Abstract
Purpose: To review the evolution of terminology describing the classification of lesions in neovascular age-related macular degeneration (nAMD) based on retinal imaging technologies. Methods: A review of the current and historical literature on imaging-guided classification of neovascularization in nAMD was performed. Results: Imaging-guided classification of neovascularization in nAMD facilitates understanding of the pathological mechanisms and disease progression. Neovascularization classification has evolved with advances in imaging technologies, from earlier classifications based on neovascularization patterns assessed by fluorescein angiography to multimodal imaging patterns, resulting in varied descriptions of lesions depending on the techniques used. Until recently, there has been a lack of consensus regarding the clinical features of choroidal neovascularization lesion types as a result of the imaging modalities initially used to define them; a recent consensus on classification has the potential to simplify and clarify descriptions of neovascularization in nAMD. The use of multimodal imaging techniques will improve lesion identification and has the potential to individualize treatment plans and improve outcomes. Conclusions: Widespread adoption of a consensus-based, image-guided classification system for neovascular lesions in nAMD and the appropriate imaging techniques used to identify them will aid clinical research and could potentially improve patient outcomes by individualizing treatment plans in the future.
Keywords: age-related macular degeneration, choroidal neovascularization, macular neovascularization, fluorescein angiography, imaging, indocyanine green angiography, multimodal imaging, neovascularization, optical coherence tomography angiography
Introduction
Age-related macular degeneration (AMD) is a chronic and progressive disease in which earlier stages can advance to geographic atrophy (GA), the development of macular neovascularization (MNV), or both.1,2 The underlying pathological mechanisms of GA and neovascular AMD (nAMD) converge on cellular pathways that lead to photoreceptor death and, ultimately, irreversible vision loss secondary to various structural damages. 1
Accurate characterization of lesions is important to guide appropriate treatments in patients with AMD. Innovations in retinal imaging technologies have supported advances in understanding AMD’s pathological mechanisms. Increasingly sophisticated imaging techniques have facilitated more detailed classification of neovascularization lesions, which informs diagnosis and personalized treatment decisions and, ultimately, has the potential to improve outcomes for patients with nAMD. As imaging technology has advanced, researchers have proposed terminologies for lesions based on their observations. Despite efforts to establish a definitive classification system for nAMD, universal adoption of a single classification remains elusive. 2 A multimodal classification proposed by Spaide et al 2 integrates recent advances in imaging technology. However, it is not broadly used, and the lack of agreed-on, coherent, and consistent language to describe neovascularization in nAMD can hinder understanding and slow the translation of potential benefits to the clinic.
In this short review of key literature on this topic, we aim to consolidate the current understanding of nAMD lesion classification, including key features of lesions, and summarize the relative merits of different imaging modalities.
Methods
Eight experts in nAMD imaging were selected to partake in this research based on their indexed publications and clinical research in the field of nAMD. The experts conducted a review of the published literature focused on the classification of neovascular lesions in nAMD, using various imaging technologies. Findings from the literature review and additional expert perspectives form the basis of this consensus review content.
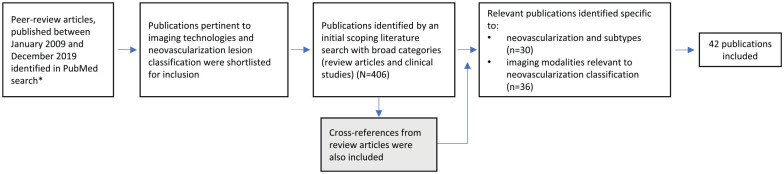
A literature search was performed on PubMed according to the predefined search parameters shown in Figure 1. Forty-two publications specific to neovascularization and imaging modalities and relevant to neovascularization classification were included. Less weight was given to recent literature that does not have the benefit of multimodality imaging, especially optical coherence tomography (OCT) and OCT angiography (OCTA).
Figure 1.
Literature search flow diagram.
*Keywords: “choroidal neovascularization” AND “review” / “neovascular age-related macular degeneration” / “geographic atrophy” / “differential diagnosis” / “optical coherence tomography” / “treatment response” / “subtypes” / “diagnosis” / “classification” / “fluorescein angiography” / “spectral domain optical coherence tomography”; “choroidal neovascularization subtype/ lesion” AND “optical coherence tomography angiography” / “optical coherence tomgography” / “indocyanine green angiography” / “infrared reflectance inaging”; “Type 1/2/3 choroidal neovascularization lesion” AND “optical coherence tomography” / “fluorescein angiography” / “optical coherence tomography angiography”; “quiescent CNV” AND “optical coherence tomography/ optical coherence tomography angiography”; “double layer sign” AND “ spectral domain optical coherence tomography” AND “neovascular age-related macular degeneration”; polypoidal choroidal vasculopathy lesion” AND “optical coherence tomography/ optical coherence tomography angiography”; “reticular pseudodrusen” AND “infrared reflectance imaging”.
Results
Co-evolution of Lesion Classification and Imaging Techniques: Brief History
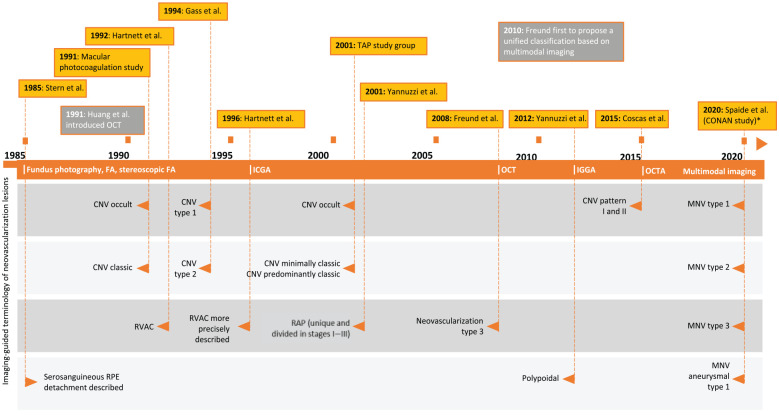
Figure 2 shows an overview of the development of classification terms in neovascularization over time. The first classification of nAMD dates back to the Macular Photocoagulation Study from 1991, which presented evidence of variable clinical responses to laser photocoagulation based on distinct angiographic patterns assessed by fluorescein angiography (FA). 3 Based on the patterns of hyperfluorescence observed, the study described choroidal neovascularization (CNV) as classic or occult. Classic CNV was characterized by well-demarcated areas of hyperfluorescence that can be discerned in the early phase of the angiogram. In the late phase, progressive pooling of dye appears in the overlying subsensory retinal space and usually obscures the boundaries of CNV. Occult CNV appears as poorly defined lesions, divided into fibrovascular pigment epithelial detachment (PED) and late leakage of an undetermined source. Fibrovascular PED was defined as areas of irregular elevation of the retinal pigment epithelium (RPE) detectable on stereoscopic FA, consisting of an area of stippled hyperfluorescence noted within 1 to 2 minutes after fluorescein injection. Late leakage of undetermined source was defined as areas of leakage at the level of the RPE in the late phase of the angiogram without well-demarcated areas of hyperfluorescence discernible in the early phase to account for the leakage.
Figure 2.
Evolution of classification of neovascularization lesions. *According to the unified classification of neovascularization proposed by Spaide et al, 2 the term CNV is inaccurate in the context of type 3 neovascular lesions and should be replaced with MNV.
Abbreviations: CNV, choroidal neovascularization; FA, fluorescein angiography; ICGA, indocyanine green angiography; MNV, macular neovascularization; RPE, retinal pigment epithelium; TAP, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography; PCV, polypoidal choroidal vasculopathy; RAP, retinal angiomatous proliferation; RVAC, retinal vascular anomalous complex.
CNV was further subclassified by the researchers in the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study in 2001 based on FA findings. 4 In this study, the terms predominantly or minimally classic CNV were used to describe lesions that made up 50% or more or less than 50% of the area, respectively, in addition to the presence of no occult or no classic CNV. This classification was based on a post hoc analysis of the pivotal TAP trial, which described lesions that had a better response to treatment. 5
An alternative method of classification, also based on FA and fundus photography, was defined by anatomic markers and the localization of the neovascular complex in relation to the RPE. In 1994, Gass 6 updated this CNV classification based on histological samples to define lesions as either type 1 CNV when neovascularization is under the RPE and type 2 CNV when neovascular proliferation is above the RPE and inside the subretinal space.
In addition to assessment by FA, the introduction of indocyanine green angiography (ICGA) in 1992 facilitated the detection and assessment of occult or poorly defined MNV. 7 ICGA has enhanced transmission through the RPE, blood, and exudative material, providing in-depth information on the architecture of the pathological vasculature beneath these tissues. 8 During late phases of ICGA, the type 1 neovascular lesion usually appears as a hyperfluorescent plaque. ICGA was used to establish the definition of polypoidal choroidal vasculopathy (PCV), a variant of type 1 CNV that was previously considered a different form of neovascularization.
The term retinal vascular anomalous complex was introduced by Hartnett et al 9 in 1992, describing a series of alternative PEDs. The differentiating characteristic of these lesions, identified by FA, was the presence of a centrally located hotspot in the RPE detachment. A more precise description was then reported in 1996 using ICGA. 10 The origin of this lesion, from choroidal or retinal vessels, was a source of continued debate. In 2001, Slakter et al 11 reported that the lesion started in the choroidal vasculature. Meanwhile, Hartnett et al 9 and later Yannuzzi et al 12 identified the initial vessel involved at the level of retinal vasculature.
The introduction of optical coherence tomography (OCT) enabled noninvasive quantitative assessment of retinal morphology, leading to a paradigm shift in assessing nAMD lesions. 13 Spectral-domain OCT (SD-OCT) resulted in visualization of details with an axial resolution of less than 10 µm and assessment of changes in the morphology of the retinal layers and the subretinal space for precise anatomic detection of structural changes corresponding to the progression or regression of neovascular lesions.14 –16
Through the use of OCT, in addition to ICGA, the term type 3 neovascularization (also described as intraretinal neovascularization or retinal angiomatous proliferation [RAP] 12 ) was introduced by Freund et al in 2008. 17 OCT enabled visualization of the relationship between choroidal vessels and retinal vessels. 18 As the understanding of type 3 neovascularization has increased, it is now known that capillary proliferation or intraretinal neovascularization may progress beyond the posterior limits of the retina to form subretinal neovascularization.
Unifying Neovascularization Classification and Clinical Features in nAMD
As a result of the differing and overlapping terminologies used to describe and the technologies used to observe lesions in nAMD, researchers have periodically attempted to present a unified nomenclature. In 2020, a unified classification of neovascularization, assessed by multimodal imaging, was proposed by the Consensus on Neovascular Age-Related Macular Degeneration Nomenclature (CONAN) study group, an international coalition of AMD and imaging experts. 2 Multimodal imaging involves the synergistic use of multiple methods to acquire images simultaneously or in a short period between the methods. It typically includes a combination of fundus photography, FA, and/or OCT, in addition to autofluorescence, when relevant, to evaluate a specific disease. 19 A multimodal imaging approach to the classification of nAMD frequently uses FA or ICGA to guide the location and SD-OCT to acquire anatomic information about the lesion.
The CONAN study group proposed nomenclature for MNV, defined as an invasion by vascular and associated tissues into the outer retina, subretinal space, or sub-RPE space. 2 They noted that the use of the term choroidal neovascularization is inaccurate in the context of type 3 neovascular lesions; as such, they recommend the types be described as neovascularization or MNV, without reference to choroidal. The classification is broadly similar to that proposed by Freund et al, 20 which itself built on the previously described classifications of types 1 to 3.
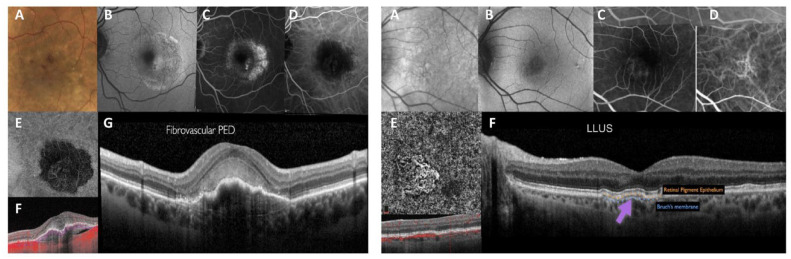
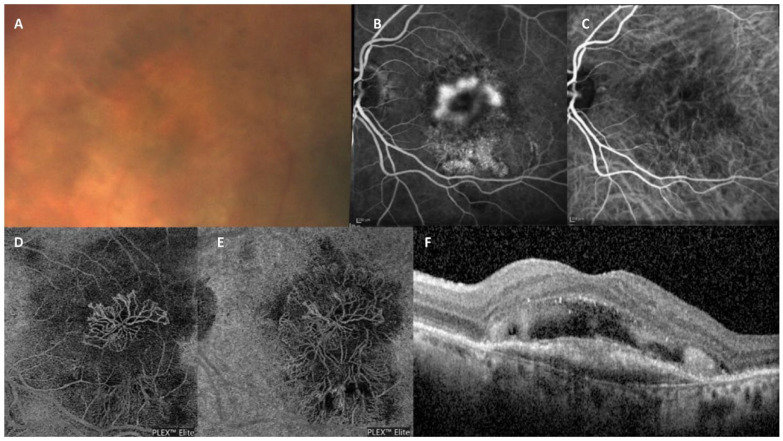
Under the CONAN recommendations, type 1 MNV is defined as ingrowth of vessels from the choriocapillaris into the sub-RPE space (Figure 3). This is the most frequent neovascularization type, found in approximately 41% of patients with nAMD. 21 On clinical examination, a type 1 MNV lesion, as assessed by fundus ophthalmoscopy, shows nonspecific signs such as pigment alterations of the RPE, RPE detachment, subretinal fluid, hard exudates, and hemorrhages that are often seen in other types of neovascularization. 22
Figure 3.
Type 1 MNV. Left image: Fibrovascular PED. (A) Color image shows increased pigmentation in the macular area with macular thickening and retinal hemorrhages. (B) Fundus autofluorescence indicating mottled hyperautofluorescence of the macular area with subretinal fluid. (C) FA shows the presence of an occult neovascular lesion. (D) ICGA shows hypofluorescence coincident with the fibrovascular PED. (E) En face structural tomographic image shows the tangled vascular network surrounded by a wide hyporeflective halo. (F) OCT structural image with flow overlay shows the presence of flow signal within the PED but absence of flow signal within the subretinal hyperreflective material. (G) Structural OCT scan through the foveal center, indicating the presence of subretinal hyperreflective material overlying a fibrovascular PED with the RPE showing no disruption. Right image: LLUS. (A) Infrared reflectance image showing pigmentary changes in the macular region. (B) FA indicating mild pigmentary changes in the macular region. (C) FA shows the correspondence of the previously described signs with no evidence of vascular leakage. (D) ICGA showing thickened choroidal vessels with anastomosis between the superior and inferior veins. (E) En face optical coherence tomography angiography proving the presence of a neovascular network. (F) Structural OCT scan showing a shallow, irregular RPE elevation corresponding to the neovascular lesion with no structural evidence of active or past exudation.
Abbreviations: FA, fluorescein angiography; ICGA, indocyanine green angiography; LLUS, late leakage of underdetermined source; OCT, optical coherence tomography; PED, pigment epithelial detachment; RPE, retinal pigment epithelium.
Nonexudative nAMD, also known as quiescent MNV, is an uncommon and usually asymptomatic form of neovascularization, which has been identified in treatment-naïve patients with nAMD. It is characterized by the absence of intraretinal/subretinal exudation on OCT but is detectable on FA, especially on ICGA.23,24 It is usually left untreated until visible exudation on OCT develops. With the imminent increase in the use of OCTA in routine clinical practice, more patients will likely be diagnosed with nonexudative MNV. The CONAN study group noted its occurrence; however, no consensus was reached regarding the designation of “quiescent” MNV.
Within the CONAN classification, PCV is deemed a subtype of type 1 MNV. PCV lesions are characterized by the presence of exudative maculopathy (with a lack of drusen) and pigmentary changes. PCV lesions are characterized by aneurysmal dilation within type 1 neovascularization, with a hallmark feature of large serohemorrhagic PED. 25 However, the study group highlighted that the nomenclature of PCV is inaccurate as it does not accurately represent the pathological manifestations of this lesion, which was based on the distribution of abnormally dilated choroidal vessels bordered by focal enlargements called polyps. 2 After that, separate reports established that the lesion had thin-walled vessels external to the RPE, above the Bruch membrane. 26 An alternative recommendation to use aneurysmal type 1 neovascularization, considering features of simple aneurysms, was proposed; however, no consensus was reached. 27 More recently, the Asia-Pacific Ocular Imaging Society PCV Workgroup published an OCT-based consensus for the nomenclature of PCV to differentiate PCV from “typical nAMD” and better describe the nature and identification of PCV. 28
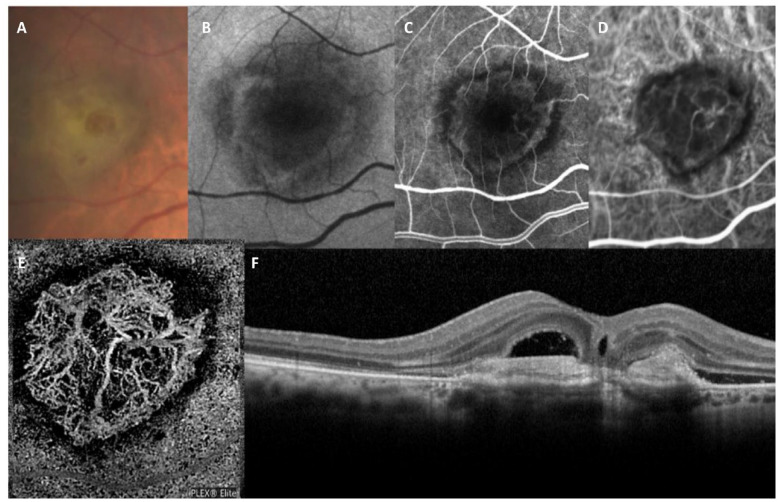
Type 2 MNV is defined as the proliferation of new vessels from the choroid into the subretinal space (Figure 4). It corresponds to classic CNV or pre-epithelial MNV, and pure type 2 MNV is the common phenotype of exudative AMD, accounting for approximately 18% of all nAMD cases. 29
Figure 4.
Type 2 MNV. (A) Color image shows decreased tessellation of the macular region with thickening and subretinal fibrinous material. (B) Fundus autofluorescence shows increased autofluorescence at the margins of the hypofluorescent lesion. (C) Fluorescein angiography shows the presence of a classic neovascular lesion. (D) Indocyanine green angiography shows the presence of a tangled network of neovessels. (E) En face OCT angiography proving the presence of a dense neovascular network. (F) Structural OCT scan showing a retinal pigment epithelium elevation associated with subretinal hyperreflective material and subretinal fluid.
Abbreviations: macular neovascularization OCT, optical coherence tomography.
Type 3 MNV, which corresponds to RAP, is defined as the downgrowth of vessels from the retinal circulation toward the outer retina and often appears with multiple small intraretinal hemorrhages (Figure 5). Yannuzzi et al 12 described the neovascularization pattern of RAP as being unique and divided into the following 3 stages: stage I, the intraretinal capillaries originating from deep retinal complex; stage II, proliferation of retinal vessels in subretinal spaces (stage IIA without PED and stage IIB with PED); and stage III, CNV clinically or angiographically. Vascularized PED was a consistent feature of stage III. These stages are identified using different imaging techniques. Type 3 MNV thus represents a distinct subgroup of AMD with intraretinal and subretinal vascularization or MNV. Of note, the 3 types of neovascularization are not mutually exclusive, and an eye can have more than 1 pathology (eg, mixed type 1 and type 2 neovascularization, corresponding to formerly minimally classic AMD) (Figure 6). Finally, late-stage neovascularization is often preceded by a morphologic feature known as reticular pseudodrusen, which represents a high-risk sign for late AMD with an estimated prevalence of 9% to 36%. 30
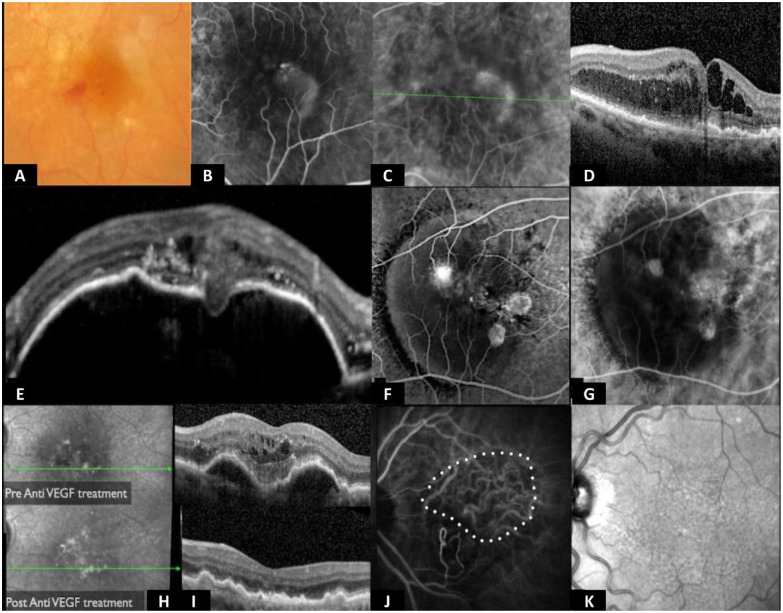
Figure 5.
Type 3 MNV. (A) Color image with intraretinal flame hemorrhage. (B) Midphase FA with intraretinal leakage. (C) Intermediate ICGA with typical leakage; other MNVs do not usually leak ICG dye. (D) Optical coherence tomography with intraretinal fluid without neurosensory retinal detachment. (E) RPE detachment with interruption and evident type 3 neovascularization. (F and G) FA and ICGA of an RPE detachment with typical position of a type 3 neovascularization in the center of the detachment. (H–K) Indirect signs such as the disappearance of RPE detachment after intravitreal injection of anti-VEGF resulting from the lack of new neovascular tissue under the RPE.
Abbreviations: anti-VEGF, antivascular endothelial growth factor; FA, fluorescein angiography; ICG, indocyanine green; ICGA, indocyanine green angiography; MNV, macular neovascularization; OCT, optical coherence tomography; RPE, retinal pigment epithelium.
Figure 6.
Type 1 and type 2 MNV. (A) Color image showing decreased tessellation of the macular region with thickening and some degree of subretinal fibrinous material. (B) Fluorescein angiography shows a minimally (<50%) classic neovascular lesion. (C) Indocyanine green angiography shows the presence of a tangled network of neovessels. (D) En face OCTA proving the presence of a dense neovascular network at the level of the avascular external retina slab. (E) En face OCTA proving the presence of a dense neovascular network at the level of the subretinal pigment epithelium layer slab. (F) Structural OCT scan shows a retinal pigment epithelium elevation with a focal disruption in close proximity to an overlying subretinal fibrovascular material associated with subretinal fluid.
Abbreviations: MNV, macular neovascularization; OCT, optical coherence tomography; OCTA, optical coherence tomography angiography.
Several conditions have the potential to be misdiagnosed as MNV in AMD, especially when older imaging techniques are used, and these other conditions should be ruled out to ensure selection of appropriate treatment. For example, hyporeflective cavities could be the result of active neovascularization or an acquired vitelliform lesion; OCTA has the potential to differentiate between them. 31 Similarly, drusenoid PED should be distinguished from fibrovascular PED, with the former tending to be smaller, shallower, and with less-defined borders. 32 With greater clarity of terminology, classification, and imaging, differentiation of distinct events should become easier for physicians.
Comparative Assessment of MNV Lesions Among Individual Imaging Techniques
The published literature suggests that the classification from different imaging modalities is not always consistent. A systematic review and meta-analysis of 8 studies with more than 400 participants found inconsistencies between OCT findings and fundus FA findings when detecting active disease in patients with nAMD. 33 The study concluded that both modalities might be needed to comprehensively monitor patients with nAMD. 33 Similarly, there were notable differences in the MNV area assessed by swept-source OCT and SD-OCT. 15 Furthermore, an observational case series that included 13 treatment-naïve patients found that in eyes with type 2 MNV, both ICGA and FA underestimated the pathological features compared with SD-OCT. 34
Recent studies have shown that OCTA has the potential to improve the visualization of MNV that was poorly defined with dye-based angiography (especially type 1 neovascularization) and enables clinicians to noninvasively identify treatment-naïve, nonactive MNV, especially in nonexudative AMD.35,36 Furthermore, OCTA has high sensitivity and specificity for diagnosing all types of MNV lesions and can be used for the dynamic assessment of the neovascularization complex during antivascular endothelial growth factor treatment. However, the use of OCTA for detecting MNV lesions has limitations, including the need for the patient to remain relatively motionless during assessments, the potential for shadow artifacts at deeper layers, and an inability to identify blood flow below a minimum threshold. Therefore, there is a possibility of overlooking the structures within which the blood flow does not reach the level of detection with the device. 37
Costanzo et al 38 performed a prospective study of 19 eyes of 17 patients to compare the size of type 1 MNV as assessed by OCTA and ICGA. In all eyes, the overlay of the 3 × 3 scanning area from OCTA to ICGA images showed a smaller lesion on OCTA than on intermediate ICGA and late ICGA. The difference between lesion size in OCTA vs ICGA was statistically significant (P< .05), although the authors could not conclude whether OCTA was underestimating or ICGA overestimating lesion size. However, the reproducibility of MNV quantification by OCTA indicates it could be a reliable tool for evaluating type 1 neovascularization. 39 A separate study, however, urged caution while analyzing results from OCTA images because attenuation from the RPE generates a smaller lesion area on OCTA images for MNV type 1 neovascularization. 40 Because of the apparent limitations of independent imaging technologies, data suggest that a combination of different techniques (ie, multimodal imaging) is required to not only provide enhanced insight into the mechanisms of retinal disease but also to increase the diagnostic sensitivity and guide treatment and retreatment decisions in routine clinical practice.
Comparative Assessment of MNV Lesions: Multimodal Imaging vs Individual Imaging Modalities
A thorough understanding of the multimodal imaging features of neovascularization will likely improve the diagnosis and management of nAMD. The literature provides evidence of comparative assessments of lesion characteristics via different imaging modalities. Ravera et al 41 compared the ability of individual imaging modalities (SD-OCT, FA, ICGA, infrared, and fundus autofluorescence) for MNV classification and treatment decisions with multimodal imaging in 52 patients. They found a strong association between SD-OCT and multimodal imaging with respect to MNV classification (interobserver coefficient 0.69 for multimodal imaging and 0.63 for SD-OCT). ICGA showed low interobserver agreement for retreatment decisions (interobserver coefficient 0.77 for multimodal imaging and 0.23 for ICGA). Soomro and Talks 42 also investigated the MNV detection efficacy of OCTA compared with a multimodal imaging approach (OCT, ICGA, and FA) in a retrospective study of 77 patients. The sensitivity of OCTA compared with FA was 71%, and the specificity was 81% (P = .108).
Soomro and Talks 42 also examined a cohort of patients with suspected MNV as ascertained by OCT (61 patients). This comparison showed that overall, ICGA was the best imaging modality for defining vascular networks followed by OCTA and then fundus FA (70%, 59%, and 49%, respectively). In contrast, when examining occult and classic nAMD, OCTA performed better than ICGA and fundus FA (72% vs 62% vs 44%).
In a prospective case series of 80 eyes, Coscas et al 43 used findings from traditional multimodal imaging (FA, ICGA, and SD-OCT) and OCTA to compare and evaluate a possible correspondence between the treatment decision and MNV classification. The imaging results from multimodal imaging and OCTA showed 95% correspondence in the detection of pattern 1 (defined as a lesion with either all or at least 3 of the following characteristics: well-defined shape, branching, numerous tiny capillaries, presence of anastomoses and loops, a perilesional hypointense halo, presence of a peripheral arcade in contrast to a “dead tree” appearance) and 90.5% for pattern 2 (fewer than the 3 features mentioned above). There was high interobserver agreement (P < .05) for both treatment decisions and MNV pattern assessment.
The accurate characterization of MNV lesions is an important pillar for determining the most appropriate treatment course for nAMD patients. As OCT-based technologies become more available in daily clinical practice, multimodal analysis using OCT-based and dye-based investigation of pathologies in nAMD is a realistic goal. A greater understanding of a lesion should be used to better guide treatment decisions and, ultimately, improve outcomes.
Conclusions
This review highlights the apparent need for more consensus regarding the clinical features of MNV lesion types given varying imaging modalities initially used to define them. Here, we have consolidated the current terminology of neovascular lesions in nAMD based on our literature review findings and clinical experience. Our goal was to provide a more standardized terminology that could decrease the bias of not accurately classifying each particular case of nAMD. We conclude that the consensus recommendations from the CONAN study group, if widely accepted by researchers, could simplify and clarify descriptions of neovascularization in nAMD as well as forming the basis for patient stratification in clinical trials and assessment of treatment success. Furthermore, the importance of using multimodal imaging techniques is evident, enabling physicians to combine results, accurately and precisely identify lesions, and individualize treatment plans based on pathological characteristics.
Acknowledgments
Catherine Lee and Ana Maria Rodriguez de Ledesma, Bedrock Healthcare Communications, Fleet, UK, provided medical writing support to the authors, funded by Novartis Pharma AG, Basel, Switzerland. Novartis was not involved in the interpretation of the literature search results.
Footnotes
Ethical Approval: This review article does not involve research conducted on human subjects; therefore, ethical approval was not required.
Statement of Informed Consent: Informed consent was not sought because no participants were recruited in this study.
Dr. Wolf received funds from Bayer, Novartis, Sandoz, and Zeiss.
Dr. Chow received consultancy fees from Bayer and Roche, research support from Opthea and RegenxBio Inc, and payment or honoraria for lectures or educational activities from Aviceda Therapeutics and Apellis Pharmaceuticals.
Dr. Souied provided expert support for AbbVie, Apellis Pharmaceuticals, Bayer, Novartis, Roche, and Teva.
Dr. Viola received consultancy fees, grants, and support for attending meetings and/or travel and participation on a data safety monitoring board or advisory board from Bayer, Novartis, and Roche.
Dr. Kaiser received consultancy fees from Alcon, Allergan, Bayer, Bausch + Lomb, Biogen Idec, Clearside Biomedical, Coherus BioSciences, Genentech/Roche, Astellas, Novartis, Ocular Therapeutix, Oculis, Ocuphire Pharma, Regeneron Pharmaceuticals Inc, and RegenxBio Inc.
Dr. Staurenghi received consultant/advisor fees from AbbVie, Apellis Pharmaceuticals, Annexon Bioscience, Bayer HealthCare Pharmaceutical, Boehringer Ingelheim, Genentech, Iveric Bio, and OraPharma Inc; consultant/advisor fees and grant support from Optos Inc and RetinAI; grant support from Quantel Medical; consultant/advisor fees, lecture fees/speakers bureau and grant support from CenterVue, Inc, Heidelberg Engineering, Hoffman La Roche, and Novartis Pharmaceuticals; lecture fees/speakers, bureau and grant support from Carl Zeiss Meditec; consultant/advisor fees, lecture fees/speakers bureau from Medscape; lecture fees/speakers bureau and grant support from Nidek Inc; and patents/royalty from Ocular Instruments Inc.
Dr. Gallego-Pinazo received consultancy fees from Apellis Pharmaceuticals and Carl Zeiss AG, consultancy fees and research support from Novartis and Roche, and research support from Ionis, Iveric Bio, Janssen, and Opthea.
Dr. Iida received consultancy fees from Bayer Yakuhin Ltd., Chugai Pharmaceutical Co, Janssen Pharmaceutical K.K., Kyowa Kirin, Nippon Boehringer Ingelheim Co, Ltd, Novartis, and Senju Pharmaceutical Co, Ltd; study funding and article processing charges from Bayer AG (Leverkusen, Germany); funding for editorial support and medical writing from Bayer Consumer Care AG (Basel, Switzerland); study funding from Regeneron Pharmaceuticals Inc; grant funding from Alcon Japan, AMO Pharma Ltd, HOYA, Nidek, Novartis, Santen Pharmaceutical, Senju Pharmaceutical Co Ltd, and Topcon Healthcare; payment or honoraria for lectures from Alcon Japan, Bayer Yakuhin Ltd, Canon Inc, Chugai Pharmaceutical Co, Nidek, Nikon, Novartis, Otsuka Pharmaceutical Co, Ltd, Santen Pharmaceutical, Senju Pharmaceutical Co, Ltd; and Topcon; patent from Topcon; and other financial rewards from Kyowa Kirin.
Funding: Novartis Pharma AG, Basel, Switzerland, funded the medical writing support for this manuscript. However, Novartis was not involved in the development of this manuscript.
ORCID iDs: Giovanni Staurenghi  https://orcid.org/0000-0002-2299-5251
https://orcid.org/0000-0002-2299-5251
Peter K. Kaiser  https://orcid.org/0000-0001-5126-045X
https://orcid.org/0000-0001-5126-045X
References
- 1. Holz FG, Schmitz-Valckenberg S, Fleckenstein M. Recent developments in the treatment of age-related macular degeneration. J Clin Invest. 2014;124(4):1430-1438. doi: 10.1172/JCI71029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spaide RF, Jaffe GJ, Sarraf D, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature study group. Ophthalmology. 2020;127(5):616-636. doi: 10.1016/j.ophtha.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol. 1991;109(9):1220-1231. doi: 10.1001/archopht.1991.01080090044025 [DOI] [PubMed] [Google Scholar]
- 4. Bressler NM, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials–TAP report 2. Arch Ophthalmol. 2001;119(2):198-207. [PubMed] [Google Scholar]
- 5. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999;117(10):1329-1345. [PubMed] [Google Scholar]
- 6. Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118(3):285-298. [PubMed] [Google Scholar]
- 7. Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12(3):191-223. [PubMed] [Google Scholar]
- 8. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). 1990. Retina. 2012;32 Suppl 1:1-8. [PubMed] [Google Scholar]
- 9. Hartnett ME, Weiter JJ, Garsd A, Jalkh AE. Classification of retinal pigment epithelial detachments associated with drusen. Graefes Arch Clin Exp Ophthalmol. 1992;230(1):11-19. doi: 10.1007/BF00166756 [DOI] [PubMed] [Google Scholar]
- 10. Hartnett ME, Weiter JJ, Staurenghi G, Elsner AE. Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology. 1996;103(12):2042-2053. doi: 10.1016/s0161-6420(96)30389-8 [DOI] [PubMed] [Google Scholar]
- 11. Slakter JS, Yannuzzi LA, Schneider U, et al. Retinal choroidal anastomoses and occult choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2000;107(4):742-753. doi: 10.1016/s0161-6420(00)00009-9 [DOI] [PubMed] [Google Scholar]
- 12. Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21(5):416-434. doi: 10.1097/00006982-200110000-00003 [DOI] [PubMed] [Google Scholar]
- 13. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181. doi: 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sayanagi K, Sharma S, Yamamoto T, Kaiser PK. Comparison of spectral-domain versus time-domain optical coherence tomography in management of age-related macular degeneration with ranibizumab. Ophthalmology. 2009;116(5):947-955. doi: 10.1016/j.ophtha.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 15. Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499-1505. doi: 10.1167/iovs.16-20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khurana RN, Dupas B, Bressler NM. Agreement of time-domain and spectral-domain optical coherence tomography with fluorescein leakage from choroidal neovascularization. Ophthalmology. 2010;117(7):1376-1380. doi: 10.1016/j.ophtha.2009.11.039 [DOI] [PubMed] [Google Scholar]
- 17. Freund KB, Ho IV, Barbazetto IA, et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina. 2008;28(2):201-211. doi: 10.1097/IAE.0b013e3181669504 [DOI] [PubMed] [Google Scholar]
- 18. Ravera V, Bottoni F, Giani A, Cigada M, Staurenghi G. Retinal angiomatous proliferation diagnosis: a multiimaging approach. Retina. 2016;36(12):2274-2281. doi: 10.1097/IAE.0000000000001152 [DOI] [PubMed] [Google Scholar]
- 19. Novais EA, Baumal CR, Sarraf D, Freund KB, Duker JS. Multimodal imaging in retinal disease: a consensus definition. Ophthalmic Surg Lasers Imaging Retina. 2016;47(3):201-205. doi: 10.3928/23258160-20160229-01 [DOI] [PubMed] [Google Scholar]
- 20. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30(9):1333-1349. doi: 10.1097/IAE.0b013e3181e7976b [DOI] [PubMed] [Google Scholar]
- 21. Jung JJ, Chen CY, Mrejen S, et al. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am J Ophthalmol. 2014;158(4):769-779.e2. doi: 10.1016/j.ajo.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 22. Sandhu SS, Talks SJ. Correlation of optical coherence tomography, with or without additional colour fundus photography, with stereo fundus fluorescein angiography in diagnosing choroidal neovascular membranes. Br J Ophthalmol. 2005;89(8):967-970. doi: 10.1136/bjo.2004.060863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Querques G, Canouï-Poitrine F, Coscas F, et al. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(3):1264-1270. doi: 10.1167/iovs.11-9063 [DOI] [PubMed] [Google Scholar]
- 24. Palejwala NV, Jia Y, Gao SS, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35(11):2204-2211. doi: 10.1097/IAE.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon HJ, Lee JJ, Park SW, Byon IS, Lee JE. Enlargement of polypoidal choroidal vasculopathy lesion without exudative findings assessed in en face optical coherence tomography images. Graefes Arch Clin Exp Ophthalmol. 2019;257(8):1621-1629. doi: 10.1007/s00417-019-04317-y [DOI] [PubMed] [Google Scholar]
- 26. Rosa RH, Davis JL, Eifrig CWG. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol. 2002;120(4):502-508. doi: 10.1001/archopht.120.4.502 [DOI] [PubMed] [Google Scholar]
- 27. Dansingani KK, Gal-Or O, Sadda SR, Yannuzzi LA, Freund KB. Understanding aneurysmal type 1 neovascularization (polypoidal choroidal vasculopathy): a lesson in the taxonomy of ‘expanded spectra’ – a review. Clin Exp Ophthalmol. 2018;46(2):189-200. doi: 10.1111/ceo.13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheung CMG, Lai TYY, Teo K, et al. Polypoidal choroidal vasculopathy: consensus nomenclature and non-indocyanine green angiograph diagnostic criteria from the Asia-Pacific Ocular Imaging Society PCV workgroup. Ophthalmology. 2021;128(3):443-452. doi: 10.1016/j.ophtha.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 29. Cohen SY, Creuzot-Garcher C, Darmon J, et al. Types of choroidal neovascularisation in newly diagnosed exudative age-related macular degeneration. Br J Ophthalmol. 2007;91(9):1173-1176. doi: 10.1136/bjo.2007.115501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wightman AJ, Guymer RH. Reticular pseudodrusen: current understanding. Clin Exp Optom. 2019;102(5):455-462. doi: 10.1111/cxo.12842 [DOI] [PubMed] [Google Scholar]
- 31. Schneider EW, Fowler SC. Optical coherence tomography angiography in the management of age-related macular degeneration. Curr Opin Ophthalmol. 2018;29(3):217-225. doi: 10.1097/ICU.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 32. Yonekawa Y, Kim IK. Clinical characteristics and current treatment of age-related macular degeneration. Cold Spring Harb Perspect Med. 2014;5(1):a017178. doi: 10.1101/cshperspect.a017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castillo MM, Mowatt G, Elders A, et al. Optical coherence tomography for the monitoring of neovascular age-related macular degeneration: a systematic review. Ophthalmology. 2015;122(2):399-406. doi: 10.1016/j.ophtha.2014.07.055 [DOI] [PubMed] [Google Scholar]
- 34. Sulzbacher F, Kiss C, Munk M, Deak G, Sacu S, Schmidt-Erfurth U. Diagnostic evaluation of type 2 (classic) choroidal neovascularization: optical coherence tomography, indocyanine green angiography, and fluorescein angiography. Am J Ophthalmol. 2011;152(5):799-806.e1. doi: 10.1016/j.ajo.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 35. Malihi M, Jia Y, Gao SS, et al. Optical coherence tomographic angiography of choroidal neovascularization ill-defined with fluorescein angiography. Br J Ophthalmol. 2017;101(1):45-50. doi: 10.1136/bjophthalmol-2016-309094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carnevali A, Cicinelli MV, Capuano V, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment-naïve quiescent choroidal neovascularization. Am J Ophthalmol. 2016;169:189-198. doi: 10.1016/j.ajo.2016.06.042 [DOI] [PubMed] [Google Scholar]
- 37. El Ameen A, Cohen SY, Semoun O, et al. Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina. 2015;35(11):2212-2218. doi: 10.1097/IAE.0000000000000773 [DOI] [PubMed] [Google Scholar]
- 38. Costanzo E, Miere A, Querques G, Capuano V, Jung C, Souied EH. Type 1 choroidal neovascularization lesion size: indocyanine green angiography versus optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9): 307-313. doi: 10.1167/iovs.15-18830 [DOI] [PubMed] [Google Scholar]
- 39. Amoroso F, Miere A, Semoun O, Jung C, Capuano V, Souied EH. Optical coherence tomography angiography reproducibility of lesion size measurements in neovascular age-related macular degeneration (AMD). Br J Ophthalmol. 2018;102(6):821-826. doi: 10.1136/bjophthalmol-2017-310569 [DOI] [PubMed] [Google Scholar]
- 40. Novais EA, Adhi M, Moult EM, et al. Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;164:80-88. doi: 10.1016/j.ajo.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ravera V, Giani A, Pellegrini M, et al. Comparison among different diagnostic methods in the study of type and activity of choroidal neovascular membranes in age-related macular degeneration. Retina. 2019;39(2):281-287. doi: 10.1097/IAE.0000000000001960 [DOI] [PubMed] [Google Scholar]
- 42. Soomro T, Talks J. The use of optical coherence tomography angiography for detecting choroidal neovascularization, compared to standard multimodal imaging. Eye (Lond). 2018;32(4):661-672. doi: 10.1038/eye.2018.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coscas GJ, Lupidi M, Coscas F, Cagini C, Souied EH. Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina. 2015;35(11):2219-2228. doi: 10.1097/IAE.0000000000000766 [DOI] [PubMed] [Google Scholar]