Abstract
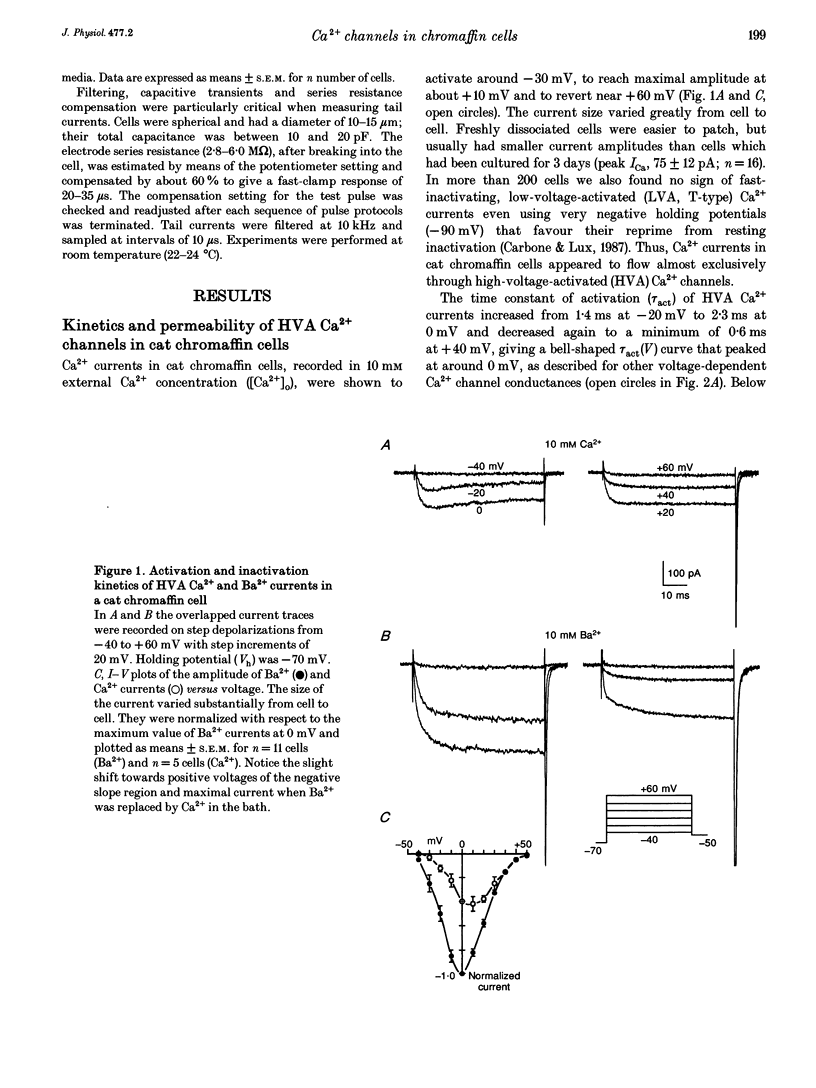
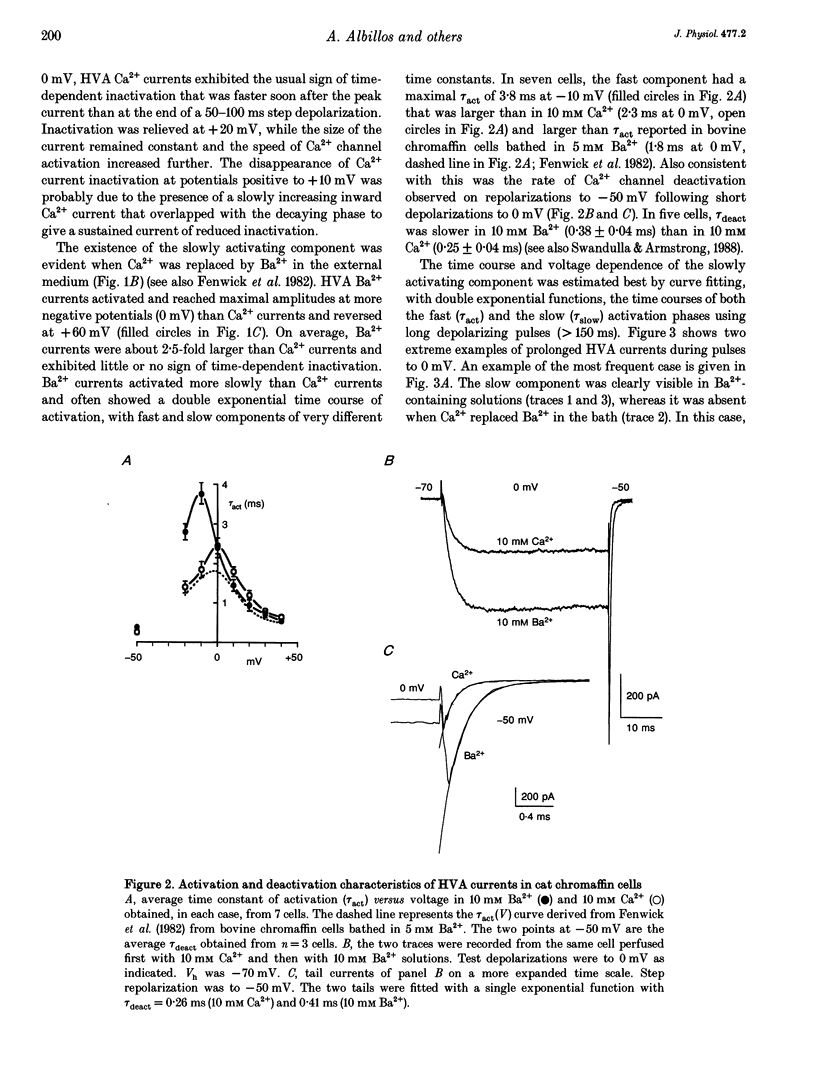
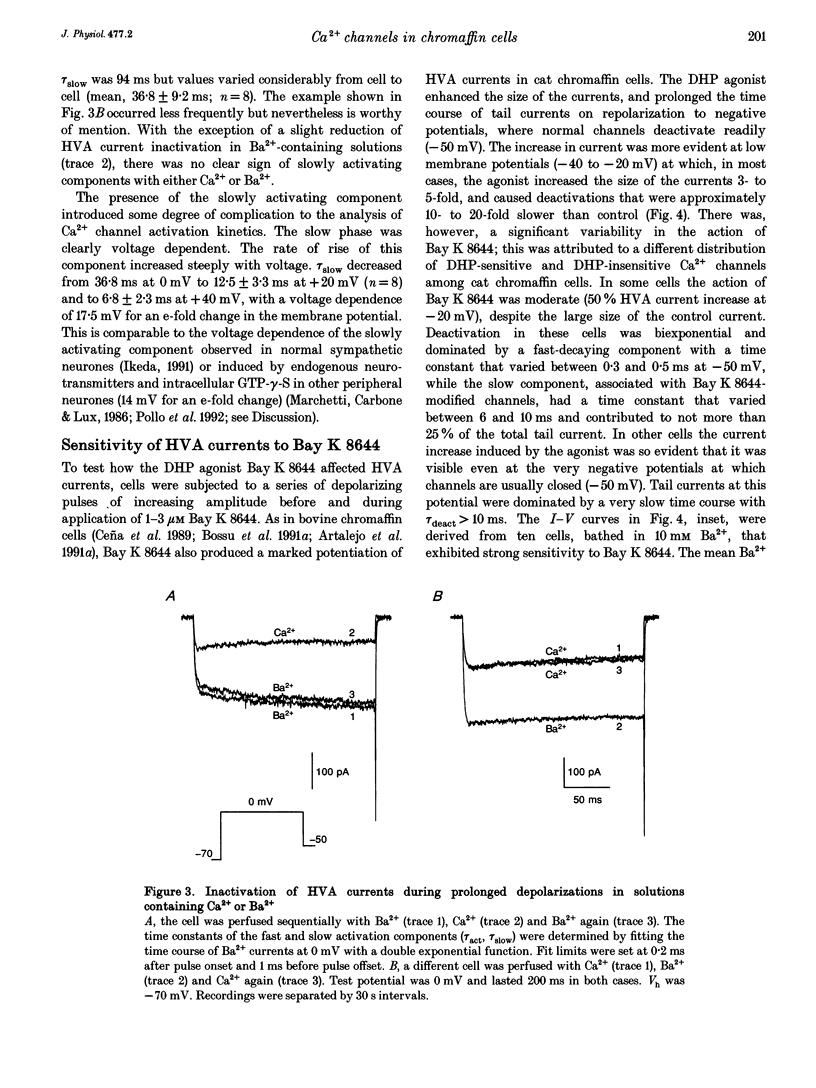
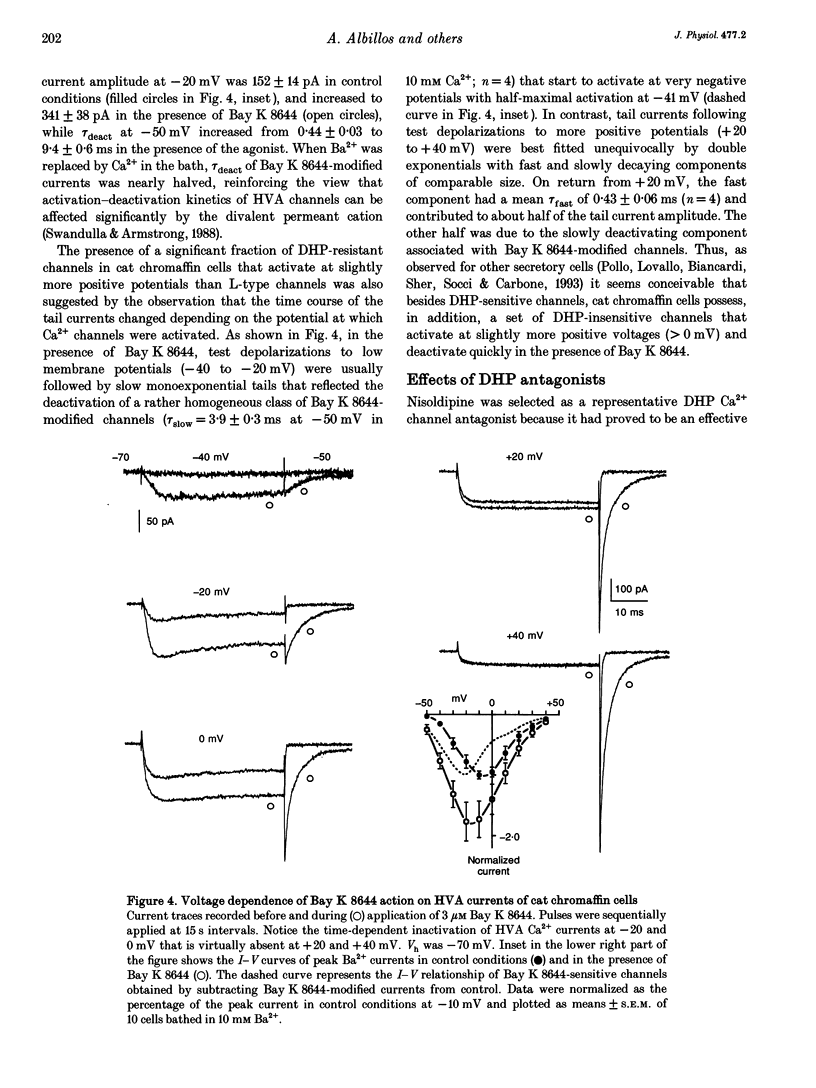
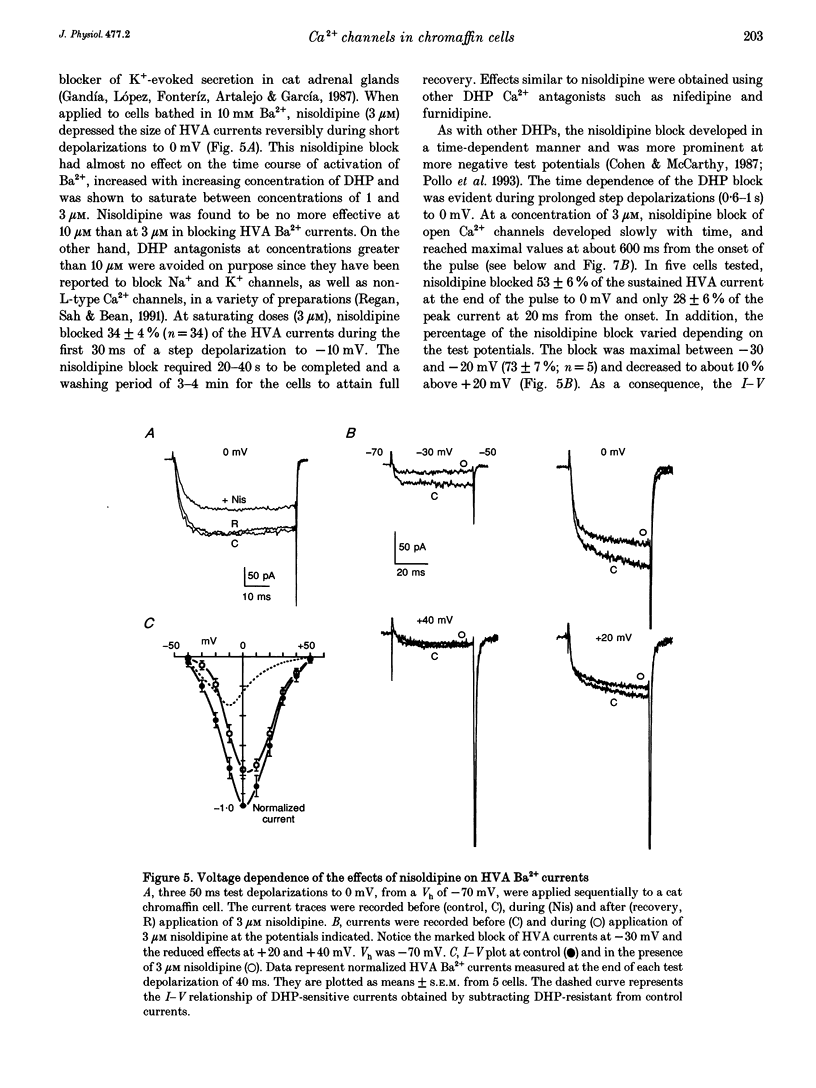
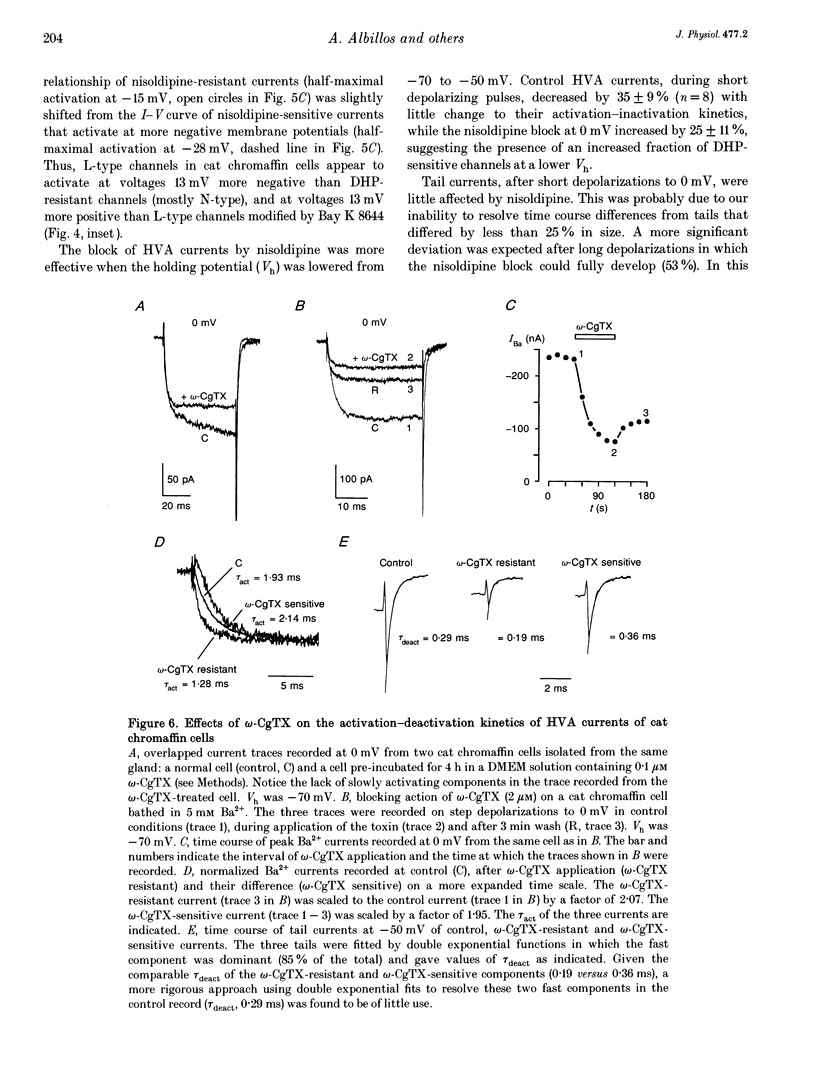
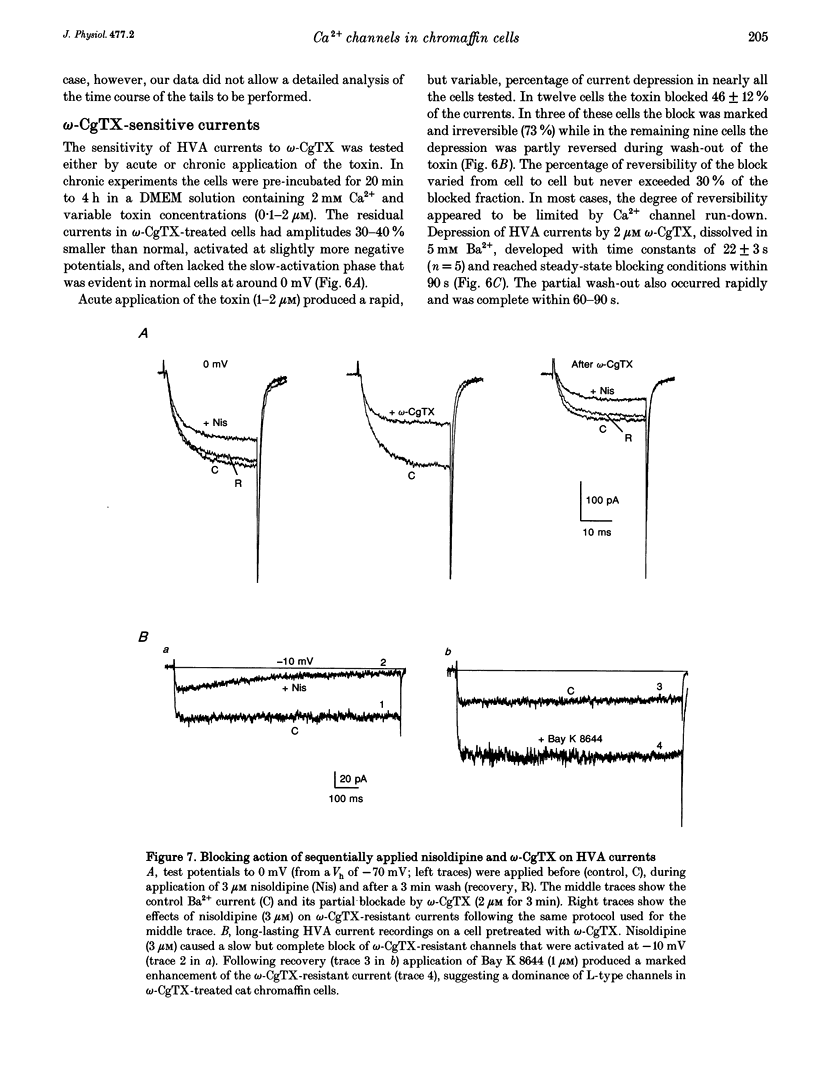
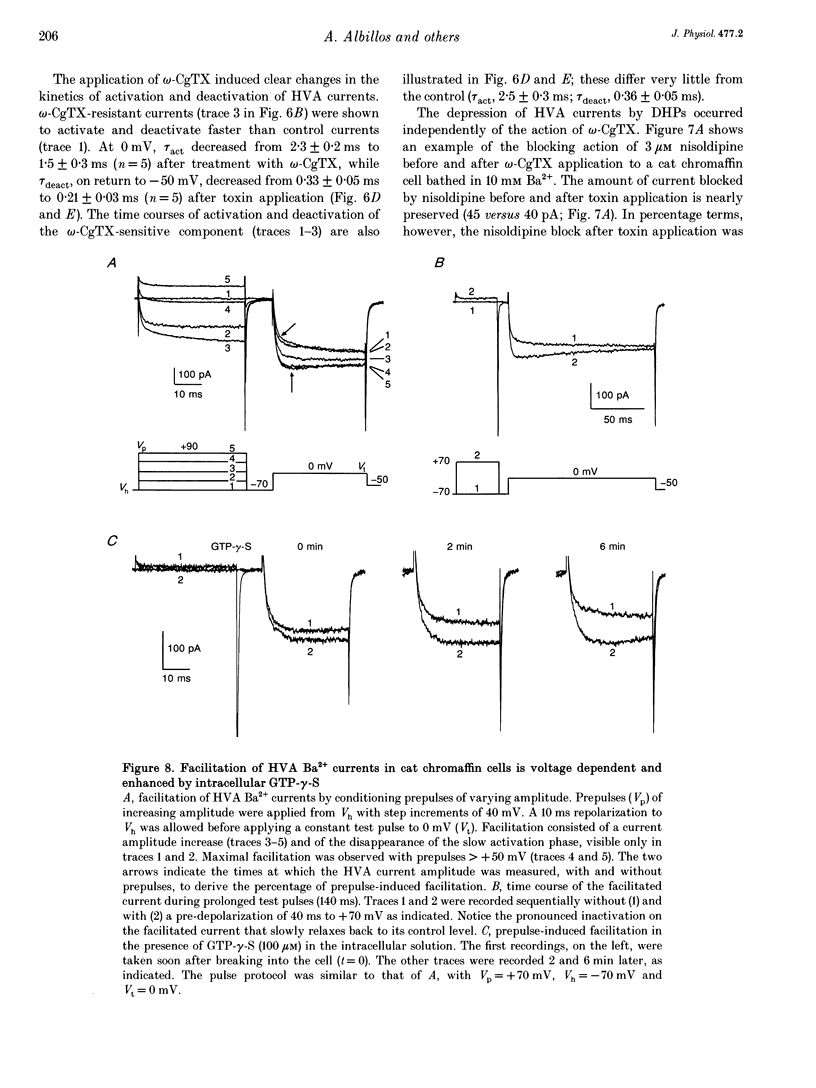
1. Using the patch-clamp technique we have investigated the kinetic and pharmacological properties of high-voltage-activated (HVA) Ca2+ channels in short-term-cultured cat chromaffin cells. 2. In 10 mM Ba2+, HVA currents activated around -40 mV, reached maximal amplitude at 0 mV and reversed at about +60 mV. At 0 mV, HVA current activation was fast (mean tau act, 2.45 ms), and followed by either an incomplete inactivation or by a second slow phase of activation (mean tau slow, 36.8 ms) that was lost when Ba2+ was replaced by Ca2+. HVA Ba2+ currents deactivate quickly on repolarization to -50 mV (mean tau deact, 0.36 ms). 3. In most cells, HVA currents were sensitive to common dihydropyridine (DHP) derivatives. Nisoldipine blocked the currents maximally at low membrane potentials (mean block 76% at -30 mV, 3 microM) and gradually less at higher voltages. Nisoldipine block was clearly time dependent (33 and 56% after 30 and 600 ms, respectively, to 0 mV). 4. Bay K 8644 (3 microM) action was variable and caused (1) a 2- to 4-fold increase of Ba2+ currents at -40 to -20 mV, (2) a -15 mV shift of the current-voltage relationship and (3) a 10- to 20-fold prolongation of HVA channel deactivation at -50 mV. 5. Nisoldipine block and Bay K 8644 potentiation of HVA currents increased markedly in omega-conotoxin GVIA (omega-CgTX)-pretreated cells, suggesting an increased fraction of DHP-sensitive currents in these cells. Nisoldipine block of residual omega-CgTX-resistant currents was almost complete (mean block, 82%) during pulses of 1 s to 0 mV. 6. The degree of inhibition produced by omega-CgTX (2 microM for 1 min) varied from cell to cell (mean block, 46%) and was partly reversible. Residual omega-CgTX-resistant currents exhibited faster activation-deactivation kinetics than control currents. 7. The slow phase of HVA current activation was abolished if a conditioning prepulse of 40 ms to +70 mV preceded a test pulse to 0 mV. Double-pulse protocols caused an average current increase (facilitation) of 37% that was voltage dependent and which correlated with the slow phase of Ca2+ channel activation. Facilitation was lost in most omega-CgTX-treated cells and was little affected by nisoldipine (3 microM) and Bay K 8644 (1 microM). Facilitation was potentiated in cells dialysed with 100 microM guanosine 5'-O-(3-thiotriphosphate) (GTP-gamma-S) and fully prevented by 1 mM guanosine 5'-O-(2-thiodiphosphate) (GDP-beta-S).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

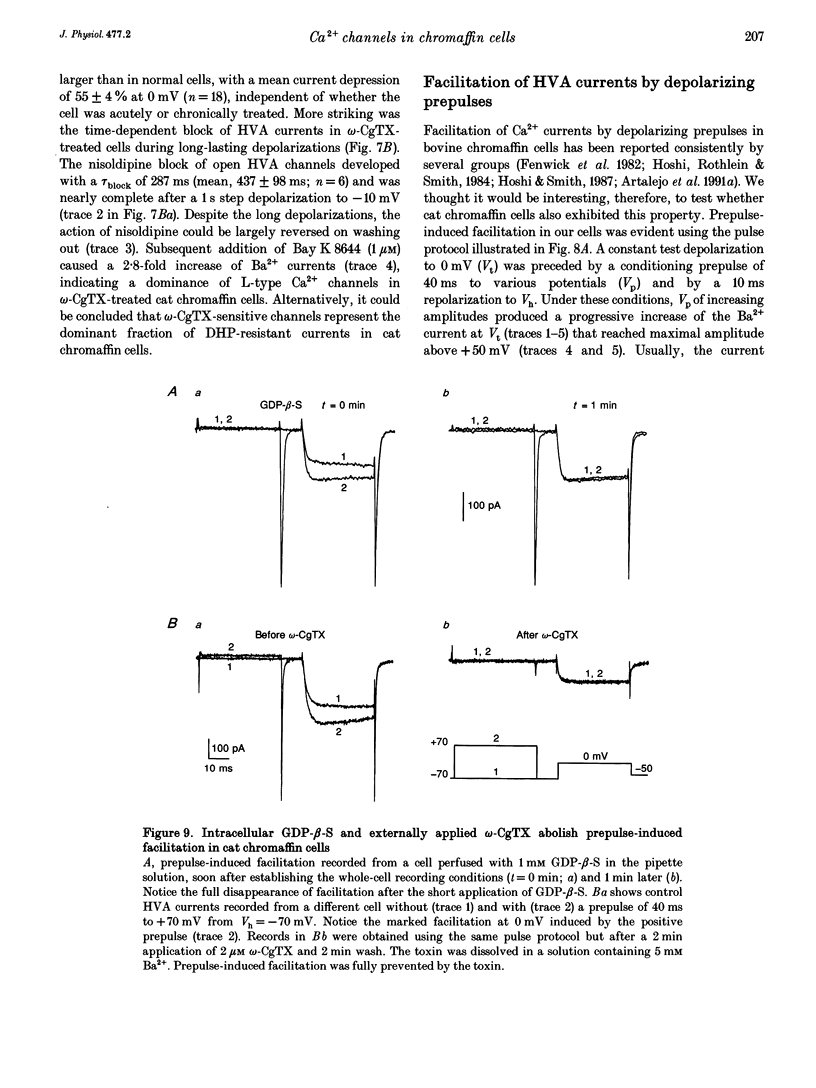
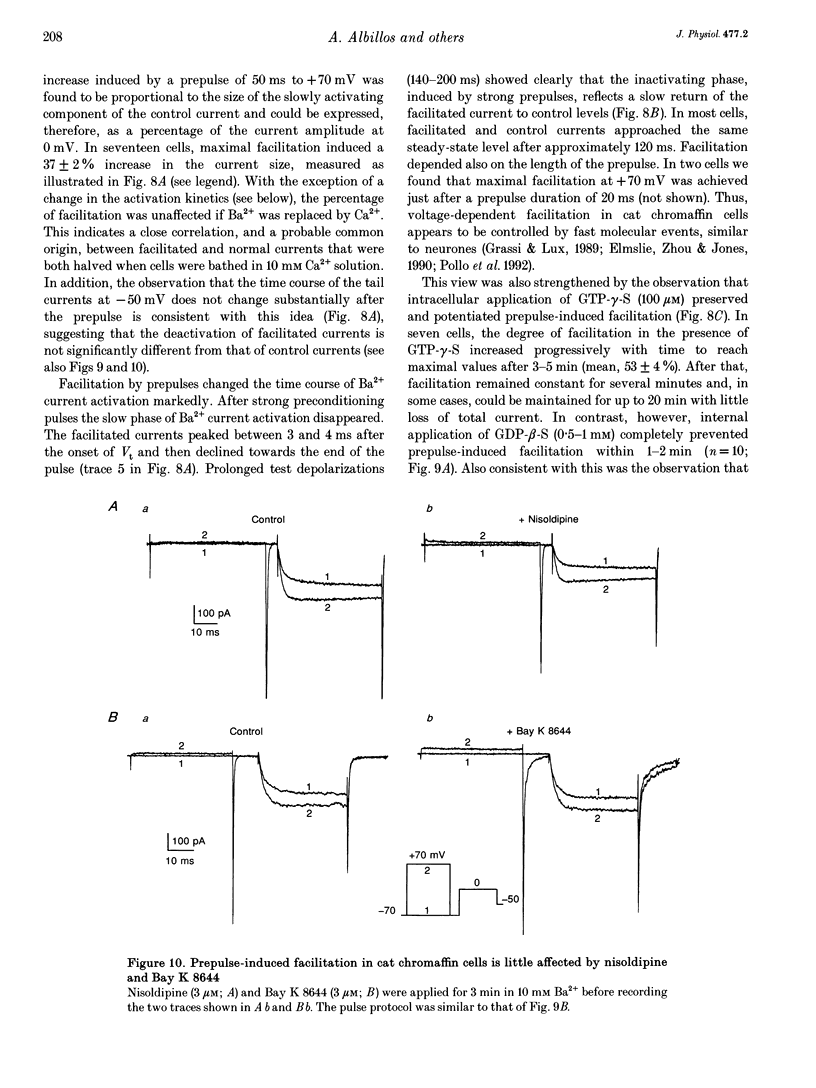





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Le Strange R., Oliff A. I., Furth M. E., Old L. J. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984 Mar 1;308(5954):69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Ariano M. A., Perlman R. L., Fox A. P. Activation of facilitation calcium channels in chromaffin cells by D1 dopamine receptors through a cAMP/protein kinase A-dependent mechanism. Nature. 1990 Nov 15;348(6298):239–242. doi: 10.1038/348239a0. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Dahmer M. K., Perlman R. L., Fox A. P. Two types of Ca2+ currents are found in bovine chromaffin cells: facilitation is due to the recruitment of one type. J Physiol. 1991 Jan;432:681–707. doi: 10.1113/jphysiol.1991.sp018406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Mogul D. J., Perlman R. L., Fox A. P. Three types of bovine chromaffin cell Ca2+ channels: facilitation increases the opening probability of a 27 pS channel. J Physiol. 1991 Dec;444:213–240. doi: 10.1113/jphysiol.1991.sp018874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo C. R., Perlman R. L., Fox A. P. Omega-conotoxin GVIA blocks a Ca2+ current in bovine chromaffin cells that is not of the "classic" N type. Neuron. 1992 Jan;8(1):85–95. doi: 10.1016/0896-6273(92)90110-y. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Rossie S., Perlman R. L., Fox A. P. Voltage-dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 1992 Jul 2;358(6381):63–66. doi: 10.1038/358063a0. [DOI] [PubMed] [Google Scholar]
- Ballesta J. J., Palmero M., Hidalgo M. J., Gutierrez L. M., Reig J. A., Viniegra S., Garcia A. G. Separate binding and functional sites for omega-conotoxin and nitrendipine suggest two types of calcium channels in bovine chromaffin cells. J Neurochem. 1989 Oct;53(4):1050–1056. doi: 10.1111/j.1471-4159.1989.tb07394.x. [DOI] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Inactivation characteristics reveal two calcium currents in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:603–620. doi: 10.1113/jphysiol.1991.sp018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossu J. L., De Waard M., Feltz A. Two types of calcium channels are expressed in adult bovine chromaffin cells. J Physiol. 1991 Jun;437:621–634. doi: 10.1113/jphysiol.1991.sp018615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Sher E., Clementi F. Ca currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Pflugers Arch. 1990 Apr;416(1-2):170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Ceña V., Nicolas G. P., Sanchez-Garcia P., Kirpekar S. M., Garcia A. G. Pharmacological dissection of receptor-associated and voltage-sensitive ionic channels involved in catecholamine release. Neuroscience. 1983 Dec;10(4):1455–1462. doi: 10.1016/0306-4522(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Ceña V., Stutzin A., Rojas E. Effects of calcium and Bay K-8644 on calcium currents in adrenal medullary chromaffin cells. J Membr Biol. 1989 Dec;112(3):255–265. doi: 10.1007/BF01870956. [DOI] [PubMed] [Google Scholar]
- Chow R. H., von Rüden L., Neher E. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 1992 Mar 5;356(6364):60–63. doi: 10.1038/356060a0. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía L., García A. G., Morad M. ATP modulation of calcium channels in chromaffin cells. J Physiol. 1993 Oct;470:55–72. doi: 10.1113/jphysiol.1993.sp019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía L., López M. G., Fonteríz R. I., Artalejo C. R., García A. G. Relative sensitivities of chromaffin cell calcium channels to organic and inorganic calcium antagonists. Neurosci Lett. 1987 Jun 26;77(3):333–338. doi: 10.1016/0304-3940(87)90523-4. [DOI] [PubMed] [Google Scholar]
- Gandía L., Michelena P., de Pascual R., López M. G., García A. G. Different sensitivities to dihydropyridines of catecholamine release from cat and ox adrenals. Neuroreport. 1990 Oct;1(2):119–122. doi: 10.1097/00001756-199010000-00009. [DOI] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hans M., Illes P., Takeda K. The blocking effects of omega-conotoxin on Ca current in bovine chromaffin cells. Neurosci Lett. 1990 Jun 22;114(1):63–68. doi: 10.1016/0304-3940(90)90429-d. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Rothlein J., Smith S. J. Facilitation of Ca2+-channel currents in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5871–5875. doi: 10.1073/pnas.81.18.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Smith S. J. Large depolarization induces long openings of voltage-dependent calcium channels in adrenal chromaffin cells. J Neurosci. 1987 Feb;7(2):571–580. doi: 10.1523/JNEUROSCI.07-02-00571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladona M. G., Aunis D., Gandía L., García A. G. Dihydropyridine modulation of the chromaffin cell secretory response. J Neurochem. 1987 Feb;48(2):483–490. doi: 10.1111/j.1471-4159.1987.tb04118.x. [DOI] [PubMed] [Google Scholar]
- López M. G., Shukla R., García A. G., Wakade A. R. A dihydropyridine-resistant component in the rat adrenal secretory response to splanchnic nerve stimulation. J Neurochem. 1992 Jun;58(6):2139–2144. doi: 10.1111/j.1471-4159.1992.tb10956.x. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carbone E., Lux H. D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch. 1986 Feb;406(2):104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Pollo A., Lovallo M., Biancardi E., Sher E., Socci C., Carbone E. Sensitivity to dihydropyridines, omega-conotoxin and noradrenaline reveals multiple high-voltage-activated Ca2+ channels in rat insulinoma and human pancreatic beta-cells. Pflugers Arch. 1993 Jun;423(5-6):462–471. doi: 10.1007/BF00374942. [DOI] [PubMed] [Google Scholar]
- Pollo A., Lovallo M., Sher E., Carbone E. Voltage-dependent noradrenergic modulation of omega-conotoxin-sensitive Ca2+ channels in human neuroblastoma IMR32 cells. Pflugers Arch. 1992 Oct;422(1):75–83. doi: 10.1007/BF00381516. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Sher E., Pandiella A., Clementi F. Omega-conotoxin binding and effects on calcium channel function in human neuroblastoma and rat pheochromocytoma cell lines. FEBS Lett. 1988 Aug 1;235(1-2):178–182. doi: 10.1016/0014-5793(88)81258-4. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Armstrong C. M. Fast-deactivating calcium channels in chick sensory neurons. J Gen Physiol. 1988 Aug;92(2):197–218. doi: 10.1085/jgp.92.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Holz R. W. Barium and calcium stimulate secretion from digitonin-permeabilized bovine adrenal chromaffin cells by similar pathways. J Neurochem. 1992 Feb;58(2):680–687. doi: 10.1111/j.1471-4159.1992.tb09771.x. [DOI] [PubMed] [Google Scholar]


