Abstract
Background
Research indicates that prehabilitation is effective in optimizing physical status before surgery, although this method may be considered “aggressive” for frail elderly patients. This study aimed to evaluate whether multimodal prehabilitation decreases postoperative complications and improves functional recovery in frail elderly patients undergoing gastric cancer surgery, in comparison to usual clinical care.
Methods
This study was a single-center, single-blind, randomized controlled trial. Patients over 65 years old with a Fried Frailty Index of 2 or higher, scheduled for gastric cancer surgery, were considered for inclusion. Eligible participants were randomized in a 1:1 ratio to either the intervention or control group. The intervention group underwent a 3-week multimodal prehabilitation program prior to surgery, in addition to perioperative care guided by ERAS protocols. The control group received only the latter. The primary outcome was the comprehensive complications index (CCI) measured at 30 days after surgery. Secondary outcomes included 30-day overall complications, functional walking capacity as assessed by 6-minute walking distance (6MWD) at 4 weeks postoperatively, and 3-month postoperative quality of life. This study was registered at ClinicalTrials.gov (No. NCT06510088).
Results
Among the 112 eligible patients, the median age was 74 years, with 58 (52.7%) being female. No between-group difference was found in the primary outcome measure, 30-day CCI. The Median (Q1-Q3) CCI for the intervention and control groups was 0 (0-12.2) and 0 (0-22.6) (P = 0.082), while the mean (SD) CCI was 6.1 (15.8) and 9.8 (12.7), respectively (P = 0.291). Notably, the incidence of severe complications (CCI > 20) was significantly lower in the intervention group compared to the control group (11.1% vs. 25.9%, P = 0.046), particularly in terms of medical complications (12.3% vs. 29.3%, P = 0.025). Preoperatively, 27 patients (47.4%) in the intervention group exhibited an increase in the 6MWD of at least 20 m, compared to 16 patients (27.6%) in the control group (P = 0.028). At 4 weeks postoperatively, more patients in the intervention group returned to their baseline 6MWD levels (63.2% vs. 43.1%, P = 0.031). Secondary parameters of functional capacity in the postoperative period generally favored the multimodal prehabilitation approach.
Conclusions
In frail elderly patients undergoing elective gastric cancer surgery, a prehabilitation program did not affect the 30-day postoperative complication rate or CCI but reduced severe complications and improved perioperative functional capacity.
Trial registration
[ClinicalTrials.gov], [NCT06510088], [07/15/2024], [Retrospectively registered]
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03490-7.
Keywords: Elderly, Frail, Gastric cancer, Multimodal prehabilitation
Introduction
Gastric cancer remains a significant health concern globally, especially among elderly individuals, where it poses a considerable burden due to its aggressive nature and limited therapeutic options [1]. Population aging, a result of demographic transition, presents a critical societal challenge [2, 3]. According to a 2022 report by the World Health Organization (WHO), the median age at gastric cancer diagnosis is 69 years [4], with patients over 75 years facing heightened risks of morbidity and mortality [2, 5]. Although surgical resection remains the primary treatment for gastric cancer, the proportion of elderly patients undergoing surgery declines with age due to preoperative frailty [6, 7]. Frailty, marked by age-related declines in energy, muscle strength, weight, and activity levels, is common among elderly gastric cancer patients and correlates with poorer surgical outcomes, including higher morbidity and mortality [7]. Therefore, interventions aimed at enhancing the functional status and resilience of frail elderly patients undergoing surgery for gastric cancer are critically important.
Numerous studies have demonstrated that prehabilitation can diminish complications, hospital readmissions, length of hospital stay (LOS), and care dependence by enhancing functional reserve. However, these studies did not differentiate between age groups and frailty status, making it difficult to interpret the relationship between the outcomes and advanced age or frailty. It is hypothesized that patients at higher risk for postoperative complications, such as frail elderly individuals, are more likely to benefit from prehabilitation. Nonetheless, conclusive evidence on multimodal rehabilitation specifically designed for this vulnerable population remains insufficient.
Prehabilitation seeks to optimize patients’ preoperative risk factors during the waiting period before surgery [8]. This preoperative phase is a critical time to modify health behaviors to reduce the stress of surgery and enhance the recovery process. Multimodal prehabilitation encompasses various interventions, including physical exercise, nutritional optimization, and psychological support, aiming to bolster physiological reserve in anticipation of the expected adverse effects of surgery and to support the postoperative recovery of functional capacity, particularly in patients with lower preoperative fitness levels [9–12]. Several studies have demonstrated that prehabilitation can diminish complications, hospital readmissions, LOS, and care dependence by enhancing functional reserve [9, 13]. However, in the course of their research, the age group and frailty status of the patients were not differentiated and the relationship between the results and advanced age/frailty cannot be well interpreted. It is hypothesized that patients at higher risk for postoperative complications, such as frail elderly individuals, are more likely to benefit from prehabilitation. Nonetheless, definitive evidence on multimodal prehabilitation specifically tailored to this vulnerable population is lacking.
Therefore, we implemented a randomized clinical trial to provide evidence regarding the potential advantages of multimodal prehabilitation on the outcomes of frail elderly patients undergoing elective gastric cancer surgery.
Materials and methods
Trial Design and Study participants
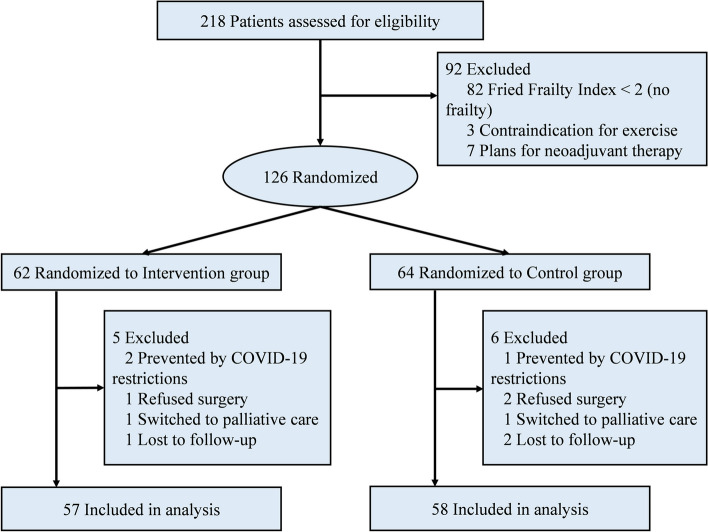
This prospective, single-blind, randomized controlled trial received approval from the Ethics Committee and Institutional Review Board of Taizhou Hospital of Zhejiang Province (ethical approval no. K20240528). Written informed consent was obtained from all participants involved in the study. The reporting of this trial adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Supplementary material, CONSORT checklist, http://www.consort-statement.org/) and was conducted in accordance with the ethical principles outlined in the Helsinki Declaration of 1975. This study was registered at ClinicalTrials.gov (No. NCT06510088). Patients over the age of 65 with a Fried Frailty Index score of 2 or higher [14], who were scheduled for surgical resection of gastric adenocarcinoma, and whose life expectancy was estimated by the surgeon to be greater than six months, were considered for inclusion in this study. Patients were excluded from the study if they: (1) were scheduled for neoadjuvant therapy; (2) had metastatic cancer; (3) were unable to swallow or participate in exercise and fitness assessments due to pre-existing conditions (e.g., orthopedic, neuromuscular, or cardiorespiratory diseases). In addition, patients who were lost to follow-up were also excluded. Enrollment began on March 1, 2019, was paused due to the COVID-19 pandemic from February 2020 through December 2020, and ultimately ended on December 31, 2023. The CONSORT diagram for this study is depicted in Fig. 1.
Fig. 1.
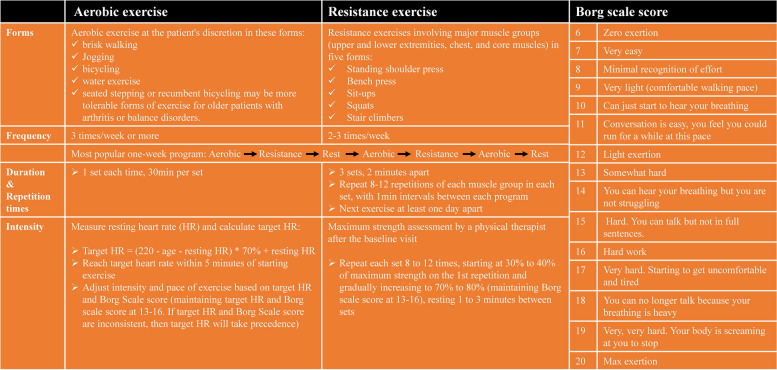
Aerobic and resistance exercise guidance of the Intervention group and the Borg scale score. HR, heart rate
Study procedures
Eligible patients were randomly assigned in a 1:1 ratio to receive either multimodal prehabilitation (intervention group) or usual clinical care (control group). Randomization was accomplished using computer-generated random numbers organized into 12 blocks of 10. An independent researcher (Mengya Zhou) placed the allocations in sealed, opaque envelopes that were consecutively numbered. The allocation remained concealed until the completion of the baseline assessment, at which point the envelopes were opened in numerical order. Outcome assessors, surgeons, and anesthesiologists were blinded to the group assignments. Due to the nature of the intervention, blinding patients or intervention staff was not feasible. Assessments were conducted at three time points: before the commencement of prehabilitation (baseline assessment), a few days before surgery (preoperative assessment), and four weeks post-surgery (4-week assessment). Perioperative management adhered to the principles of the Enhanced Recovery After Surgery (ERAS) program.
Multimodal Prehabilitation
Participants in the intervention group engaged in a 3-week, individualized, supervised multimodal prehabilitation program. This program included four components: aerobic and resistance exercises, respiratory training, nutritional counseling with whey protein supplementation, and psychological adjustment. Smoking cessation interventions were also included if necessary. The intervention was based on a combination of home and hospital settings. A multidisciplinary team comprising surgeons, anesthesiologists, kinesiologists, dietitians, physiotherapists, and nurses with psychology training will propose an individualized treatment strategy to the patient. The prehabilitation program commenced immediately following the baseline visit, which was scheduled approximately four weeks prior to the planned operation.
Aerobic and resistance exercises
Aerobic and resistance exercises represent the core activities of multimodal prehabilitation program. During these sessions, patients performed 30 min of moderate aerobic exercise and 25 min of resistance exercises using an elastic band. A 30-minute home-based exercise of aerobic endurance exercise (jogging, walking, cycling, at discretion) was required for at least 3 days per week. Aerobic exercise intensity was gauged using two primary metrics: target heart rate (HR) and perceived rate of exertion (Borg Scale) [15]. The program aimed to achieve a moderate-to-high training intensity, corresponding to a Borg Scale score of 13 to 16, and to maintain the target heart rate (HR), calculated using the formula: (220 - age - resting HR) × 70% + resting HR. Furthermore, patients were supervised and guided by a trained physiotherapist on a one-on-one basis during the entire resistance exercise phase at the hospital rehabilitation unit. Resistance exercises focusing on the major muscle groups, including the upper and lower extremities, chest, and core muscles, were conducted 2–3 times per week. Each exercise was repeated 8–12 times in 3 sets. A designated physiotherapist supervised the resistance exercises and offered corrective guidance. Training intensity was continuously monitored and fine-tuned based on Borg scale assessments. A detailed description of the aerobic and resistance exercises is shown in Fig. 2.
Fig. 2.
CONSORT Flow Diagram
Respiratory training
Respiratory training was conducted using a TRI-BALL® respiratory trainer. Easy to handle and train with small size and light design. The initial session of respiratory training was conducted under the demonstration and guidance of a clinical physician. Subsequent sessions were performed by the patients at home, who also kept a daily log of their activities. Patients were instructed to engage in respiratory training at least three times daily, with each session lasting 10 min. TRI-BALL® encourages the patient to achieve a maximum prolonged inhalation, named SMI technique (Sustained Maximal Inspiration). SMI is a deep, slow breathing exercise repeated several times a day that strengthens the respiratory muscles, causing the intercostal muscles and diaphragm to contract, thereby expanding the chest cavity. This process increases lung capacity, enhances lung expansion, and improves coughing ability, helping to prevent mucus accumulation and aiding in the recovery of lung function after surgery.
Nutrition intervention
A registered dietitian assessed the nutritional status of all participants. Nutritional status was evaluated using the Nutritional Risk Screening 2002 (NRS2002) scale, which is both valid and reliable for identifying nutritional risk in elderly hospitalized patients. NRS 2002 score of ≥ 3 indicated nutritional risk. At baseline, participants completed a 3-day total food recall questionnaire to evaluate their daily caloric and protein intake. The dietary goal for participants is to achieve a daily caloric intake that supports a balanced diet, emphasizing a reduction in calories from fats and sugars while ensuring adequate intake of essential nutrients. Specifically, in accordance with the recommended daily intake of fruits and vegetables outlined in the Chinese Dietary Guidelines, participants are instructed to consume at least 300–500 g of vegetables and 200–350 g of fruit per day. Additionally, target protein intake was 1.5 g/kg/d [16]. If the patient did not meet the protein requirement by diet al.one, they were provided with whey protein supplementation (Details are provided in Supplementary Material 1). Thirteen participants required daily whey protein supplementation to meet the recommended protein intake. These participants were instructed to consume the supplement within one hour after exercise to optimize muscle protein synthesis [17, 18].
The research team instructed patients and their families to use the FatSecret APP or the website https://www.fatsecret.cn/ for daily calorie tracking (Details are provided in Supplementary Material 1). This approach aimed to enhance patients’ self-management skills, enabling them to make appropriate dietary adjustments based on their individual needs.
Psychological intervention
Anxiety-coping interventions included relaxation techniques and deep breathing exercises, administered in a one-to-one format by a nurse trained in psychological care. Participants identified as being at greater risk for psychological distress were referred to a medical psychologist for further treatment. Additionally, the intervention encompassed counseling on smoking and alcohol cessation.
Control conditions - usual clinical care
Perioperative care of the control group was based on standardized ERAS recommendations that have been widely implemented to minimize heterogeneity in perioperative care. Typically, participants did not receive any preoperative interventions related to exercise, nutrition, or mental health.
Outcomes
The primary outcome was 30-day postoperative complications determined using the Comprehensive Complication Index (CCI). The CCI, a validated index for assessing morbidity and mortality, aggregates all complications using the Clavien-Dindo classification system, resulting in a score from 0 (no complications) to 100 (death) [19]. The CCI was calculated using the online CCI calculator available at http://www.assessurgery.com. A CCI score more than 20 was considered to define severe complications significantly impacting surgical outcomes based on prior research involving a similar surgical cohort [9]. Secondary outcomes included postoperative 6-minute walking distance (6MWD), other postoperative endpoints (such as transfusion requirements, recovery of gastrointestinal function, LOS, hospital readmission), 3-month postoperative quality of life (QoR-9 scale), physical activity [Short Form (36) health survey (SF-36)] [20], and psychological status (Hospital Anxiety and Depression scale) [21]. Functional capacity was assessed using the 6-MWD, a reliable measure of exercise tolerance in patients undergoing colorectal surgery [22], with changes of at least 20 m deemed clinically significant [23].
Additionally, in our original trial registration (No. NCT06510088), both postoperative complications and functional capacity were listed as co-primary outcomes. However, during the analysis, we recognized that postoperative complications should be the primary focus because of their critical impact on patient recovery. Therefore, we have made a post-hoc decision to designate postoperative complications as the sole primary outcome. We explicitly clarify this change to ensure transparency regarding the deviation from the original registration and to align our analysis with clinical priorities.
Sample size and statistical analysis
An intention-to-treat population analysis was conducted. The calculation for the sample size was derived from the mean (standard deviation, SD) CCI (10.5 [14]) observed in our population, with a 30% reduction in the CCI deemed clinically significant. By employing an α of 0.05, a power of 0.90 (2-sided test), and accounting for an anticipated dropout rate (e.g., surgeries not performed) of 10%, the target was set to enroll 120 participants (60 per group).
Statistical analyses were performed using the SPSS (version 26.0) and R software (version 4.2.3). Continuous variables are depicted as the mean (SD) or median [first quartile (Q1) - third quartile (Q3)] and were compared using either independent sample t-tests or Mann-Whitney U tests. Qualitative variables were expressed as percentages and compared across groups utilizing either Pearson’s chi-square test or Fisher’s exact test. To identify independent predictors of postoperative complications, a multivariate logistic regression analysis was conducted. Statistical significance was established at P < 0.05.
Results
Patient characteristics
From March 1, 2019, to December 31, 2023, a total of 218 potential patients were screened for eligibility. Of these, 92 patients were excluded for various reasons: 82 did not meet the frailty criteria, 3 had contraindications for exercise, and 7 were scheduled for neoadjuvant treatment. Consequently, 126 frail patients were randomized into the intervention group (n = 62) or the control group (n = 64). After randomization, an additional 11 patients were excluded due to surgery refusal, COVID-19 restrictions, transition to palliative care, or loss to follow-up. Thus, the final analysis included 115 patients, with 57 in the intervention group and 58 in the control group.
The baseline physical conditions and surgical characteristics were similar between the intervention and control groups, with no statistically significant differences observed (Table 1). Both groups exhibited a high prevalence of minimally invasive surgery, at 87.7% in the intervention group and 82.8% in the control group. Adverse events were reported by 7 of the 123 participants, primarily consisting of lightheadedness or nausea attributed to the exercise intervention (n = 4) or protein supplements (n = 3). Notably, no serious adverse events related to the program were recorded.
Table 1.
Baseline and Surgical characteristics in the intervention vs. control groups
| Variables | Intervention Group (n = 57) | Control Group (n = 58) |
|---|---|---|
| Age, Median (Q1-Q3), y | 73 [70.5, 77] | 74 [68, 78] |
| Sex | ||
| Female | 24 (42.1) | 30 (51.7) |
| Male | 33 (57.9) | 28 (48.3) |
| Body mass index, Median (Q1-Q3), kg/m2 a | 25.1 [23.0, 27.2] | 25.5 [23.7, 27.8] |
| Education level above high school | 40 (70.2) | 35 (60.3) |
| Smoking status | ||
| None | 15 (26.3) | 12 (20.7) |
| Former | 30 (52.6) | 30 (51.7) |
| Current | 12 (21.1) | 16 (27.6) |
| Alcohol use (current), No. (%), d | 9 (15.8) | 6 (10.3) |
| ASA physical status classification, No. (%) | ||
| II | 37 (64.9) | 33 (56.9) |
| III | 20 (35.1) | 25 (43.1) |
| Fried Frailty Index, No. (%) b | ||
| 2 | 23 (40.4) | 24 (41.4) |
| 3 | 27 (47.4) | 26 (44.8) |
| 4 | 6 (10.5) | 8 (13.8) |
| 5 | 1 (1.8) | 0 (0) |
| Comorbidities, No. (%) | ||
| Hypertension | 29 (50.9) | 33 (56.9) |
| Diabetes | 18 (31.6) | 21 (36.2) |
| Cardiovascular diseases | 16 (28.1) | 19 (32.8) |
| Dyslipidemia | 25 (43.9) | 26 (44.8) |
| Asthma | 2 (3.5) | 1 (1.7) |
| Chronic obstructive pulmonary disease | 6 (10.5) | 3 (5.2) |
| Obstructive sleep apnea | 5 (8.8) | 5 (8.6) |
| Others | 11 (19.3) | 15 (25.8) |
| Charlson comorbidity index, Median (Q1-Q3) c | 3 (2–5) | 3 (3–5) |
| Nutritional status—NRS2002, No. (%) | ||
| < 3 | 18 (31.6) | 23 (39.7) |
| ≥ 3 | 39 (68.4) | 35 (60.3) |
| Metabolic status | ||
| Hemoglobin level, Median (Q1-Q3), g/L | 127.9 [118.5, 139] | 124.8 [119.4, 137.7] |
| C-reactive protein level, Median (Q1-Q3), mg/L | 5.4 [2.1, 12.8] | 4.5 [1.3, 10.7] |
| Albumin level, Mean (SD), g/L | 42.1 (3.35) | 41.6 (4.8) |
| Pathological TNM stage | ||
| I | 7 (12.3) | 11 (19.0) |
| II | 27 (47.4) | 28 (48.3) |
| III | 23 (40.4) | 19 (32.8) |
| Aerobic capacity | ||
| Baseline 6MWD, Mean (SD), m | 332.8 [309, 376.4] | 328 [300.5, 369] |
| EORTC QLQ-C30-global health, Median (Q1-Q3) | 66 [66, 83] | 66 [50, 83] |
| Surgical data | ||
| Duration of surgery, Mean (SD), min | 156.2 (11.7) | 151.8 (12.1) |
| Estimated blood loss, Median (Q1-Q3), mL | 80 [50, 100] | 100 [50, 100] |
| Intraoperative transfusion | 0 (0) | 1 (1.7) |
| Surgical approach, No. (%) | ||
| Open | 7 (12.3) | 10 (17.2) |
| Minimally invasive | 50 (87.7) | 48 (82.8) |
Abbreviation: 6-MWD 6-minute walking distance, ASA American Society of Anesthesiologists, EORTC QLQ-C30 European Organsation for Research and Treatment Of Cancer, Quality Of Life Of Cancer Patients Module, Q1 first quartile, Q3 third quartile, SD standard deviation, TNM tumor, node, metastasis
aCalculated as weight in kilograms divided by square of height in meters
bHigher scores indicate greater frailty
cScores range from 0 to 24, with higher scores indicating greater comorbidities
In addition, the following data summarizes adherence to each component of the intervention: 85% of participants completed three sessions of moderate-intensity aerobic exercise each week. Additionally, 83% of participants performed at least two resistance training sessions per week, with 68% achieving three sessions. Adherence to respiratory training was 92%. Moreover, all participants consistently followed the recommended daily intake of whey protein.
Primary outcomes
Complications
A detailed description of postoperative complications is presented in Table 3. No between-group difference was found in the primary outcome measure, 30-day CCI. The Median (Q1-Q3) CCI for the intervention and control groups was 0 (0-12.2) and 0 (0-22.6) (P = 0.082), while the mean (SD) CCI was 6.1 (15.8) and 9.8 (12.7), respectively (P = 0.291). Notably, the incidence of severe complications (CCI > 20) was notably lower in the intervention group compared to the control group (11.1% vs. 25.9%, P = 0.046). We also did not identify between-group differences in 30-day overall complications (25.9% vs. 39.7%, P = 0.123) and surgical complications (14.0% vs. 17.2%, P = 0.636). Additionally, patients in the intervention group experienced fewer medical complications (12.3% vs. 29.3%, P = 0.025), particularly those related to cardiovascular or respiratory issues. In addition, multivariable logistic regression analyses revealed that a Charlson Comorbidity Index score < 3 (odds ratio, OR [95% confidence interval, CI] 2.017 [1.241–3.816]; P = 0.027) and multimodal prehabilitation (OR [95% CI] 1.971 [1.232–3.195], P = 0.039) were found to be independent protective factors for 30-day overall complications, whereas Fried Frailty Index and surgical approach, were not (Table 2).
Table 3.
Impact of the intervention on aerobic capacity
| 6-MWD | Intervention Group (n = 57) | Control Group (n = 58) | P value |
|---|---|---|---|
| Preoperative, Median (Q1-Q3), m | 374 [320.5, 417] | 337 [296, 378] | 0.044* |
| Difference from baseline ≥ 20 m, No. (%) a | 27 (47.4) | 16 (27.6) | 0.028* |
| Postoperative (4 weeks), Median (Q1-Q3), m | 348.5 [311, 405] | 327 [295, 362] | 0.274 |
| Recovered at 4 weeks postoperatively, No. (%) b | 34 (63.2) | 25 (43.1) | 0.031* |
Abbreviation: 6-MWD 6-minute walking distance, Q1 first quartile, Q3 third quartile
aImprovement of 6MWD of 20 m or more compared with baseline
bRecovered to 6MWD within 20 m of the baseline value or above
Table 2.
Postoperative outcomes in the intervention vs. control groups
| Variables | Intervention Group (n = 57) | Control Group (n = 58) | P value |
|---|---|---|---|
| Comprehensive Complication Index | |||
| Mean (SD) | 6.1 (15.8) | 9.8 (12.7) | 0.291 |
| Median (Q1-Q3) | 0 (0-12.2) | 0 (0-22.6) | 0.082 |
| Comprehensive Complication Index > 20, No. (%) | 6 (10.5) | 15 (25.9) | 0.033 |
| Delayed gastric emptying | 1 (1.8) | 1 (1.7) | 0.990 |
| Anastomotic leakage | 1 (1.8) | 1 (1.7) | 0.990 |
| Intra-abdominal abscess | 1 (1.8) | 2 (3.4) | 0.569 |
| Bleeding | 1 (1.8) | 1 (1.7) | 0.990 |
| Ileus | 0 (0) | 2 (3.4) | 0.157 |
| Pulmonary complications | 1 (1.8) | 5 (8.6) | 0.098 |
| Cardiovascular complications | 1 (1.8) | 2 (3.4) | 0.569 |
| Neurological complications | 0 (0) | 1 (1.7) | 0.319 |
| Overall complications, No. (%) | 14 (24.6) | 23 (39.7) | 0.083 |
| Number of patients with medical complication, No. (%) a | 7 (12.3) | 17 (29.3) | 0.025 |
| Cardiovascular | 2 (3.5) | 4 (6.9) | 0.414 |
| Neurological | 2 (3.5) | 1 (1.7) | 0.548 |
| Pulmonary | 4 (7.0) | 9 (15.5) | 0.257 |
| Urological | 2 (3.5) | 3 (5.2) | 0.662 |
| Thromboembolic | 0 (0) | 1 (1.7) | 0.319 |
| Others | 1 (1.8) | 4 (6.9) | 0.176 |
| Number of patients with surgical complication, No. (%) a | 8 (14.0) | 10 (17.2) | 0.636 |
| Delayed gastric emptying | 2 (3.5) | 3 (5.2) | 0.662 |
| Anastomotic leakage | 1 (1.8) | 1 (1.7) | 0.990 |
| Intra-abdominal abscess | 2 (3.5) | 2 (3.4) | 0.986 |
| Bleeding | 1 (1.8) | 1 (1.7) | 0.990 |
| Ileus | 0 (0) | 2 (3.4) | 0.157 |
| Cholecystitis | 1 (1.8) | 0 (0) | 0.311 |
| Abdominal wound complication | 1 (1.8) | 2 (3.4) | 0.569 |
| Number of patients with both medical and surgical complication, No. (%) | 4 (7.0) | 7 (12.1) | 0.357 |
| Time to first, Median (Q1-Q3), d c | |||
| Aerofluxus | 3 [3, 4] | 3 [3, 4] | 0.361 |
| Defecation | 5 [5, 6] | 6 [5, 6] | 0.169 |
| Liquid diet | 3 [3, 4] | 4 [3, 4] | 0.129 |
| Half-liquid diet | 7 [6, 8] | 8 [7, 8] | 0.176 |
| In-hospital mortality | 1 (1.8) | 0 (0) | 0.311 |
| Length of postoperative hospital stay, Median (Q1-Q3), d | 8 [6, 10] | 8 [7, 11] | 0.493 |
| ICU admission | 7 (12.3) | 8 (13.8) | 0.810 |
| ICU days of stay | 2 [1, 3] | 2 [1, 4] | 0.224 |
| Hospital readmission < 30 days, No. (%) | 3 (5.3) | 3 (5.2) | 0.983 |
Abbreviation: ICU Intensive Care Unit, Q1 first quartile, Q3 third quartile
aMore than one complication may be present in a patient
bDelirium, collapse, decubitus
cData on relevant variables were missing for very few participants, and all missing values were filled in with the corresponding median values in each group
Secondary outcomes
Functional walking capacity
Post-intervention changes in the 6MWD for both groups are presented in Table 4. During the preoperative period, the median 6MWD increased by 40.5 m in the intervention group compared to an increase of only 9 m in the control group (P = 0.047). In the intervention group, 27 out of 57 patients (47.4%) experienced a preoperative increase in 6MWD of 20 m or more from baseline, considered the minimal important difference, while this improvement was seen in only 16 out of 58 patients (27.6%) in the control group (P = 0.028). At 4 weeks post-surgery, 34 patients (63.2%) in the intervention group had either recovered to or exceeded their baseline 6MWD, whereas only 25 patients (43.1%) in the control group achieved this level of improvement or maintenance in their walking capacity (P = 0.031).
Table 4.
Impact of the intervention on lung function, quality of life, physical activity, and Psychological Status
| Variables | Intervention Group (n = 57) | Control Group (n = 58) | P value |
|---|---|---|---|
| Pulmonary function test | |||
| FEV1 (L), Mean (SD) | |||
| Baseline | 2.41 (0.62) | 2.38 (0.55) | 0.870 |
| Before surgery | 2.59 (0.51) | 2.42 (0.53) | 0.748 |
| 4 weeks after surgery | 2.04 (0.58) | 1.89 (0.50) | 0.776 |
| FVC (L), Mean (SD) | |||
| Baseline | 3.20 (0.75) | 3.02 (0.69) | 0.853 |
| Before surgery | 3.38 (0.77) | 2.99 (0.62) | 0.532 |
| 4 weeks after surgery | 2.74 (0.66) | 2.38 (0.68) | 0.561 |
| FEV1 / FVC (%), Mean (SD) | |||
| Baseline | 76.1 (11.5) | 80.2 (7.8) | 0.607 |
| Before surgery | 74.0 (14.5) | 79.6 (8.2) | 0.583 |
| 4 weeks after surgery | 75.7 (10.1) | 79.9 (8.7) | 0.650 |
| PEF (L/min), Mean (SD) | |||
| Baseline | 316.4 (112.3) | 335.1 (106.9) | 0.812 |
| Before surgery | 376.8 (89.2) | 368.3 (110.1) | 0.902 |
| 4 weeks after surgery | 289.5 (81.9) | 259.5 (86.0) | 0.688 |
| Quality of Life | |||
| QoR-9, Median (Q1-Q3) a | |||
| 1 week after surgery | 14 [12–15] | 13 [12–15] | 0.796 |
| 2 weeks after surgery | 16 [15–17] | 15.5 [14–17] | 0.647 |
| 4 weeks after surgery | 17 [17–18] | 17 [16–18] | 0.562 |
| Physical Activity | |||
| Total Physical SF-36 subscale, Mean (SD) b | |||
| Baseline | 49.5 (19.3) | 51.8 (17.5) | 0.836 |
| Before surgery | 56.7 (18.6) | 58.6 (19.5) | 0.921 |
| 4 weeks after surgery | 49.3 (20.2) | 51.2 (14.6) | 0.648 |
| Total Mental SF-36 subscale, Mean (SD) b | |||
| Baseline | 53.7 (22.4) | 58.5 (20.4) | 0.901 |
| Before surgery | 59.3 (20.6) | 66.2 (19.6) | 0.933 |
| 4 weeks after surgery | 54.1 (21.9) | 62.5 (15.4) | 0.647 |
| Psychological Status | |||
| HADS Anxiety score, Median (Q1-Q3) c | |||
| Baseline | 5 [3, 8] | 5 [2, 7] | 0.769 |
| Before surgery | 4 [2, 6] | 4 [2, 6.5] | 0.835 |
| 4 weeks after surgery | 3 [2, 5] | 3.5 [2, 5] | 0.851 |
| HADS Depression score, Median (Q1-Q3) c | |||
| Baseline | 4 [2, 5] | 3 [2, 5] | 0.811 |
| Before surgery | 2 [0, 4] | 3 [0, 6] | 0.627 |
| 4 weeks after surgery | 2 [1, 3] | 2 [1, 3] | 0.714 |
Abbreviations: 6MWD 6-minute walk distance, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, HADS Hospital Anxiety and Depression Scale, PEF peak expiratory flow, Q1 first quartile, Q3 third quartile, SD standard deviation
aScores range from 0 to 18, with higher values indicating better recovery
bScores range from 0 to 100, with higher values indicating better health
cScores range from 0 to 21, with higher values indicating worse health
Surgical outcomes
The surgical outcome data are summarized in Table 3. There were no significant differences in the LOS, with a Median (Q1-Q3) of 8 [6–10] days in the intervention group and 8 [7–11] days in the control group (P = 0.493). Hospital readmission rates within 30 days were 5.3% in the intervention group and 5.2% in the control group (P = 0.983 ). Admissions to the intensive care unit (ICU) were 12.3% for the intervention group and 13.8% for the control group (P = 0.810), with ICU stays lasting a Median (Q1-Q3) of 2 [1–3] days versus 2 [1–4] days (P = 0.224). One participant in the intervention group died within 30 days post-surgery due to anastomotic leakage. No statistically significant differences were observed between the two groups in terms of the Median (Q1-Q3) time to first aerofluxus (3 [3, 4] vs. 3 [3, 4] days, P = 0.361), first defecation (5 [5, 6] vs. 6 [5, 6] days, P = 0.169), first liquid diet (3 [3, 4] vs. 4 [3, 4] days, P = 0.129), or first half-liquid diet (8 [7, 8] vs. 8 [7-8.5] days, P = 0.176).
Functional outcomes
Table 5 presents the predetermined secondary functional outcomes over time. There were no significant differences in various pulmonary function indices between the control and intervention groups, nor were there notable differences between pre- and post-intervention conditions within each group. Similarly, QoR-9 scores did not differ significantly between the intervention and control groups. No significant between-group differences were observed for physical activity (SF-36 scale) or for psychological status (HADS Anxiety and Depression score).
Table 5.
Univariable and Multivariable Logistic Regression Analysis of Postoperative complications
| Variable | Univariable Logistic Analysis | Multivariable Logistic Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age, y | ||||||
| < 75 | 1 | Reference | - | - | - | - |
| ≥ 75 | 1.652 | 0.604–4.562 | 0.329 | - | - | - |
| Sex | ||||||
| Male | 1 | Reference | - | - | - | - |
| Female | 0.723 | 0.216–2.117 | 0.598 | - | - | - |
| Preoperative BMI | 0.806 | 0.547–1.311 | 0.369 | - | - | - |
| ASA physical status classification | ||||||
| II | 1 | Reference | - | - | - | - |
| III | 0.875 | 0.407–2.083 | 0.766 | - | - | - |
| IV | 1.072 | 0.374–3.158 | 0.910 | - | - | - |
| Fried Frailty Index | ||||||
| 2 | 1 | Reference | - | - | - | - |
| 3 | 1.465 | 0.545–3.831 | 0.446 | - | - | - |
| 4 | 1.725 | 0.722–4.156 | 0.219 | - | - | - |
| 5 | 1.594 | 0.333–2.942 | 0.611 | - | - | - |
| Charlson comorbidity index | ||||||
| < 3 | 1 | Reference | - | - | - | - |
| ≥ 3 | 2.351 | 1.380–4.022 | 0.002 | 2.017 | 1.241–3.816 | 0.027 |
| Smoking status | ||||||
| None | 1 | Reference | - | - | - | - |
| Current | 1.079 | 0.371–2.425 | 0.872 | - | - | - |
| Alcohol use | ||||||
| None | 1 | Reference | - | - | - | - |
| Current | 1.158 | 0.564–2.397 | 0.708 | - | - | - |
| Metabolic status | ||||||
| Preoperative C-reactive protein level | 0.939 | 0.804–1.131 | 0.476 | - | - | - |
| Preoperative albumin level | 0.815 | 0.573–1.169 | 0.244 | - | - | - |
| Preoperative hemoglobin level | 0.901 | 0.636–1.318 | 0.281 | - | - | - |
| Pathological TNM stage a | ||||||
| I | 1 | Reference | - | - | - | - |
| II | 1.532 | 0.862–2.747 | 0.155 | - | - | - |
| III | 1.056 | 0.531–2.104 | 0.921 | - | - | - |
| Aerobic capacity | ||||||
| Baseline 6MWD ≥ 400 m | 1 | Reference | - | - | - | - |
| Baseline 6MWD < 400 m | 1.498 | 0.526–2.208 | 0.262 | - | - | - |
| Multimodal prehabilitation | ||||||
| Yes | 1 | Reference | - | - | - | - |
| No | 2.009 | 1.061–3.512 | 0.008 | 1.971 | 1.232–3.195 | 0.039 |
| Estimated blood loss | 0.425 | 0.151–1.239 | 0.121 | - | - | - |
| Duration of surgery | 0.820 | 0.459–1.261 | 0.593 | - | - | - |
| Intraoperative transfusion | 1.026 | 0.702–1.479 | 0.918 | |||
| Surgical approach | ||||||
| Minimally invasive | 1 | Reference | - | |||
| Open | 0.815 | 0.483–1.625 | 0.581 | - | ||
| Intraoperative transfusion | 1.018 | 0.714–1.463 | 0.922 | |||
Abbreviations: 6-MWD 6-minute walking distance, ASA American Society of Anesthesiologists, BMI body mass index, CI confidence interval, OR odds ratio, TNM tumor, node, metastasis
Discussion
In this randomized clinical trial, multimodal prehabilitation did not appear to reduce the overall 30-day complication rate in frail elderly patients undergoing elective gastric cancer surgery in the context of the ERAS, with no between-group difference in the primary outcome measure, 30-day CCI. However, in further subgroup analyses, multimodal prehabilitation significantly reduced the incidence of severe complications (CCI > 20) and medical complications. Furthermore, multimodal prehabilitation could improve the perioperative functional walking capacity, indicating better functional recovery after prehabilitation. No between-group differences were observed regarding disability, psychological status, and mortality, except for LOS. Additionally, the multivariable logistic regression analysis indicated that multimodal prehabilitation functioned as an independent protective factor against complications.
Preoperative prehabilitation seeks to improve patients’ preoperative functionality and physiological reserves, thereby reducing surgical stress and facilitating postoperative recovery [13, 24]. Compared with previously published preoperative prehabilitation protocols for thoracic and colorectal surgery [9, 25], we have made some improvements to the intervention measures to facilitate clinical implementation and dissemination. Firstly, the duration of the multimodal prehabilitation program we proposed is only 2 weeks, aiming to maximize patient compliance, which is also one of the innovations. Previous studies mostly recommended intervention programs lasting 4 weeks or more [8, 14], but considering that most patients wish to undergo surgery as soon as possible, such a long duration may lead to lower acceptability and compliance. In this study, only a very small number of participants were excluded due to low compliance. Secondly, patients were allowed to select their preferred mode of aerobic exercise rather than adhering to a fixed regimen as seen in most studies. Provided the exercise recommendations’ frequency, duration, and intensity requirements are met, patients could make slight adjustments to their training plans. Thirdly, recognizing the challenges in implementing and disseminating a supervised hospital-based exercise program, we developed a straightforward home-based regimen, incorporating simple images, completion diaries, and regular visits to promote adherence.
Elderly patients have an increased risk of surgical complications because of malnutrition, underlying diseases, and decreased physical performance [26]. Preoperative frailty has been shown to be associated with an increased risk of postoperative complications and increased LOS after abdominal surgery [27]. However, evidence regarding the effect of multimodal prehabilitation before abdominal surgery in frail patients is limited. Previous studies supported the validity of the CCI as a measure of postoperative morbidity and suggested that, compared with traditional morbidity measures (e.g., overall rate of complications, rate of severe complications), it provides a more comprehensive and sensitive endpoint for surgical research [28, 29]. In this study, the percentage of patients who experienced severe complications decreased by nearly 50% following the multimodal prehabilitation program. Other studies have also reported a reduction in medical complications, which is consistent with the hypothesis that rehabilitation specifically targeting cardiorespiratory health and metabolic balance [12]. The precise underlying mechanisms, however, remain unclear. Additionally, Chia et al. [30] conducted an observational study comparing prehabilitation to standard clinical care, showing a reduction in LOS from 11 to 8 days among frail patients undergoing colorectal surgery. Conversely, our study did not find significant differences in LOS between the groups. Notably, the median LOS in our study was considerably shorter than that observed by Chia et al. [30], potentially due to differences in the care context, such as the implementation of ERAS. In the past decades, the implementation of ERAS has significantly improved the prognosis of surgical patients. However, ERAS interventions are limited, and multimodal prehabilitation serves as a direct complement to this program.
Enhanced cardiorespiratory fitness prior to surgery is believed to augment physiological reserve and increase the patient’s ability to tolerate surgical stress [31]. Aerobic exercise can improve cardiopulmonary function and reduce the incidence of cardiovascular complications. Respiratory training effectively decreases the risk of postoperative pulmonary complications by enhancing lung capacity and airway patency. A comprehensive prehabilitation program improves the overall health status of patients, thereby reducing both the incidence and severity of postoperative complication. These mechanisms collectively facilitate postoperative recovery and enhance patients’ quality of life, further underscoring the significance of prehabilitation programs in postoperative recovery. In terms of aerobic training modalities utilized in prehabilitation programs, the literature describes endurance exercise training, resistance training, inspiratory muscle training, or a combination of these methods. However, there is a scarcity of studies examining the impact of improved aerobic capacity on postoperative adverse events [32]. While lung function tests suggested a potential benefit of these interventions, no significant differences were observed in major lung function indicators. This finding can largely be attributed to the fact that baseline pulmonary function was predominantly within normal ranges. Additionally, the incidence of pulmonary complications was notably higher in the control group compared to the intervention group. Nevertheless, the stability of the results of the lung function indexes is still questionable due to the limited sample size, necessitating further investigation.
There is no universally accepted measure of perioperative functional capacity. We selected this measure as it effectively mirrors daily activities, involves a wide range of bodily systems engaged in exercise, and is both well-tolerated and easy to administer. The 6MWD has previously been shown to predict postoperative complications and cancer survival in patients undergoing pulmonary surgery [33]. Previously, 6MWD has also been shown to predict postoperative complications and cancer survival in a pulmonary surgery population [12]. Our findings showed improved functional recovery both pre- and postoperatively compared to baseline levels. Specifically, the intervention group exhibited a preoperative mean 6MWD surpassing baseline levels, whereas the control group’s performance did not improve significantly. Following multimodal rehabilitation, a greater number of participants experienced a minimum 20-meter enhancement in the postoperative 6MWD than their baseline levels. At the 4-week postoperative assessment, the 6-minute walk distance (6MWD) showed a significant difference between the two groups, favoring prehabilitation. Overall, prehabilitation leads to a faster return to baseline functional levels, and in some cases, even surpassing the initial values—a trend not observed in the control group.
It is worth noting that multimodal prehabilitation had no detectable impact on quality of life and psychological status. On the one hand, it could be argued that the short duration of the program and the lack of more comprehensive psychological interventions may explain the lack of effect on both quality of life and psychological status. On the other hand, most patients experienced good perioperative recovery without signs of anxiety or depression, hence short-term assessments of health-related quality of life and psychological well-being did not show significant improvements.
It is noteworthy that multimodal prehabilitation did not demonstrate a discernible impact on quality of life and psychological status. One possible explanation is the short duration of the program and the absence of more comprehensive psychological interventions, which may account for the lack of effect on both quality of life and psychological status. Conversely, the majority of patients experienced satisfactory perioperative recovery without exhibiting signs of anxiety or depression. Consequently, short-term assessments of health-related quality of life and psychological well-being did not reveal significant improvements.
The findings of this randomized trial should be interpreted with certain limitations in mind. First, due to the nature of the intervention, neither participants nor intervention staff were blinded, thereby introducing a potential risk of performance bias. It is important to note, however, that outcome assessors were blinded to group assignments. Second, the small sample size may have increased the likelihood of false-positive and false-negative results. Third, the majority of our sample consisted of cases undergoing minimally invasive surgery, which may limit the generalizability of our findings to settings with a higher prevalence of open surgery. Additionally, this trial was conducted at a center with well-established ERAS protocols; therefore, our results are applicable only to similar care contexts.
Conclusions
In frail elderly patients undergoing elective gastric cancer surgery in the context of ERAS, a prehabilitation program involving exercise, nutritional, and psychological interventions did not appear to affect 30-day postoperative complications rate and 30-day CCIs. It did, however, reduce the incidence of severe and medical complications while improving perioperative functional capacity.
Supplementary Information
Acknowledgements
We thank Muhammad Ali, Ph.D., for editing the English text of the draft of this manuscript.
Abbreviations
- CCI
Comprehensive Complication Index
- CI
Confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- ERAS
Enhanced Recovery After Surgery
- HR
Heart rate
- LOS
Length of hospital stay
- ICU
Intensive care unit
- IQR
Interquartile range
- OR
Odds ratio
- WHO
World Health Organization
- 6MWD
6-minute walking distance
- SF-36
Short Form (36) health survey
- SD
Standard deviation
Authors’ contributions
Jianhui Chen: Conceptualization, Data curation, Formal analysis, Writing – original draft. Chen Hong: Conceptualization, Data curation. Rui Chen: Conceptualization, Writing – review & editing. Mengya Zhou: Conceptualization, Randomization. Senbin Lin: Conceptualization, Supervision, Writing – review and editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
Data availability
The data used in this paper will be provided by the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University. Informed consent was obtained from each participant before enrollment in the study.
Consent for publication
This is not applicable for this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Tian Z, Xia M, Cheng Y, Zhou J, Li R, Zhao S, Sun Q, Wang D. Surgical options and survival prognosis in geriatric patients beyond average lifespan with locally advanced gastric cancer: a propensity score-matched analysis. Surg Endosc. 2024;38(5):2756–69. [DOI] [PubMed] [Google Scholar]
- 3.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120–32. [DOI] [PubMed] [Google Scholar]
- 4.Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, Yasui H, Yabusaki H, Afanasyev S, Park YK, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25(2):212–24. [DOI] [PubMed] [Google Scholar]
- 5.Ushimaru Y, Nagano S, Nishikawa K, Kawabata R, Takeoka T, Kitagawa A, Ohara N, Tomihara H, Maeda S, Imazato M, et al. A comprehensive study on non-cancer-related mortality risk factors in elderly gastric cancer patients post-curative surgery. BMC Gastroenterol. 2024;24(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Zheng HL, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, et al. High preoperative modified frailty index has a negative impact on short- and long-term outcomes of octogenarians with gastric cancer after laparoscopic gastrectomy. Surg Endosc. 2018;32(5):2193–200. [DOI] [PubMed] [Google Scholar]
- 7.Lee DU, Kwon J, Han J, Fan GH, Hastie DJ, Lee KJ, Karagozian R. The clinical impact of frailty on the postoperative outcomes of patients undergoing gastrectomy for gastric cancer: a propensity-score matched database study. Gastric Cancer. 2022;25(2):450–8. [DOI] [PubMed] [Google Scholar]
- 8.Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, Momblán D, Balust J, Blanco I, Martínez-Pallí G. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a Randomized Blinded Controlled Trial. Ann Surg. 2018;267(1):50–6. [DOI] [PubMed] [Google Scholar]
- 9.Molenaar CJL, Minnella EM, Coca-Martinez M, Ten Cate DWG, Regis M, Awasthi R, Martínez-Palli G, López-Baamonde M, Sebio-Garcia R, Feo CV, et al. Effect of Multimodal Prehabilitation on reducing postoperative complications and enhancing functional capacity following colorectal Cancer surgery: the PREHAB Randomized Clinical Trial. JAMA Surg. 2023;158(6):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrell-Vega J, Esparza Gutierrez AG, Humeidan ML. Multimodal Prehabilitation Programs for Older Surgical patients. Anesthesiol Clin. 2019;37(3):437–52. [DOI] [PubMed] [Google Scholar]
- 11.Bolshinsky V, Li MH, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum. 2018;61(1):124–38. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, Liang N, Li S, Chen W, Huang Y. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg. 2020;131(3):840–9. [DOI] [PubMed] [Google Scholar]
- 13.Molenaar CJ, van Rooijen SJ, Fokkenrood HJ, Roumen RM, Janssen L, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database Syst Rev. 2022;5(5):Cd013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, Stein B, Charlebois P, Ghitulescu G, Morin N, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(3):233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 16.McClave SA, Kozar R, Martindale RG, Heyland DK, Braga M, Carli F, Drover JW, Flum D, Gramlich L, Herndon DN, et al. Summary points and consensus recommendations from the North American Surgical Nutrition Summit. JPEN J Parenter Enter Nutr. 2013;37(5 Suppl):s99–105. [DOI] [PubMed] [Google Scholar]
- 17.Gorissen SH, Horstman AM, Franssen R, Crombag JJ, Langer H, Bierau J, Respondek F, van Loon LJ. Ingestion of Wheat Protein Increases in Vivo Muscle Protein Synthesis Rates in healthy older men in a Randomized Trial. J Nutr. 2016;146(9):1651–9. [DOI] [PubMed] [Google Scholar]
- 18.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26(6):s696–703. [DOI] [PubMed] [Google Scholar]
- 19.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 20.Hopman WM, Towheed T, Anastassiades T, Tenenhouse A, Poliquin S, Berger C, Joseph L, Brown JP, Murray TM, Adachi JD, et al. Canadian normative data for the SF-36 health survey. Canadian multicentre osteoporosis study research group. Cmaj. 2000;163(3):265–71. [PMC free article] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 22.Pecorelli N, Fiore JF Jr, Gillis C, Awasthi R, Mappin-Kasirer B, Niculiseanu P, Fried GM, Carli F, Feldman LS. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties. Surg Endosc. 2016;30(6):2199–206. [DOI] [PubMed] [Google Scholar]
- 23.Antonescu I, Scott S, Tran TT, Mayo NE, Feldman LS. Measuring postoperative recovery: what are clinically meaningful differences? Surgery. 2014;156(2):319–27. [DOI] [PubMed] [Google Scholar]
- 24.Gillis C, Ljungqvist O, Carli F. Prehabilitation, enhanced recovery after surgery, or both? A narrative review. Br J Anaesth. 2022;128(3):434–48. [DOI] [PubMed] [Google Scholar]
- 25.Wade-Mcbane K, King A, Urch C, Jeyasingh-Jacob J, Milne A, Boutillier CL. Prehabilitation in the lung cancer pathway: a scoping review. BMC Cancer. 2023;23(1):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesari M, Calvani R, Marzetti E. Frailty in older persons. Clin Geriatr Med. 2017;33(3):293–303. [DOI] [PubMed] [Google Scholar]
- 27.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–86. [DOI] [PubMed] [Google Scholar]
- 28.Artiles-Armas M, Roque-Castellano C, Conde-Martel A, Marchena-Gómez J. The comprehensive complication index is related to frailty in elderly surgical patients. J Surg Res. 2019;244:218–24. [DOI] [PubMed] [Google Scholar]
- 29.Borchert DH, Federlein M, Müller VA, Wagenpfeil S, Eisele RM. Comprehensive complication index for NOTES procedures: results from a randomized controlled trial and comparison to published NOTES complication data. Surg Endosc. 2015;29(10):2928–33. [DOI] [PubMed] [Google Scholar]
- 30.Chia CL, Mantoo SK, Tan KY. Start to finish trans-institutional transdisciplinary care’: a novel approach improves colorectal surgical results in frail elderly patients. Colorectal Dis. 2016;18(1):O43–50. [DOI] [PubMed] [Google Scholar]
- 31.Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Ann Thorac Cardiovasc Surg. 2011;17(5):461–8. [DOI] [PubMed] [Google Scholar]
- 32.Bruce J, Mazuquin B, Canaway A, Hossain A, Williamson E, Mistry P, Lall R, Petrou S, Lamb SE, Rees S, et al. Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation. BMJ. 2021;375:e066542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granger CL, Holland AE, Gordon IR, Denehy L. Minimal important difference of the 6-minute walk distance in lung cancer. Chron Respir Dis. 2015;12(2):146–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this paper will be provided by the corresponding author upon reasonable request.