ABSTRACT
Transient global amnesia (TGA) is a rare, temporary, sudden-onset disturbance in anterograde episodic memory with unclear pathophysiology. Previous brain volumetric analysis in TGA patients showed varied results.
Objective:
To explore brain morphometry, hypothesizing that patients with TGA exhibit structural alterations.
Methods:
A case-control study was performed involving TGA subjects (n=50) and matched healthy controls (n=50). Both groups underwent a 3D-T1 weighted structural MRI on a 3T scanner, and voxel-based morphometry (VBM), region-based morphometry (RBM), and surface-based morphometry (SBM) were analyzed.
Results:
After performing the VBM, RBM, and SBM analyses, no consistent and statistically significant differences were found after applying multiple corrections.
Conclusion:
Despite previous studies showing volumetric changes in TGA patients, our results differ from this. The discrepancy could be due to sample size and timing of MRI scans. While our findings do not explain TGA pathophysiology, they support a network dysfunction as a possible mechanism and discards a structural alteration as a predisposing factor for TGA.
Keywords: Amnesia, Transient Global, Magnetic Resonance Imaging
RESUMO
Amnésia global transitória (AGT) é uma alteração rara, temporária e de início súbito na memória episódica anterógrada, com uma fisiopatologia pouco clara. Análises volumétricas cerebrais anteriores em pacientes com AGT apresentaram resultados variados.
Objetivo
Explorar a morfometria cerebral, com a hipótese de que pacientes com AGT exibam alterações estruturais.
Métodos:
Realizou-se um estudo de caso-controle envolvendo indivíduos com AGT (n=50) e controles saudáveis (n=50). Os dois grupos foram submetidos a uma ressonância magnética ponderada em 3D-T1 em um scanner de 3T, e analisou-se a morfometria baseada em voxel (MBV), morfometria baseada em região (MBR) e morfometria baseada em superfície (MBS).
Resultados:
Após realizar as análises de MBV, MBR e MBS, não foram observadas diferenças consistentes e estatisticamente significativas após a aplicação de correções múltiplas.
Conclusão:
Apesar de estudos anteriores mostrarem alterações volumétricas em pacientes com AGT, os resultados deste estudo diferem disso. A discrepância pode ser atribuída ao tamanho da amostra e ao momento em que as ressonâncias foram realizadas. Embora os presentes achados não expliquem a fisiopatologia da AGT, eles apoiam a disfunção de rede como um possível mecanismo e descartam uma alteração estrutural como fator predisponente para AGT.
Palavras-chave: Amnésia Global Transitória, Imageamento por Ressonância Magnética
INTRODUCTION
Transient global amnesia (TGA) is an episodic memory disorder affecting middle-aged and older adults 1 , characterized by difficulty in acquiring new information (anterograde amnesia) and recalling recent events preceding the episode (retrograde amnesia) 2 .
Involvement of the hippocampus can be considered the structural correlate of the amnestic deficit. The region of the hippocampus mostly affected is the cornu ammonis CA1 subfield, reported to have a particular vulnerability to metabolic stress and cytotoxic damage, often presenting with a restrictive lesion in diffusion-weighted magnetic resonance imaging (MRI) sequences that becomes visible 24–48 hours after the episode and may persist for 7–10 days 3 .
Nevertheless, Single Photon Emission Computed Tomography (SPECT) 4 and network 5 studies described other regions involved in TGA, beyond the hippocampus, including the cingulate gyrus, the anterior thalamic nucleus, the putamen and caudate nucleus, the corpus callosum and fornix, among others.
The underlying pathophysiological mechanism of TGA remains uncertain, encompassing potential causes such as isolated arterial ischemia, venous congestion linked to mesial temporal ischemia, mechanisms related to epilepsy, migraines, cortical spreading depression, and psychogenic factors 3 .
Despite this, TGA generally carries a favorable prognosis, as patients fully recover within 24 hours. However, some individuals may report lingering cognitive deficits even after the resolution of acute episodes 6,7,8,9 .
The morphometric characteristics of cortical structures and hippocampal subfields in individuals with TGA have been examined in prior studies, yielding inconsistent findings 10,11,12,13 .
Considering that although the brain region expected to be most affected is the hippocampus, other regions were also described to be involved. Our hypothesis is that individuals diagnosed with TGA might exhibit wide structural alterations. Therefore, we decided to conduct a comprehensive analysis of brain structures in subjects after a TGA episode, comparing them to a sample of healthy controls. To scrutinize local variations and subtle changes in gray matter, voxel-based morphometry (VBM) was employed for the analysis of cerebral structure. Additionally, region-based morphometry (RBM) was utilized with a specific focus on hippocampal structures, and surface-based morphology (SBM) to investigate potential alterations in cortical thickness and surface area.
METHODS
Subjects
Participants who experienced a TGA event were enrolled, identified, and recruited from the emergency department of a neurological tertiary center in Buenos Aires, Argentina, spanning the period between December 2019 and June 2022. The events were defined according to the Hodges and Warlow diagnostic criteria 14 :
anterograde amnesia witnessed by an observer, with symptoms resolving within 24 hours;
cognitive impairment confined to amnesia, without clouding of consciousness, loss of personal identity, focal neurological or epileptic signs, or recent head trauma or seizures; and
absence of structural brain lesions.
To ensure accurate diagnoses, all subjects underwent neurological examination and detailed assessment to rule out differential diagnoses, including an electroencephalogram, jugular venous doppler with reflux measurement, and an on-call structural brain MRI, which specifically included a diffusion-weighted sequence.
To avoid confounders, the 3D-T1 weighted structural MRI was performed and analyzed at least ten days after the event. To facilitate patient recruitment and ensure optimal adherence, a maximum limit of three months was established for performing MRI scans.
Control group
The control group comprised 50 individuals matched for age and gender, who consulted at the Memory Clinic, all of whom had cognitive complaints and underwent brain MRI and routine cognitive assessments for this reason. Normality among participants was defined by the absence of any history of neurological disorders, the absence of abnormal findings in MRI scans as evaluated by a trained neuroradiologist, and a performance score higher than -1.5 Z score (adjusted for age, gender, and education) for all neurocognitive tests included in the Uniform Data Set version 3 (UDS3) neuropsychological battery 15 .
Magnetic resonance imaging acquisition and conversion
MRI scans were acquired using a GE Discovery 750 3T scanner with a 32-channel head coil. 3D T1-weighted images parameters are summarized in Table 1.
Table 1. 3D Inversion time-weighted sequence parameters.
| Parameter | Value |
|---|---|
| NEX | 1 |
| Matrix | 256 x 256 |
| Slice thickness | 1.1 mm |
| Field of view | 230 mm |
| Voxel size | 1.1 x 1.1 x 1.1 mm |
| TR | 8.26 ms |
| TE | 3.26 ms |
| TI | 450 ms |
Abbreviations: NEX, Number of excitations; TR, Repetition time; TE, Echo time; TI, Inversion time.
Images were downloaded from our institutional Picture Archiving and Communication Systems (PACS), anonymized, and converted to NifTI format using the dcm2niix module form MRIcroGL 1.2.2.
Processing
NifTI-converted T1-weighted images were normalized to a template space and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Spatial registration was performed using the default CAT12 approach (geodesic shooting). The cortical thickness of GM segmentations was calculated using the standard CAT12 pipeline. After segmentation and parcellation, volume and surface data were smoothed with 8- and 15-mm kernels, respectively.
VBM, RBM, and SBM analyses were performed on each MRI, based on the Cobra 16 and Neuromorphometrics 17 atlases, to ensure that the results were consistent across the different analysis methods. VBM, RBM, and SBM steps, as well as quality check and statistical analyses, were performed using Computational Anatomy Toolbox 12 (CAT12) 18 , an extension to Statistical Parametrical Mapping (SPM12) software package 19 .
VBM overlays were warped to a template made from the averaged 100 brains included in this study, while RBM were warped to the MNI 2009c NLIN asymmetric T2 template.
Quality check
Input images and preprocessing quality were checked using the quality assurance reports generated by CAT12. Image resolution, noise, and bias were graded and summarized in a weighted average grade (WAG) by CAT12. WAG is defined as following:
Excellent,
Good,
Satisfactory,
Sufficient,
Critical, and
Unacceptable/failed.
WAG from most open-access databases typically ranges between 2 and 3. Additionally, all input images were visually inspected by an experienced neuroradiologist (HC).
Total intracranial volume (TIV) as well as absolute and relative CSF, GM, and WM volumes were estimated. A global thickness estimation was also recorded in the quality assurance report. To check whether the normalization and spatial registration was successful across all subjects, a single slice display of the GM segmentation masks was generated and visually inspected. Lastly, the homogeneity of the sample was examined by generating a correlation matrix and boxplot. Processing time was recorded in minutes.
Group-level analysis
For VBM and RBM, an independent two-sample t-test with TIV, gender, and age as covariates was modeled and estimated. VBM was implemented without Jacobian modulation of the intensities or global scaling. For SBM, an independent two-sample t-test without covariates was modeled and estimated. A p<0.05, corrected for multiple comparisons applying the Holm-Bonferroni test, was considered statistically significant.
RESULTS
Subjects
A total of 100 subjects were included, with 50 in the TGA group and 50 in the healthy control group. Fifty-four percent of the subjects were female. Mean age was 67 years old (range 57–77). Regarding cognitive results, the Montreal Cognitive Assessment (MoCA) showed no statistically significant results between TGA and control patients. Demographic characteristics of TGA and control group are summarized in Table 2.
Table 2. Demographic characteristics.
| TGA (n=50) | Control (n=50) | p-value* | q-value † | ||
|---|---|---|---|---|---|
| Age (mean, SD) | 67 (10) | 68 (6) | >0.9 | >0.9 | |
| Sex | Female (n, %) | 27 (54) | 28 (56) | 0.8 | |
| Male (n, %) | 23 (46) | 22 (44) | |||
| Education level | 14.8 (3.5) | 14.6 (3.4) | 0.6 | >0.9 | |
| MoCA (Z score) (mean, SD) | 0.16 (0.88) | 0.49 (0.52) | 0.13 | 0.5 | |
| TGA recurrence (n, %) | 7 (14%) | NA | |||
| CA1 diffusion lesion (n, %) | 10 (20%) | NA | |||
Abbreviations: TGA, transient global amnesia; SD, standard deviation; MoCA, Montreal Cognitive Assessment; CA1, Cornus ammonis 1; NA, not applicable.
Notes: *Wilcoxon rank sum test; Pearson’s χ 2 test;
†False discovery rate correction for multiple testing.
Gross morphometric data (TIV, GM, WM, and CSF volumes) are summarized in Table 3.
Table 3. Gross volumetric data summarized by group.
| TGA (n=50) | HC (n=50) | p-value | |
|---|---|---|---|
| Mean TIV±SD | 1,486±143 mL | 1,466±120 mL | 0.482 |
| Mean GM volume±SD | 623±53 mL | 608±46 mL | 0.141 |
| Mean WM volume±SD | 494±63 mL | 480±65 mL | 0.301 |
| Mean CSF volume±SD | 396±85 mL | 378±74 mL | 0.595 |
Abbreviations: TGA, transient global amnesia; HC, healthy controls; TIV, total intracranial volume; SD, standard deviation; GM, grey matter; WM, white matter; CSF, cerebrospinal fluid.
Magnetic resonance imaging quality control
Input 3D T1- weighted images had a mean WAG of 2.5 (range 1.8–5.3), which is defined as good. Only two subjects had critical quality images (with values higher than or equal to 5), and no subjects had unacceptable quality images (higher than or equal to 6). Mean processing time was 32±4 minutes.
Voxel-based morphometry
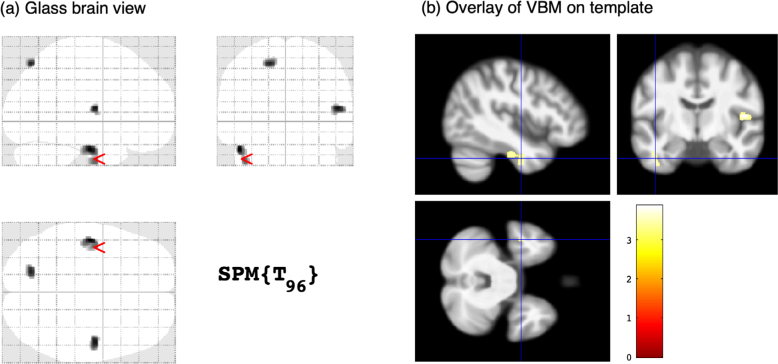
Using a p<0.001 and a minimum threshold of 80 voxels cluster (88 mm 3 ), three regions with reduced GM were identified in subjects with TGA compared to controls, with peak coordinates at the left fusiform gyrus, right opercular precentral gyrus, and left superior parietal lobe (Figure 1). However, no suprathreshold clusters were found using a p<0.05 corrected for multiple comparisons.
Figure 1. Voxel-based morphometry results. Regions with reduced grey matter in subjects with transient global amnesia compared to controls, uncorrected p<0.001, 80 voxels cluster threshold. (A) Glass brain maximum intensity projection view showing 3 suprathreshold clusters with peak coordinates at left fusiform gyrus, right precentral gyrus (opercular), and left superior parietal lobe. (B) Corresponding overlay of thresholder Statistical Parametrical Mapping on average template, with cross-hairs at the left fusiform gyrus cluster.
Region-based morphometry
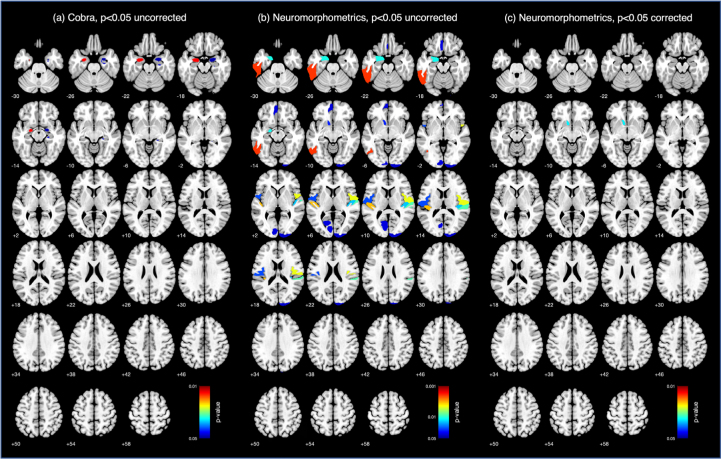
Using an uncorrected p<0.05, decreased GM volume was observed in cortical and subcortical regions of interest (ROI) in the TGA group compared to controls. However, only the volume difference in the left accumbens nucleus on the Neuromorphometrics atlas subsisted after applying the Holm-Bonferroni correction (Figure 2).
Figure 2. Region-based morphometry results. Group comparisons showing decreased volume in the transient global amnesia group compared to controls using uncorrected p<0.05 in the Cobra (A) and Neuromorphometrics (B).
Surface-based morphometry
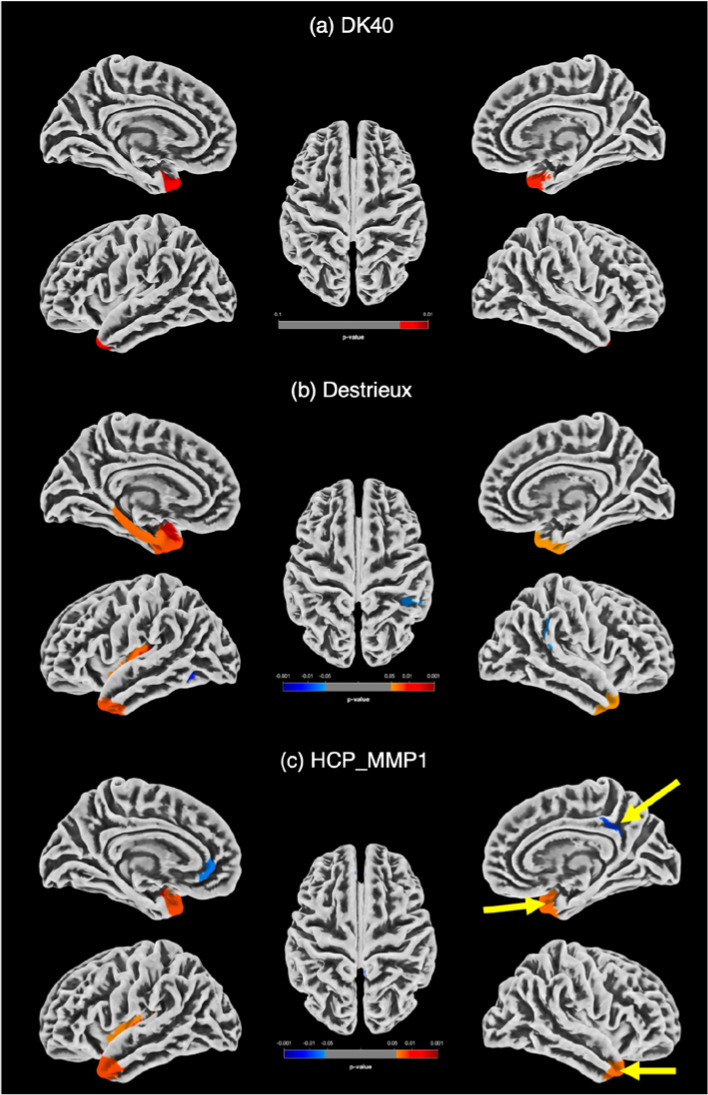
Using an uncorrected p<0.05, decreased cortical thickness was found in the TGA group compared to controls in the DK40, Destrieux, and HCP MMP 1.0 atlases, mainly in the temporal poles. However, only the cortical thickness differences observed in the right temporal pole and ventromedial visual area 2 on the HCP atlas subsisted after applying Holm-Bonferroni correction (Figure 3).
Figure 3. Surface based morphometry results. Group comparisons showing decreased cortical thickness (orange-red) in the transient global amnesia group compared to controls (uncorrected p<0.05) in the DK40 (A), Destrieux (B), and HCP MMP 1.0 (C) atlases. Only the right temporal pole and ventromedial visual area 2 differences subsisted after Holm-Bonferroni corrected p<0.05 on the HCP atlas (arrows in C).
DISCUSSION
The objective of this study was to investigate brain cortical structure in individuals with a history of TGA in comparison to a control group. Utilizing VBM, RBM, and SBM analyses, and after applying multiple corrections, the findings revealed no consistent and statistically significant differences in gray matter morphometry.
This study encompasses a relatively large sample of individuals with TGA, where three distinct types of analyses were conducted. The examination of cortical volume enables an exploration of brain structure, acknowledging that cortical volume integrates both surface area and thickness. VBM faces challenges in accurately measuring cortical morphology. Surface-based analysis (SBA) offers enhanced accuracy in representing cortical geometry and analyzing cortical shape and folding patterns, which are not attainable through VBM. The hippocampus plays a crucial role in memory, especially in episodic memory. Region-based analysis allowed for a targeted regional investigation with a focus on the hippocampal region.
Despite the findings across all three analytical approaches, the results deviate from those reported in previous works. For instance, Pirlich et al. 11 reported an increase in GM volume within the bilateral hippocampus. In contrast, Park et al. 10 observed a significant decrease in GM volume in the left hippocampus, left amygdala, bilateral cingulum, and the right posterior lobe of the cerebellum in TGA patients compared to control subjects. Hodel et al. 13 reported lower cortical thickness compared with controls in the orbitofrontal, cingulate, and inferior temporal cortices. Additionally, Kim et al. 12 identified widespread alterations, involving regions with reduced and increased cortical thickness across both cerebral hemispheres. These results are attributed to the relatively large sample size, which includes a great number of patients compared to previous works. Furthermore, the decision to conduct brain structural MRI in the subacute stage contributes to the elimination of potential confounding factors.
Cortical spreading depression (CSD) is one of the most widely recognized explanations for the pathogenesis of TGA 4 . Through a mechanism of glutamate-mediated neuronal and glial depolarization, CSD causes transient hypoxia and metabolic stress that might affect the CA1 hippocampal subfield. This mechanism has the potential to disrupt the normal functioning of neural networks in the brain, altering the synchrony and connectivity between brain areas 4 .
Previous studies showed cortical volume reduction in default mode network (DMN) regions and cerebellum in TGA patients compared to controls 10,12,20 . Various functional MRI studies suggest the engagement of the episodic memory network 21 , the executive network 22 , and the limbic network 23 in TGA episodes.
While this study’s results do not offer insights into the pathophysiology, the absence of asymmetries in our triadic approach analysis supports network dysfunction as a possible mechanism underlying the clinical findings and dismisses a structural cause as a predisposing factor for the occurrence of an episode.
The present study has certain limitations. If the volume reduction is very subtle, it could explain the lack of statistical significance in our results. Conducting a study with a larger sample size would likely provide more insight into this aspect. Besides, as TGA is a multifactorial condition, cortical volume may not be the primary marker of structural changes in TGA and other imaging modalities may be more informative in early stages. Because the syndrome is infrequent, patients are unlikely to have a brain MRI scan before the event that would allow comparison with the current one to determine if there is a volume variation in the patient.
In conclusion, no significant gray matter asymmetries were found in TGA patients compared to controls after VBM, RBM, and SBM analyses. The main contribution of our work is that it was performed on a relatively large sample of TGA patients using three different morphometric analyses, demonstrating that our results are consistent.
Footnotes
Funding: none.
REFERENCES
- 1.Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264–72. doi: 10.1016/j.mayocp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi: 10.3389/fnhum.2020.602496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205–14. doi: 10.1016/S1474-4422(09)70344-8. [DOI] [PubMed] [Google Scholar]
- 4.Schmidtke K, Reinhardt M, Krause T. Cerebral perfusion during transient global amnesia: findings with HMPAO SPECT. J Nucl Med. 1998;39(1):155–9. [PubMed] [Google Scholar]
- 5.Wang T, Xu Y, Li D, Tu W, Li Y, Miao S, et al. Network localization of transient global amnesia beyond the hippocampus. Neurol Sci. 2023;44(2):649–57. doi: 10.1007/s10072-022-06439-4. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel DR, Smith J, Wade RR, Cherukuru N, Ursani A, Dobruskina Y, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691–703. doi: 10.2147/NDT.S130710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch T, Alfke K, Stingele R, Rohr A, Freitag-Wolf S, Jansen O, et al. Selective affection of hippocampal CA-1 neurons in patients with transient global amnesia without long-term sequelae. Brain. 2006;129(Pt 11):2874–84. doi: 10.1093/brain/awl248. [DOI] [PubMed] [Google Scholar]
- 8.Borroni B, Agosti C, Brambilla C, Vergani V, Cottini E, Akkawi N, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125–7. doi: 10.1007/s00415-004-0497-x. [DOI] [PubMed] [Google Scholar]
- 9.Schöberl F, Irving S, Pradhan C, Bardins S, Trapp C, Schneider E, et al. Prolonged allocentric navigation deficits indicate hippocampal damage in TGA. Neurology. 2019;92(3):e234–e243. doi: 10.1212/WNL.0000000000006779. [DOI] [PubMed] [Google Scholar]
- 10.Park KM, Han YH, Kim TH, Mun CW, Shin KJ, Ha SY, et al. Pre-existing structural abnormalities of the limbic system in transient global amnesia. J Clin Neurosci. 2015;22(5):843–7. doi: 10.1016/j.jocn.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Pirlich M, Höfer C, Weise CM, Stockert A, Thöne-Otto A, Garthe A, et al. Hippocampal gray matter volume in the long-term course after transient global amnesia. Neuroimage Clin. 2021;30:102586. doi: 10.1016/j.nicl.2021.102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HC, Lee BI, Kim SE, Park KM. Cortical morphology in patients with transient global amnesia. Brain Behav. 2017;7(12):e00872. doi: 10.1002/brb3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodel J, Leclerc X, Zuber M, Gerber S, Besson P, Marcaud V, et al. Structural connectivity and cortical thickness alterations in transient global amnesia. AJNR Am J Neuroradiol. 2020;41(5):798–803. doi: 10.3174/ajnr.A6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834–43. doi: 10.1136/jnnp.53.10.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Alzheimers’s Coordinating Center Uniform Data Set (UDS) [Internet] [[cited on Mar 15, 2023]]. Available from: https://naccdata.org/data-collection/forms-documentation/uds-3 .
- 16.Computational Brain Anatomy Laboratory MRI-based digital brain atlases [Internet] [[cited on Mar 15, 2023]]. Available from: https://www.cobralab.ca/atlases .
- 17.Neuromorphometrics, Inc. Building a Model of the Living Human Brain Modeiling the living human brain [Internet] [[cited on Mar 15, 2023]]. Available from: https://www.neuromorphometrics.com .
- 18.Gaser C, Dahnke R, Thompson PM, Kurth F, Luders E. CAT – a computational anatomy toolbox for the analysis of structural MRI data. Alzheimer’s Disease Neuroimaging Initiative. bioRxiv. 2022 doi: 10.1101/2022.06.11.495736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistical Parametric Mapping Introduction. 2023. [[cited on Mar 15, 2023]]. Available from: https://www.fil.ion.ucl.ac.uk/spm/
- 20.Nakada T, Kwee IL, Fujii Y, Knight RT. High-field, T2 reversed MRI of the hippocampus in transient global amnesia. Neurology. 2005;64(7):1170–4. doi: 10.1212/01.WNL.0000156158.48587.EA. [DOI] [PubMed] [Google Scholar]
- 21.Peer M, Nitzan M, Goldberg I, Katz J, Moshe Gomori J, Ben-Hur T, et al. Reversible functional connectivity disturbances during transient global amnesia. Ann Neurol. 2014;75(5):634–43. doi: 10.1002/ana.24137. [DOI] [PubMed] [Google Scholar]
- 22.Zidda F, Griebe M, Ebert A, Ruttorf M, Roßmanith C, Gass A, et al. Resting-state connectivity alterations during transient global amnesia. Neuroimage Clin. 2019;23:101869. doi: 10.1016/j.nicl.2019.101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang J, Lee DA, Lee HJ, Park KM. Limbic covariance network alterations in patients with transient global amnesia. J Neurol. 2022;269(11):5954–62. doi: 10.1007/s00415-022-11263-z. [DOI] [PubMed] [Google Scholar]