Abstract
1. The sarcoplasmic reticulum (SR) membrane of skeletal muscle contains potassium channels which are thought to support charge neutralization during calcium release by providing a permeability pathway for counter-ion movement. To describe the behaviour of the SR K+ channel under physiological conditions, single channel activity was recorded from excised patches of SR membrane. Patches were made from membrane blebs extruded from contracted muscle fibres whose surface membranes had been removed previously by mechanical dissection. 2. The channel was active over a large voltage range from -80 to +100 mV. The current-voltage relationship of the channel was linear over most of this voltage range (slope conductance equal to 60 pS in 130 mM potassium), but showed rectification at voltages below -50 mV. 3. The activity of the channel (number of state transitions per unit time) was greater at positive voltages than at negative voltages. Analysis of dwell-time distributions showed that the time spent in the open state is best fitted by a double Gaussian, suggesting that the channel possesses both a long (l)- and a short (s)-lived open state with identical conductances. The dwell times for the two states were Ts = 0.3 ms and Tl = 2.6 ms at +90 mV and Ts = 0.1 ms and Tl = 15.1 ms at -40 mV. Thus, positive voltage decreased the long open time significantly which was consistent with the observed increase in channel activity at positive potentials. 4. The permeability sequence of the channel to various monovalent cations was deduced from the channel reversal potential under bi-ionic conditions and was found to be: K+ > Rb+ > Na+ > Cs+ > Li+. 5. Channel activity was reduced when the patch was perfused with 1,10-bis-guanidino-n-decane (BisG10), a drug reported to block the SR K+ channel with high affinity. The drug concentration necessary to reduce the open probability (P(o)) by 50% was 19.8 microM at -40 mV and 338.2 microM at +50 mV. The zero voltage dissociation constant (Kd) was calculated to be 48 microM. 6. Pharmacological agents known to affect surface membrane K+ channels, such as 0.5 mM Ba2+ or 3.0 mM 4-aminopyridine, were much less effective in blocking the channel than BisG10. Physiological calcium concentrations (pCa = 8.0 and 3.0) did not affect channel behaviour.4
Full text
PDF

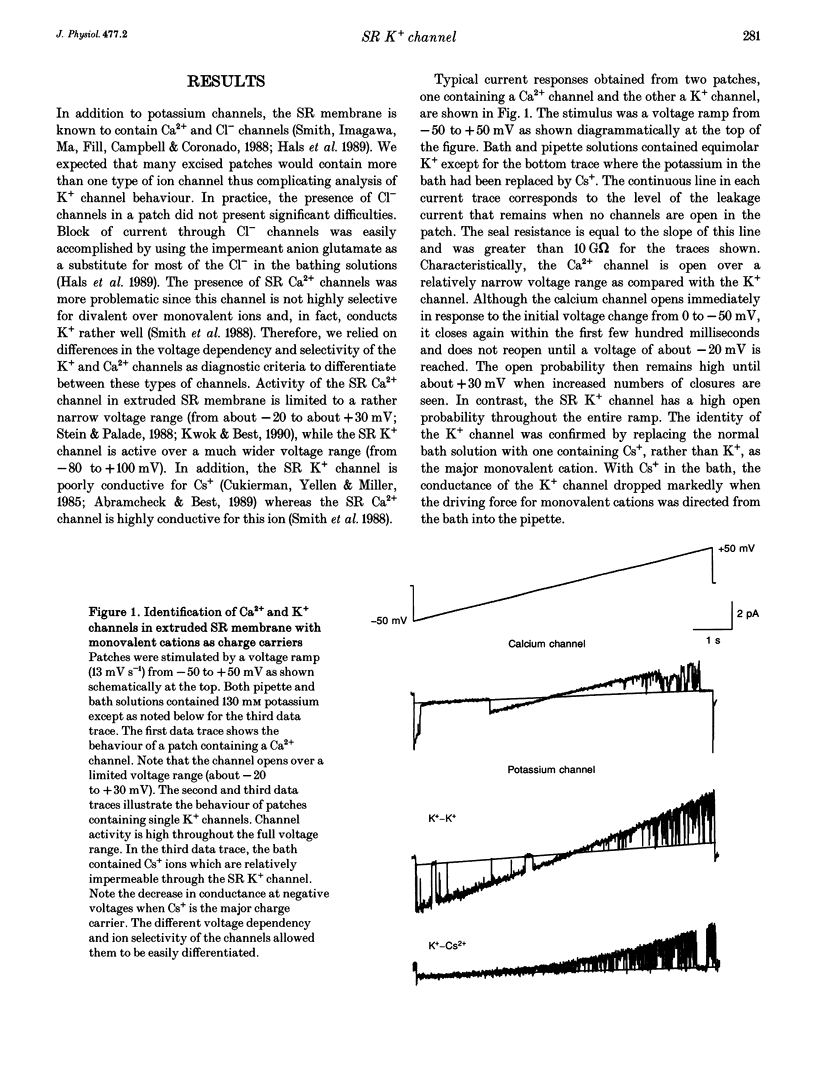
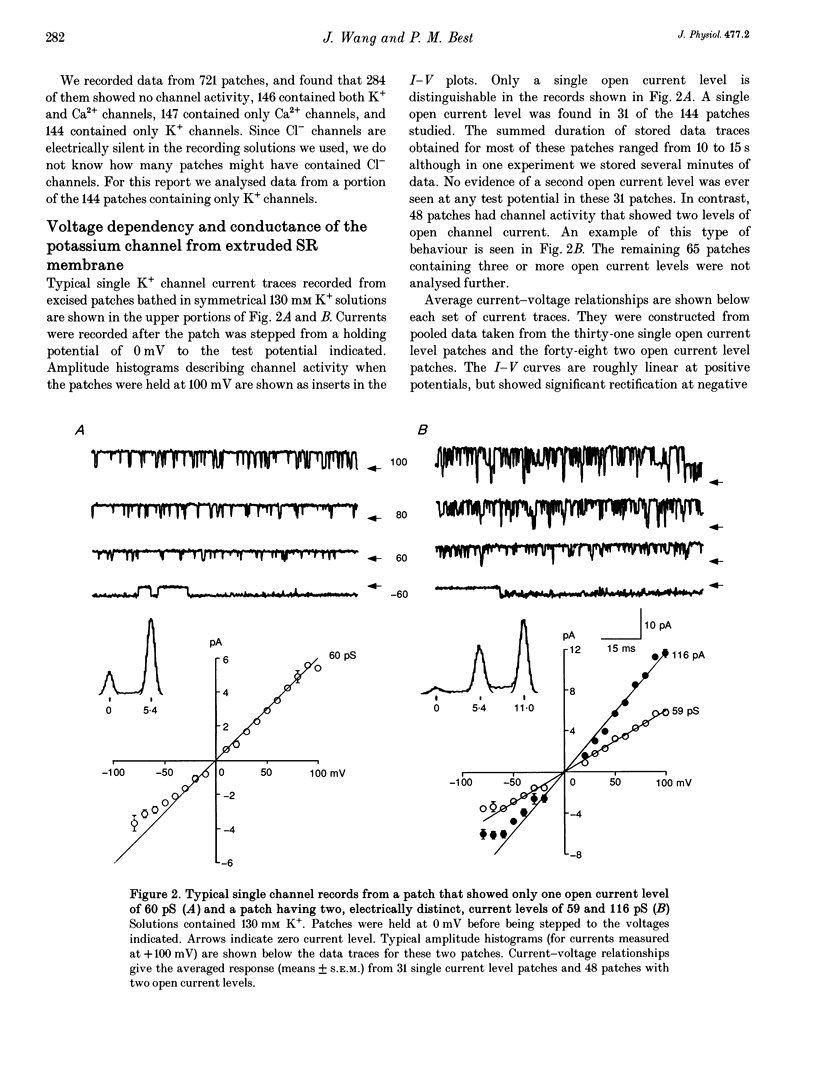
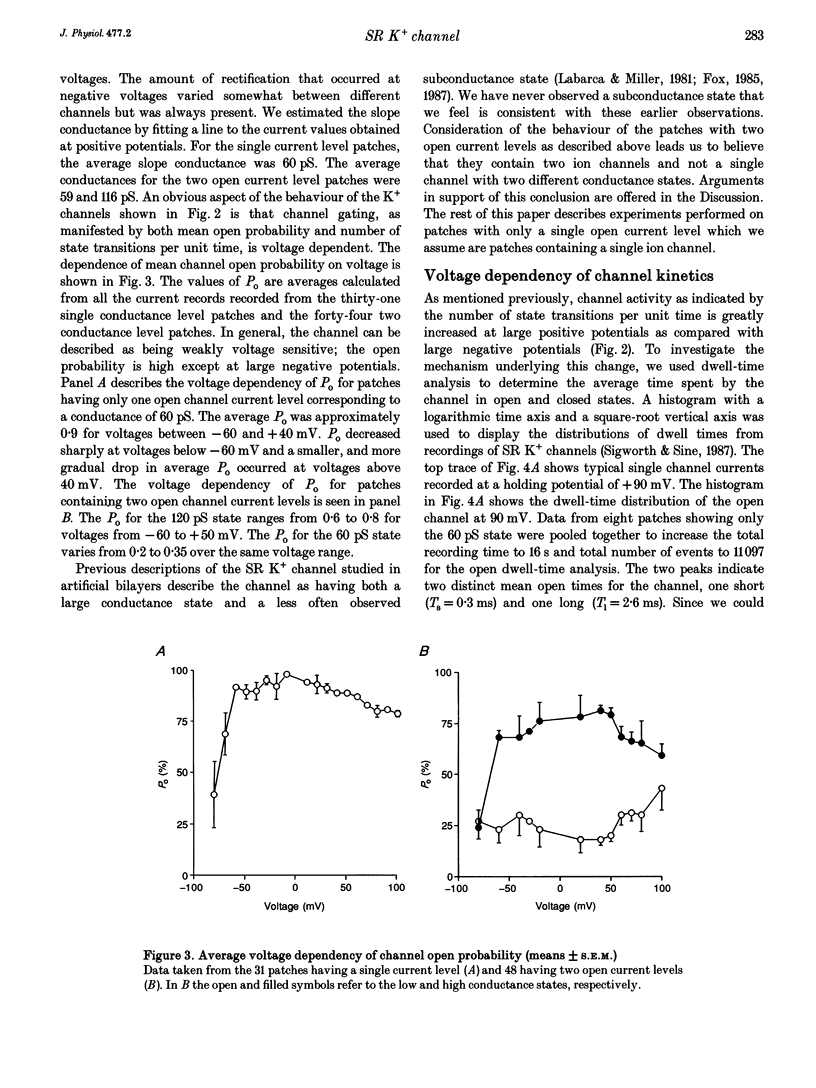
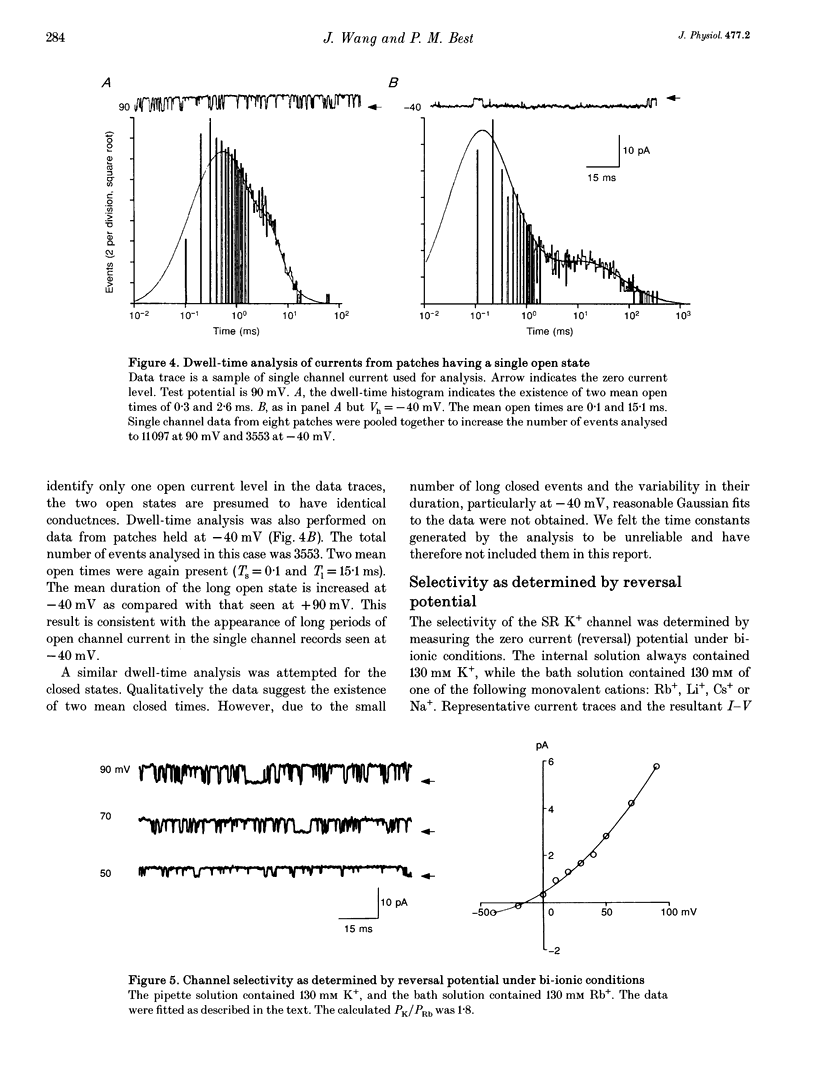
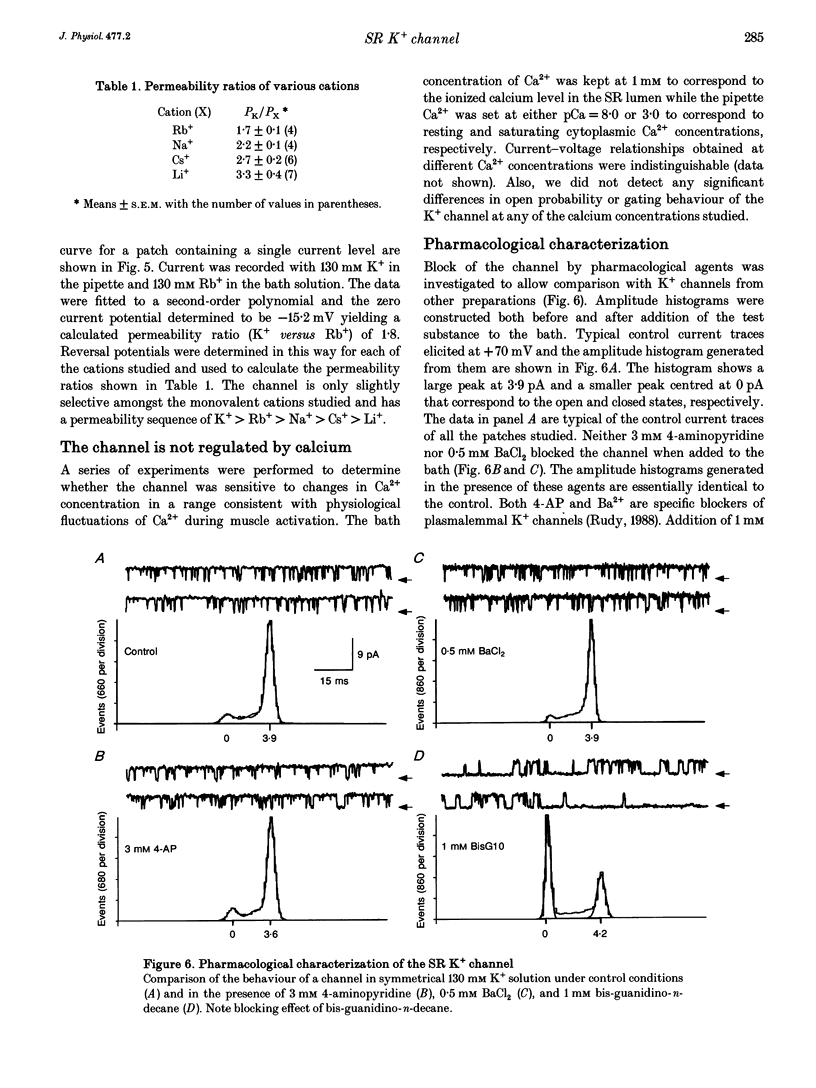
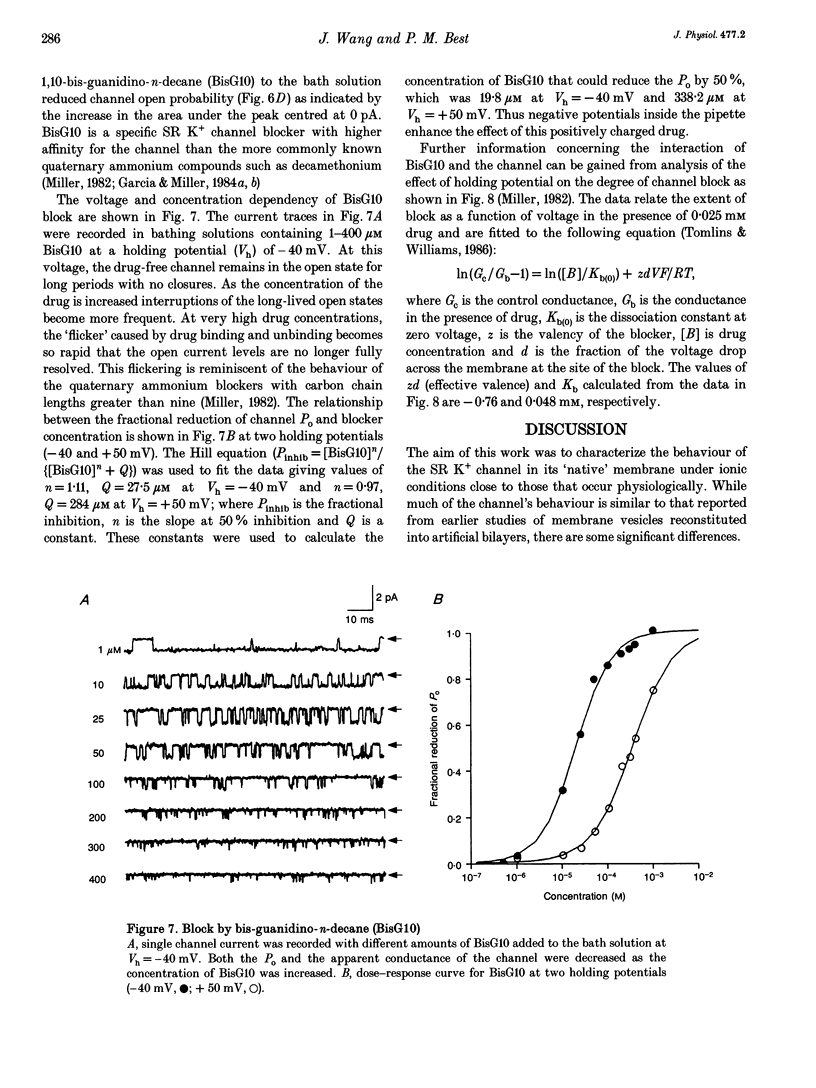
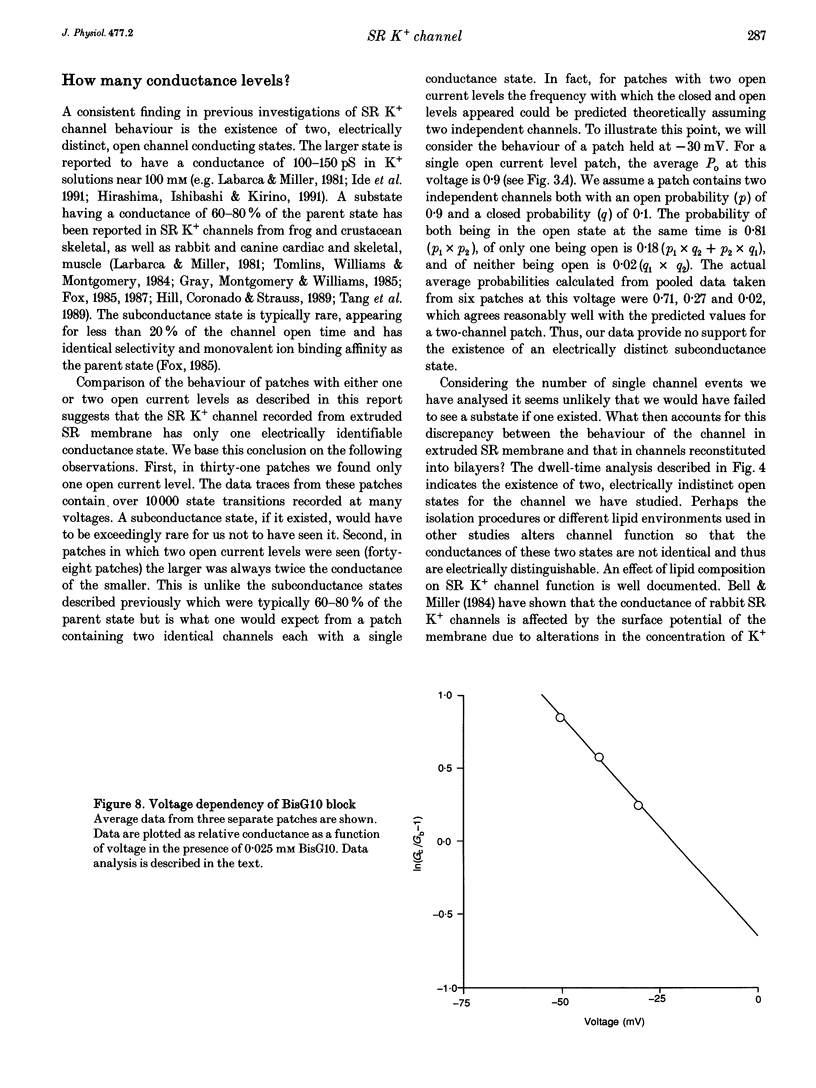



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck C. W., Best P. M. Physiological role and selectivity of the in situ potassium channel of the sarcoplasmic reticulum in skinned frog skeletal muscle fibers. J Gen Physiol. 1989 Jan;93(1):1–21. doi: 10.1085/jgp.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. E., Miller C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys J. 1984 Jan;45(1):279–287. doi: 10.1016/S0006-3495(84)84154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado R., Rosenberg R. L., Miller C. Ionic selectivity, saturation, and block in a K+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980 Oct;76(4):425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman S., Yellen G., Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophys J. 1985 Sep;48(3):477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A. Conductance and selectivity properties of a substate of the rabbit sarcoplasmic reticulum channel. Biophys J. 1985 Apr;47(4):573–576. doi: 10.1016/S0006-3495(85)83953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Garcia A. M., Miller C. Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol. 1984 Jun;83(6):819–839. doi: 10.1085/jgp.83.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. M., Miller C. Channel-mediated tl fluxes in sarcoplasmic reticulum vesicles. Biophys J. 1984 Jan;45(1):49–51. doi: 10.1016/S0006-3495(84)84103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Montgomery R. A., Williams A. J. Asymmetric block of a monovalent cation-selective channel of rabbit cardiac sarcoplasmic reticulum by succinyl choline. J Membr Biol. 1985;88(1):85–95. doi: 10.1007/BF01871216. [DOI] [PubMed] [Google Scholar]
- Hals G. D., Stein P. G., Palade P. T. Single channel characteristics of a high conductance anion channel in "sarcoballs". J Gen Physiol. 1989 Mar;93(3):385–410. doi: 10.1085/jgp.93.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima N., Ishibashi H., Kirino Y. Comparative electrophysiological study of reconstituted giant vesicle preparations of the rabbit skeletal muscle sarcoplasmic reticulum K+ channel. Biochim Biophys Acta. 1991 Aug 26;1067(2):235–240. doi: 10.1016/0005-2736(91)90049-e. [DOI] [PubMed] [Google Scholar]
- Ide T., Morita T., Kawasaki T., Taguchi T., Kasai M. Purification of a K(+)-channel protein of sarcoplasmic reticulum by assaying the channel activity in the planar lipid bilayer system. Biochim Biophys Acta. 1991 Aug 26;1067(2):213–220. doi: 10.1016/0005-2736(91)90046-b. [DOI] [PubMed] [Google Scholar]
- Kometani T., Kasai M. Ionic permeability of sarcoplasmic reticulum vesicles measured by light scattering method. J Membr Biol. 1978 Jul 18;41(4):295–308. doi: 10.1007/BF01871994. [DOI] [PubMed] [Google Scholar]
- Labarca P. P., Miller C. A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol. 1981;61(1):31–38. doi: 10.1007/BF01870750. [DOI] [PubMed] [Google Scholar]
- Lewis T. M., Dulhunty A. F., Junankar P. R., Stanhope C. Ultrastructure of sarcoballs on the surface of skinned amphibian skeletal muscle fibres. J Muscle Res Cell Motil. 1992 Dec;13(6):640–653. doi: 10.1007/BF01738254. [DOI] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Evidence for a K+, Na+ permeable channel in sarcoplasmic reticulum. J Membr Biol. 1978 Dec 15;44(2):159–186. doi: 10.1007/BF01976037. [DOI] [PubMed] [Google Scholar]
- McKinley D., Meissner G. Sodium and potassium ion permeability of sarcoplasmic reticulum vesicles. FEBS Lett. 1977 Oct 1;82(1):47–50. doi: 10.1016/0014-5793(77)80882-x. [DOI] [PubMed] [Google Scholar]
- Meissner G. Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol Cell Biochem. 1983;55(1):65–82. doi: 10.1007/BF00229243. [DOI] [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol. 1978 Apr 20;40(1):1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- Oetliker H. An appraisal of the evidence for a sarcoplasmic reticulum membrane potential and its relation to calcium release in skeletal muscle. J Muscle Res Cell Motil. 1982 Sep;3(3):247–272. doi: 10.1007/BF00713037. [DOI] [PubMed] [Google Scholar]
- Pape P. C., Konishi M., Baylor S. M. Valinomycin and excitation-contraction coupling in skeletal muscle fibres of the frog. J Physiol. 1992 Apr;449:219–235. doi: 10.1113/jphysiol.1992.sp019083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P., Palade P. Sarcoballs: direct access to sarcoplasmic reticulum Ca2+-channels in skinned frog muscle fibers. Biophys J. 1988 Aug;54(2):357–363. doi: 10.1016/S0006-3495(88)82967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. M., Wang J., Eisenberg R. S. K+-selective channel from sarcoplasmic reticulum of split lobster muscle fibers. J Gen Physiol. 1989 Aug;94(2):261–278. doi: 10.1085/jgp.94.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J., Montgomery R. A. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80(2):191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- Tomlins B., Williams A. J. Solubilisation and reconstitution of the rabbit skeletal muscle sarcoplasmic reticulum K+ channel into liposomes suitable for patch clamp studies. Pflugers Arch. 1986 Sep;407(3):341–347. doi: 10.1007/BF00585312. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]


