibody, Anti-RNP anti-ribonucleoproteins antibody, ANuA anti-nucleosome antibody, AHA anti-histone antibody, SLE Systemic lupus erythematosus, n number
As shown in Table 4, complement C4 was decreased in 56.67% of SLE patients, and 73.33% had an abnormal ESR. The counts and percentages of lymphocytes and their subsets were generally within the normal range.
Table 4.
Systemic inflammatory indicators of SLE patients
| Peripheral blood index | SLE, n = 30 |
|---|---|
| C4 (g/L) | |
| < 0.7 | 17 (56.67) |
| 0.7–1.4 | 13 (43.33) |
| > 1.4 | 0 |
| ESR (mm/h) | |
| 0–20 | 8 (26.67) |
| > 20 | 22 (73.33) |
| T cells percentage (%) | 74.51 ± 8.69 |
| T cells count (/μL) | 1075.09 ± 598.21 |
| CD4+ T cells percentage (%) | 31.30 ± 8.30 |
| CD4+ T cells count (/μL) | 449.30 ± 249.61 |
| CD8+ T cells percentage (%) | 40.94 ± 11.98 |
| CD8+ T cells count (/μL) | 594.17 ± 415.56 |
| B cells percentage (%) | 15.02 ± 8.89 |
| B cells count (/μL) | 215.22 ± 155.10 |
| NK cells percentage (%) | 7.14 ± 5.95 |
| NK cells count (/μL) | 100.65 ± 98.67 |
C4 Complement C4, ESR erythrocyte sedimentation rate, SLE Systemic lupus erythematosus, L litre, g gram, mm millimeter, h hour, μL microlitre, n number
Correlation analysis
DED is often associated with elevated tear cytokines [42]. In SLE patients (Table 5), CFS levels were positively correlated with tear chemokine CXCL11 concentration (r = 0.68, P = 0.001). MGS was positively correlated with tear cytokine IL-6 concentration (r = 0.54, P = 0.002). LIPCOF was positively correlated with tear chemokine CXCL8 and CCL4 concentration (r = 0.55, P = 0.002; r = 0.41, P = 0.023). There were no significant correlations between OSDI, Schirmer I, TBUT, CGCs, DED severity, and tear cytokines or chemokines among SLE patients.
Table 5.
Association between ocular surface disorders and tear cytokines among SLE patients
| CCL4 | CXCL8 | CXCL11 | IL-6 | IL-7 | |
|---|---|---|---|---|---|
| OSDI | -0.13 | -0.14 | -0.26 | -0.05 | -0.13 |
| Schirmer I | 0.07 | -0.19 | 0.25 | -0.03 | 0.26 |
| TBUT | -0.23 | -0.16 | -0.11 | -0.29 | -0.13 |
| CFS | -0.03 | 0.02 | 0.68** | 0.27 | 0.21 |
| MGS | 0.26 | 0.33 | -0.29 | 0.54** | 0.04 |
| LIPCOF | 0.41* | 0.55** | -0.18 | -0.05 | -0.03 |
| CGCs | -0.15 | -0.33 | 0.13 | -0.03 | -0.06 |
| Severity of DED | -0.05 | -0.03 | 0.16 | 0.15 | 0.01 |
OSDI Ocular Surface Disease Index, TBUT Tear film break-up time, CFS Corneal fluorescein staining, MGS Meibomian gland secretion, LIPCOF Lid-parallel conjunctival folds, CGCs Conjunctival goblet cells, DED Dry eye disease, CXCL11 chemokine (C-X-C motif) ligand 11, IL interleukin, CXCL8 chemokine (C-X-C motif) ligand 8, CCL4 chemokine (C–C motif) ligand 4, SLE Systemic lupus erythematosus
*and bold mean P < 0.05, ** and bold mean P < 0.01
In addition, DED was also associated with systemic conditions in SLE patients. As shown in Table 6, OSDI score was positively correlated with the systemic indicator C4 (r = 0.42, P = 0.023). There was a significant positive correlation between CFS level and anti-SSB antibody (r = 0.58, P = 0.001). CGCs were negatively correlated with B cell count (r = -0.51, P = 0.013). There was a significant positive correlation between severity of DED and anti-SSB antibody, C4 (r = 0.49, P = 0.006; r = 0.39, P = 0.035).
Table 6.
Association between ocular surface disorders and typical peripheral blood indicators among SLE patients
| Anti-SSB | Anti-dsDNA | ANuA | AHA | C4 |
B cell count |
|
|---|---|---|---|---|---|---|
| OSDI | 0.01 | -0.27 | -0.36 | -0.33 | 0.42* | -0.41 |
| Schirmer I | 0.13 | -0.05 | -0.10 | -0.12 | 0.12 | 0.06 |
| TBUT | -0.08 | 0.14 | -0.10 | 0.08 | 0.03 | -0.18 |
| CFS | 0.58** | 0.10 | 0.12 | -0.07 | 0.17 | 0.14 |
| MGS | -0.12 | -0.24 | -0.31 | -0.11 | -0.11 | 0.01 |
| LIPCOF | 0.17 | -0.15 | -0.09 | 0.16 | 0.17 | -0.25 |
| CGCs | 0.05 | 0.36 | -0.01 | 0.21 | -0.06 | -0.51* |
| Severity of DED | 0.49** | -0.19 | -0.13 | -0.32 | 0.39* | -0.19 |
OSDI Ocular Surface Disease Index, TBUT Tear film break-up time, CFS Corneal fluorescein staining, MGS Meibomian gland secretion, LIPCOF Lid-parallel conjunctival folds, CGCs Conjunctival goblet cells, DED Dry eye disease, Anti-SSB anti-Sjogren's syndrome B antibody, Anti-dsDNA anti-double stranded DNA antibody, ANuA anti-nucleosome antibody, AHA anti-histone antibody, C4 Complement C4, SLE Systemic lupus erythematosus
*and bold mean P < 0.05, ** and bold mean P < 0.01
Furthermore, a correlation was found between tear cytokines and systemic factors in SLE patients. There was a significant positive correlation between tear chemokine CXCL11 and anti-dsDNA antibody (r = 0.42, P = 0.022; Fig. 2A). Tear chemokine CCL4 was correlated positively with AHA (r = 0.41, P = 0.026; Fig. 2B). CD8+ T cells were correlated positively with tear chemokine CX3CL1, cytokines IL-1β, IL-2, IL-10, IL-17A, IFN-γ, and TNF-α in SLE patients (r = 0.77, r = 0.82, r = 0.81, r = 0.85, r = 0.84, r = 0.74, r = 0.79, all P < 0.01; Fig. 2C). No significant correlations were observed between other tear cytokine or chemokine and systemic indicators among SLE patients.
Fig. 2.
Correlation analysis between the tear chemokine CXCL11 and anti-dsDNA antibody A, the level of tear chemokine CCL4 and AHA antibody B, the CD8.+ T cell count and tear cytokines IFN-γ, IL-1β, IL-2, IL-10, IL-17A, TNF-α, and chemokine CX3CL1 in SLE patients C
Discussion
Our study reported that more severe ocular surface damage was shown in SLE patients compared to normal controls, including significantly higher OSDI score, percentage of abnormal TBUT and CFS, prevalence and severity of DED, and lower density of CGCs. Additionally, levels of tear chemokine CXCL11 and cytokine IL-7 were elevated in SLE patients compared to normal population. Moreover, in SLE patients, severity of DED was significantly positively correlated with specific SLE-associated autoantibody anti-SSB, and elevated level of tear chemokine CXCL11 was positively correlated with anti-dsDNA antibody.
Dry eye is the most common ophthalmic manifestation for SLE [10]. Our study shown that 36.67% of SLE patients exhibited DED. A meta-analysis similarly reported dry eye in approximately one third of SLE patients [43]. The pathogenesis of SLE-related dry eye remains incompletely understood. Recent studies suggest a link between dry eye pathogenesis and tear cytokines [15, 44]. Concurrently, SLE pathogenesis involves cytokine dysregulation [45]. IL-7, crucial for immune homeostasis, supports naive T cell survival, T and B cell development, and memory T cell maintenance. In DED, IL-7 plays a key role in inflammation. Studies have detected increased tear IL-7 concentrations in DED patients [46]. In a C57BL/6 mice model of chronic dry eye disease, IL-7 gene and protein levels were elevated in drainage lymph nodes and conjunctiva. Topical IL-7 blockade provided sustained relief for DED [47]. IL-7 dysregulation also disrupts lymphoid development and contributes to autoimmune diseases pathophysiology [48–50]. IL-7 promotes Th17 and Th1 proliferation and induces cytokine secretion (IFN-γ and IL-17) in SLE [51]. Additionally, IL-7 overexpression has been observed in Sjögren's syndrome (SS) patients and in joints or serum of rheumatoid arthritis patients, correlating with disease severity [52–56]. Currently, there is limited research on tear IL-7 and SLE. Our study found significantly higher tear IL-7 levels in SLE patients compared to controls.
CXCL11, which was elevated in SLE group compared to controls in our study, belongs to Th1 chemokine family and regulates immune cell migration, differentiation, and activation, such as differentiating naive T cells to Th1 cells, and recruiting natural killer (NK) cells and macrophages [57]. Studies have implicated CXCL11 in the pathogenesis of autoimmune diseases and cancer, including Graves disease (GD), autoimmune thyroiditis (AT), and colorectal cancer [58–60]. Currently, research on CXCL11 and dry eye is limited. However, both animal models and patients with DED have shown significantly increased tear levels of CXCL11, which correlate with disease severity [61, 62]. In our study, we measured tear cytokines and found that elevated level of CXCL11 were shown in patients with SLE compared to normal group, positively correlating with corneal fluorescein staining. Tear chemokine CXCL11 may thus serve as a potential pathogenic factor in SLE-related dry eye and could indicate the extent of ocular surface damage associated with this condition.
We futher found that tear chemokine CXCL11 and CCL4 was associated with SLE-associated autoantibodies. The immune pathogenesis of SLE includes production of autoantibodies, B lymphocyte abnormalities, T lymphocyte abnormalities and complement system abnormalities [63]. Anti-dsDNA antibodies, as the classical autoantibody specificity of SLE, fluctuate with disease activity or flare development [64, 65]. In SLE, anti-dsDNA antibodies contribute to multiple end-organ injuries, especially lupus nephritis [66]. Moreover, they also can promote thrombosis by directly activating platelets and futher increase the cardiovascular risk [67, 68]. Studies have shown that precautionary change of therapy is effective in preventing flares when anti-dsDNA levels are increased by > 50% [69]. In addition, AHA have also been reported to be positively correlated with SLE disease activity [70, 71]. Our study showed that tear chemokine CXCL11 was moderately positively correlated with anti-dsDNA and CCL4 was positively correlated with AHA among SLE patients. Tear chemokine CXCL11 and CCL4 may be a potential indicator of SLE disease activity.
Additionally, CGCs loss or death has been observed in ocular surface inflammatory diseases such as DED, SS, ocular cicatritic pyeloid (OCP), Stevens Johnson syndrome (SJS), and blepharitis [21, 72]. CGCs play role in innate immune system, secreting defensins, soluble mucins (MUC), and trefoil factors that form a barrier in the tear film. This barrier prevents invasion by external microorganisms, limits exposure to commensal bacteria, and thereby prevents chronic inflammatory responses [21]. Studies have shown that tear protein of SS patients were significantly reduced compared to controls [73]. Of course, some different views have indicated that SS subjects displayed a significant increase in both soluble MUC1 and MUC16 concentrations due to the stimulation of inflammatory mediators [74, 75]. Our study also demonstrated a loss of CGCs, which was significantly negatively correlated with B cell count among SLE patients.
During the study, we observed ocular surface injury and inflammation in some SLE patients who did not meet current diagnostic criteria for dry eye. However, our study found that tear cytokines and CGCs were more highly correlated with the ocular surface. Therefore, we suggest that tear cytokines and CGCs could serve as potential adjunctive tests to better understand ocular surface injury in SLE-related dry eye.
In addition, as research has shown, dry eye is associated with ocular surface inflammation. The level of cytokine in tears in dry eye patients is higher than that in normal eyes. In patients with rheumatic immune diseases, including SLE, aberrant immune system activity leads to an expansion of autoantibody specificities and elevated levels of serum cytokines and chemokines, further ocular surface inflammation or dry eye. Many studies have also shown that disease activity in such patients is positively correlated with the severity of dry eye or ocular inflammation [76–79]. Systemic treatment with drugs such as glucocorticoid or immunosuppressant to control rheumatoid immune diseases can also control ocular inflammation or dry eye to a certain extent. Studies have confirmed that, in patients with systemic autoimmune diseases, systemic therapy, such as methotrexate, cyclosporine A, chloroquine, and prednisone, alleviates symptoms and signs of dry eye and decreases levels of tear cytokines [80–82]. However, due to the presence of the blood-ocular barrier, the drugs that eventually reach the eye surface are still relatively limited, so eye drops are still the first-line drugs for dry eye patients in the guidelines. Our study also calls for a combination of systemic and topical treatments.
In conclusion, among SLE patients, dry eye and tear cytokine-mediated ocular surface inflammation persist and are associated with systemic conditions. Thus, it is essential for SLE patients to undergo combined systemic and ocular assessments.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ADDE
Aqueous deficient dry eye
- AHA
Anti-histone antibody
- ANuA
Anti-nucleosome antibody
- anti-dsDNA
Anti-dsDNA antibody
- anti-RNP
Anti-ribonucleoproteins antibody
- anti-Ro-52
Anti-Ro-52 antibody
- anti-Sm
Anti-Smith antibody
- anti-SSA
Anti-Sjogren's syndrome A antibody
- anti-SSB
Anti-Sjogren's syndrome B antibody
- AT
Autoimmune thyroiditis
- C4
Complement C4
- CCL3
Chemokine (C–C motif) ligand 3
- CFS
Corneal fluorescein staining
- CGCs
Conjunctival goblet cells
- CX3CL1
Chemokine (C-X3-C motif) ligand 1
- CXCL11
Chemokine (C-X-C motif) ligand 11
- DED
Dry eye disease
- EDE
Evaporative dry eye
- ESR
Erythrocyte sedimentation rate
- GD
Graves disease
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- IL-7
Interleukin-7
- IFN-γ
Interferon-γ
- LIPCOF
Lid-parallel conjunctival folds
- MDE
Mixed dry eye
- MGS
Meibomian gland secretion
- MUC
Mucin
- NK
Natural killer
- OCP
Ocular cicatritic pyeloid
- OSDI
Ocular Surface Disease Index
- PAS
Periodic Acid-Schiff
- SLE
Systemic lupus erythematosus
- SJS
Stevens Johnson syndrome
- SS
Sjögren's syndrome
- TBUT
Tear break-up time
- TNF-α
Tumor necrosis factor-α
Authors’ contributions
Yuerong Ren: Writing-Review and Editing , Conceptualization. Jing Tian: Writing-Original draft, Methodology. Wen Shi: Data collection, Formal analysis. Jianing Feng: Data collection, Investigation. Yingyi Liu: Data curation. Huanmin Kang: Software. Yan He: Writing-Review, Supervision, Project administration, Funding acquisition. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China grants 82371034 (Y.H.), 82171028 (Y.H.), Natural Science Foundation of Hunan Province grants 2022JJ30065 (Y.H.), Beijing Physician Scientist Training Project BJPSTP-2024–06 (Y.H.).
Data availability
The data that support the findings of this study is provided within the supplementary information file.
Declarations
Ethics approval and consent to participate
All subjects participated in the program voluntarily and signed informed consent forms. This study received approval from the clinical Research Ethics Committee of The Second Xiangya Hospital of Central South University (Ethics No.: Ocular Surface Observation in patients with systemic immune Diseases, LYF2021028). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Narvaez J. Systemic lupus erythematosus 2020. Med Clin (Barc). 2020;155(11):494–501. Lupus eritematoso sistemico 2020. 10.1016/j.medcli.2020.05.009. [DOI] [PubMed]
- 2.Barber MRW, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(9):515–32. 10.1038/s41584-021-00668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Pomar C, Pintor J, Colligris B, Carracedo G. Therapeutic inhibitors for the treatment of dry eye syndrome. Expert Opin Pharmacother. 2017;18(17):1855–65. 10.1080/14656566.2017.1403584. [DOI] [PubMed] [Google Scholar]
- 4.Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172(11):ITC81–96. 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 5.Pan L, Lu MP, Wang JH, Xu M, Yang SR. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr. 2020;16(1):19–30. 10.1007/s12519-019-00229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capecchi R, Puxeddu I, Pratesi F, Migliorini P. New biomarkers in SLE: from bench to bedside. Rheumatology (Oxford). 2020;59(Suppl5):v12–8. 10.1093/rheumatology/keaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update omicronn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14–25. 10.1136/annrheumdis-2020-218272. [DOI] [PubMed] [Google Scholar]
- 8.Preble JM, Silpa-archa S, Foster CS. Ocular involvement in systemic lupus erythematosus. Curr Opin Ophthalmol. 2015;26(6):540–5. 10.1097/ICU.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 9.Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol. 2016;100(1):135–41. 10.1136/bjophthalmol-2015-306629. [DOI] [PubMed] [Google Scholar]
- 10.Lee I, Zickuhr L, Hassman L. Update on ophthalmic manifestations of systemic lupus erythematosus: pathogenesis and precision medicine. Curr Opin Ophthalmol. 2021;32(6):583–9. 10.1097/ICU.0000000000000810. [DOI] [PubMed] [Google Scholar]
- 11.Chen A, Chen HT, Hwang YH, Chen YT, Hsiao CH, Chen HC. Severity of dry eye syndrome is related to anti-dsDNA autoantibody in systemic lupus erythematosus patients without secondary Sjogren syndrome: A cross-sectional analysis. Medicine (Baltimore). 2016;95(28): e4218. 10.1097/MD.0000000000004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang A, Gu Z, Liao R, Shuai Z. Dry Eye Indexes Estimated by Keratograph 5M of Systemic Lupus Erythematosus Patients without Secondary Sjogren’s Syndrome Correlate with Lupus Activity. J Ophthalmol. 2019;2019:8509089. 10.1155/2019/8509089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. Jul2017;15(3):276–83. 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Suarez-Cortes T, Merino-Inda N, Benitez-Del-Castillo JM. Tear and ocular surface disease biomarkers: A diagnostic and clinical perspective for ocular allergies and dry eye disease. Exp Eye Res. 2022;221: 109121. 10.1016/j.exer.2022.109121. [DOI] [PubMed] [Google Scholar]
- 15.Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 2017;124(11S):S4–13. 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, Hu B, Peng RM, Huang JF, Hong J. Tear Cytokines as Biomarkers for Acute Ocular Graft-Versus-Host Disease. Cornea. 2022. 10.1097/ICO.0000000000002959. [DOI] [PubMed]
- 17.Yamaguchi T. Inflammatory Response in Dry Eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES192–9. 10.1167/iovs.17-23651. [DOI] [PubMed] [Google Scholar]
- 18.Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197: 108115. 10.1016/j.exer.2020.108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puro DG. How goblet cells respond to dry eye: adaptive and pathological roles of voltage-gated calcium channels and P2X7 purinoceptors. Am J Physiol Cell Physiol. 2020;318(6):C1305–15. 10.1152/ajpcell.00086.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao C, Golebiowski B, Stapleton F, Zhou X, Chen S, Madigan MC. Conjunctival MUC5AC+ goblet cell index: relationship with corneal nerves and dry eye. Graefes Arch Clin Exp Ophthalmol. 2018;256(11):2249–57. 10.1007/s00417-018-4065-y. [DOI] [PubMed] [Google Scholar]
- 21.Swamynathan SK, Wells A. Conjunctival goblet cells: Ocular surface functions, disorders that affect them, and the potential for their regeneration. Ocul Surf. 2020;18(1):19–26. 10.1016/j.jtos.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26(7):938–44. 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng X, Lu Y, Wei J, et al. A cohort study of T helper 17 cell-related cytokine levels in tear samples of systemic lupus erythematosus and Sjogren's syndrome patients with dry eye disease. Clin Exp Rheumatol. 2021;39 Suppl 133(6):159–165. 10.55563/clinexprheumatol/tlnr4z. [DOI] [PubMed]
- 24.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78(9):1151–9. 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- 25.Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatology (Oxford). 2020;59(Suppl5):v4–11. 10.1093/rheumatology/keaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akowuah PK, Adjei-Anang J, Nkansah EK, et al. Comparison of the performance of the dry eye questionnaire (DEQ-5) to the ocular surface disease index in a non-clinical population. Cont Lens Anterior Eye. 2022;45(3): 101441. 10.1016/j.clae.2021.101441. [DOI] [PubMed] [Google Scholar]
- 27.Pult H, Wolffsohn JS. The development and evaluation of the new Ocular Surface Disease Index-6. Ocul Surf. 2019;17(4):817–21. 10.1016/j.jtos.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21. 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 29.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539–74. 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Tian J, Shi W, et al. Evaluation and correlation analysis of ocular surface disorders and quality of life in autoimmune rheumatic diseases: a cross-sectional study. BMC Ophthalmol. 2023;23(1):229. 10.1186/s12886-023-02959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Methodologies to diagnose and monitor dry eye disease. report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):108–52. 10.1016/s1542-0124(12)70083-6. [DOI] [PubMed] [Google Scholar]
- 32.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–50. 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–49. 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pult H, Bandlitz S. Lid-Parallel Conjunctival Folds and Their Ability to Predict Dry Eye. Eye Contact Lens. 2018;44(Suppl 2):S113–9. 10.1097/ICL.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Chaurasiya D, Sultan S, et al. Therapeutic Profile of Human Umbilical Cord Blood Serum and Autologous Serum Therapies in Treatment of Ocular Surface Disorders: A Pilot Study. J Ocul Pharmacol Ther. 2023;39(1):36–47. 10.1089/jop.2022.0107. [DOI] [PubMed] [Google Scholar]
- 36.Javed A, Aslam T, Jones SA, Ashworth J. Objective Quantification of Changes in Corneal Clouding Over Time in Patients With Mucopolysaccharidosis. Invest Ophthalmol Vis Sci. 2017;58(2):954–8. 10.1167/iovs.16-20647. [DOI] [PubMed] [Google Scholar]
- 37.Komai S, Inatomi T, Nakamura T, et al. Long-term outcome of cultivated oral mucosal epithelial transplantation for fornix reconstruction in chronic cicatrising diseases. Br J Ophthalmol. 2022;106(10):1355–62. 10.1136/bjophthalmol-2020-318547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma B, Zhou Y, Liu R, et al. Pigment epithelium-derived factor (PEDF) plays anti-inflammatory roles in the pathogenesis of dry eye disease. Ocul Surf. 2021;20:70–85. 10.1016/j.jtos.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Management and therapy of dry eye disease. report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163–78. 10.1016/s1542-0124(12)70085-x. [DOI] [PubMed] [Google Scholar]
- 40.Dijkstra DJ, Joeloemsingh JV, Bajema IM, Trouw LA. Complement activation and regulation in rheumatic disease. Semin Immunol. 2019;45: 101339. 10.1016/j.smim.2019.101339. [DOI] [PubMed] [Google Scholar]
- 41.Goldblatt F, O’Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382(9894):797–808. 10.1016/S0140-6736(13)61499-3. [DOI] [PubMed] [Google Scholar]
- 42.Roda M, Corazza I, Bacchi Reggiani ML, et al. Dry Eye Disease and Tear Cytokine Levels-A Meta-Analysis. Int J Mol Sci. 2020;21(9). 10.3390/ijms21093111. [DOI] [PMC free article] [PubMed]
- 43.Turk MA, Hayworth JL, Nevskaya T, Pope JE. Ocular Manifestations in Rheumatoid Arthritis, Connective Tissue Disease, and Vasculitis: A Systematic Review and Metaanalysis. J Rheumatol. 2021;48(1):25–34. 10.3899/jrheum.190768. [DOI] [PubMed] [Google Scholar]
- 44.Wang HH, Chen WY, Huang YH, et al. Interleukin-20 is involved in dry eye disease and is a potential therapeutic target. J Biomed Sci. 2022;29(1):36. 10.1186/s12929-022-00821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aringer M. Inflammatory markers in systemic lupus erythematosus. J Autoimmun. 2020;110: 102374. 10.1016/j.jaut.2019.102374. [DOI] [PubMed] [Google Scholar]
- 46.Hu B, Qiu Y, Hong J. Tear cytokine levels in the diagnosis and severity assessment of ocular chronic graft-versus-host disease(GVHD). Ocul Surf. 2020;18(2):298–304. 10.1016/j.jtos.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Chauhan SK, Tan X, Dana R. Interleukin-7 and -15 maintain pathogenic memory Th17 cells in autoimmunity. J Autoimmun. 2017;77:96–103. 10.1016/j.jaut.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markovic I, Savvides SN. Modulation of Signaling Mediated by TSLP and IL-7 in Inflammation, Autoimmune Diseases, and Cancer. Front Immunol. 2020;11:1557. 10.3389/fimmu.2020.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barata JT, Durum SK, Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. 2019;20(12):1584–93. 10.1038/s41590-019-0479-x. [DOI] [PubMed] [Google Scholar]
- 50.Lundstrom W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24(3):218–24. 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XS, Li BZ, Hu LF, et al. Perspectives of the relationship between IL-7 and autoimmune diseases. Clin Rheumatol. 2013;32(12):1703–9. 10.1007/s10067-013-2360-x. [DOI] [PubMed] [Google Scholar]
- 52.Meyer A, Parmar PJ, Shahrara S. Significance of IL-7 and IL-7R in RA and autoimmunity. Autoimmun Rev. 2022;21(7):103120. 10.1016/j.autrev.2022.103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riviere E, Pascaud J, Virone A, et al. Interleukin-7/Interferon Axis Drives T Cell and Salivary Gland Epithelial Cell Interactions in Sjogren’s Syndrome. Arthritis Rheumatol. 2021;73(4):631–40. 10.1002/art.41558. [DOI] [PubMed] [Google Scholar]
- 54.Burska AN, Neilan J, Chisman RE, et al. Serum IL-7 as diagnostic biomarker for rheumatoid arthritis, validation with EULAR 2010 classification criteria. Clin Exp Rheumatol. 2018;36(1):115–20. [PubMed] [Google Scholar]
- 55.Riviere E, Pascaud J, Tchitchek N, et al. Salivary gland epithelial cells from patients with Sjogren’s syndrome induce B-lymphocyte survival and activation. Ann Rheum Dis. 2020;79(11):1468–77. 10.1136/annrheumdis-2019-216588. [DOI] [PubMed] [Google Scholar]
- 56.Liang Y, Zhang Z, Li J, Luo W, Jiang T, Yang Z. Association between IL-7 and primary Sjogren’s syndrome: A single-center study and a systematic scoping review. Int Immunopharmacol. 2022;108:108758. 10.1016/j.intimp.2022.108758. [DOI] [PubMed] [Google Scholar]
- 57.Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–7. 10.1016/j.ctrv.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fallahi P, Ferrari SM, Ragusa F, et al. Th1 Chemokines in Autoimmune Endocrine Disorders. J Clin Endocrinol Metab. 2020;105(4). 10.1210/clinem/dgz289. [DOI] [PubMed]
- 59.Fallahi P, Ferrari SM, Elia G, et al. Cytokines as Targets of Novel Therapies for Graves’ Ophthalmopathy. Front Endocrinol (Lausanne). 2021;12: 654473. 10.3389/fendo.2021.654473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang C, Chen W, Shen J. CXCR7 Targeting and Its Major Disease Relevance. Front Pharmacol. 2018;9:641. 10.3389/fphar.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Y, Asbell PA. sPLA2-IIa participates in ocular surface inflammation in humans with dry eye disease. Exp Eye Res. 2020;201: 108209. 10.1016/j.exer.2020.108209. [DOI] [PubMed] [Google Scholar]
- 62.Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-gamma in experimental dry eye. J Immunol. 2014;193(10):5264–72. 10.4049/jimmunol.1400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwata S, Tanaka Y. Therapeutic perspectives on the metabolism of lymphocytes in patients with rheumatoid arthritis and systemic lupus erythematosus. Expert Rev Clin Immunol. 2021;17(10):1121–30. 10.1080/1744666X.2021.1964957. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Xia Y. Anti-double Stranded DNA Antibodies: Origin, Pathogenicity, and Targeted Therapies. Front Immunol. 2019;10:1667. 10.3389/fimmu.2019.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Enocsson H, Sjowall C, Wirestam L, et al. Four Anti-dsDNA Antibody Assays in Relation to Systemic Lupus Erythematosus Disease Specificity and Activity. J Rheumatol. 2015;42(5):817–25. 10.3899/jrheum.140677. [DOI] [PubMed] [Google Scholar]
- 66.Rekvig OP. The dsDNA, Anti-dsDNA Antibody, and Lupus Nephritis: What We Agree on, What Must Be Done, and What the Best Strategy Forward Could Be. Front Immunol. 2019;10:1104. 10.3389/fimmu.2019.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patino-Trives AM, Perez-Sanchez C, Perez-Sanchez L, et al. Anti-dsDNA Antibodies Increase the Cardiovascular Risk in Systemic Lupus Erythematosus Promoting a Distinctive Immune and Vascular Activation. Arterioscler Thromb Vasc Biol. 2021;41(9):2417–30. 10.1161/ATVBAHA.121.315928. [DOI] [PubMed] [Google Scholar]
- 68.Andrianova IA, Ponomareva AA, Mordakhanova ER, et al. In systemic lupus erythematosus anti-dsDNA antibodies can promote thrombosis through direct platelet activation. J Autoimmun. 2020;107: 102355. 10.1016/j.jaut.2019.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Floris A, Piga M, Cauli A, Mathieu A. Predictors of flares in Systemic Lupus Erythematosus: Preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun Rev. 2016;15(7):656–63. 10.1016/j.autrev.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Zeng Y, Xiao Y, Zeng F, et al. Assessment of anti-nucleosome antibody (ANuA) isotypes for the diagnosis and prediction of systemic lupus erythematosus and lupus nephritis activity. Clin Exp Med. 2022. 10.1007/s10238-022-00942-w. [DOI] [PubMed]
- 71.Shang X, Ren L, Sun G, et al. Anti-dsDNA, anti-nucleosome, anti-C1q, and anti-histone antibodies as markers of active lupus nephritis and systemic lupus erythematosus disease activity. Immun Inflamm Dis. 2021;9(2):407–18. 10.1002/iid3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018;11(6):1551–7. 10.1038/s41385-018-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caffery B, Joyce E, Boone A, et al. Tear lipocalin and lysozyme in Sjogren and non-Sjogren dry eye. Optom Vis Sci. 2008;85(8):661–7. 10.1097/OPX.0b013e318181ae4f. [DOI] [PubMed] [Google Scholar]
- 74.Caffery B, Heynen ML, Joyce E, Jones L, Ritter R 3rd, Senchyna M. MUC1 expression in Sjogren’s syndrome, KCS, and control subjects. Mol Vis. 2010;16:1720–7. [PMC free article] [PubMed] [Google Scholar]
- 75.Caffery B, Joyce E, Heynen ML, et al. MUC16 expression in Sjogren’s syndrome, KCS, and control subjects. Mol Vis. 2008;14:2547–55. [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Wu M, Ren Y, et al. Evaluation of Dry Eye Severity and Ocular Surface Inflammation in Patients with Autoimmune Rheumatic Diseases. Ocul Immunol Inflamm. 2024:1–13. 10.1080/09273948.2024.2315196. [DOI] [PubMed]
- 77.Zhang LW, Zhou PR, Wei P, Cong X, Wu LL, Hua H. Expression of interleukin-17 in primary Sjogren’s syndrome and the correlation with disease severity: A systematic review and meta-analysis. Scand J Immunol. 2018;87(4): e12649. 10.1111/sji.12649. [DOI] [PubMed] [Google Scholar]
- 78.Chung JK, Kim MK, Wee WR. Prognostic factors for the clinical severity of keratoconjunctivitis sicca in patients with Sjogren’s syndrome. Br J Ophthalmol. 2012;96(2):240–5. 10.1136/bjo.2011.202812. [DOI] [PubMed] [Google Scholar]
- 79.Akcay Usta S, Icoz M. Evaluation of Ocular Surface Parameters and Systemic Inflammatory Biomarkers in Hazelnut Harvesters. Ocul Immunol Inflamm. 2024:1–6. 10.1080/09273948.2024.2336598. [DOI] [PubMed]
- 80.Lee HJ, Shin S, Yoon SG, Cheon EJ, Chung SH. The Effect of Chloroquine on the Development of Dry Eye in Sjogren Syndrome Animal Model. Invest Ophthalmol Vis Sci. 2019;60(12):3708–16. 10.1167/iovs.19-27469. [DOI] [PubMed] [Google Scholar]
- 81.Villani E, Galimberti D, Del Papa N, Nucci P, Ratiglia R. Inflammation in dry eye associated with rheumatoid arthritis: cytokine and in vivo confocal microscopy study. Innate Immun. 2013;19(4):420–7. 10.1177/1753425912471692. [DOI] [PubMed] [Google Scholar]
- 82.Cordero-Coma M, Anzaar F, Sobrin L, Foster CS. Systemic immunomodulatory therapy in severe dry eye secondary to inflammation. Ocul Immunol Inflamm Mar-Apr. 2007;15(2):99–104. 10.1080/09273940701299354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study is provided within the supplementary information file.



