Abstract
Despite great progress in imaging, genetics, surgery, and therapeutics, frontal lobe epilepsy (FLE) continues to be a challenge for neurologists and epileptologists. This manuscript summarizes the latest advancements in FLE discussed at the 2023 Epilepsy Specialist Symposium during the American Epilepsy Society Annual meeting. Correlation between stereoelectroencephalography and clinical symptoms has reinvigorated symptomatology literature in FLE, allowing for more precise aura anatomical localization. Neuropsychological assessments permit the identification of different FLE cognitive phenotypes, with language being the most prominent domain-specific impairment. These tests can help develop psychotherapeutic and cognitive support systems for these patients. Genetic and molecular studies have uncovered specific genes associated with FLE susceptibility, offering prospects for targeted therapies. Advanced neuroimaging techniques such as high field magnetic resonance imaging (MRI), functional MRI (fMRI), magnetoencephalography and colocalization of multiple imaging techniques have led to more precise localization of the epileptogenic zone providing insights into the dynamic neural networks underlying frontal lobe seizures. This has facilitated guided therapeutic surgical interventions that can be employed around the world, expanding access of these technologies to multiple populations. Despite many advances, prognosis of FLE remains poor for some patients. The biggest determinant for poor prognosis continues to be nonlesional FLE. Newer technological advancements aim to pass these barriers and offer FLE patients a better quality of life with lower seizure burden and higher cognitive outcomes.
Keywords: frontal, epilepsy, genetics, prognosis
Introduction
The frontal lobe is the brain area located anteriorly to the central sulcus on the lateral surface. With its enormous size and enigmatic functions including movement, volition, behavior, speech and reasoning, the frontal lobe has puzzled scientists for centuries. The symptoms of epilepsy arising from the frontal lobe can also vary widely due to the extensive neocortex. Its size and complexity have made it difficult to localize epileptogenic lesions when compared to other brain areas such as the temporal lobe. Neurologists and epileptologists have tried to better localize seizures and epilepsies to improve their treatment and outcome. The invention of electroencephalography (EEG) in the early 20th century facilitated the separation between focal and generalized epilepsies. An earlier, coronal triangular montage that aimed to monitor deep brain structures was later discouraged due to the possibility of false lateralization. 1 The development of computed tomography and magnetic resonance imaging permitted visualization of epileptogenic lesions like never before. Newer multimodal imaging can combine anatomic with physiological principles to hone the localization of the epileptogenic zone (EZ). Advances in molecular biology and genetics in the 21st century have led to genetic markers linked to susceptibility for frontal lobe epilepsy (FLE) giving a better understanding to begin precision medicine.
This manuscript describes the latest developments regarding FLE, aiming to provide clinicians and researchers with a current understanding of the rapidly evolving landscape.
From Anatomy to Auras
Knowledge of the functional anatomy of the frontal lobe is key to understanding frontal lobe seizure semiology. Interictal and ictal EEG findings together with seizure semiology are key to successful seizure localization and good outcome after resective epilepsy surgery. Structural and functional imaging are important for presurgical planning only when they are concordant with seizure semiology and EEG. Lüders defined the EZ as the area of cortex that is necessary and sufficient for initiating seizures and whose removal is necessary for complete abolition of seizures. 2
Electrical brain stimulation has demonstrated the somatotopic organization of the primary and supplemental sensorimotor areas, frontal eye field, language and second sensory areas. Stereoelectroencephalography (SEEG) recordings and stimulation show that the anterior cingulate is involved in emotional and autonomic responses along with repetitive and fluid motor movements, vocalization and pronation and ictal pouting (Chapeau Sign). Stimulation of the anterior cingulate and anterior mid-cingulate cortex (aMCC) results in grasping movements, repetitive behaviors and getting up. Stimulation of the posterior mid-cingulate cortices (pMCC) result in tonic, dystonic movements which may be asymmetric with preserved awareness. 3
Auras occur in about two-thirds of patients with frontal lobe seizures; cephalic sensation, fear or an indescribable sensation are more common, followed by autonomic or somatosensory sensations. 4 A constricting feeling in the throat accompanied by guttural vocalizations occurs with anterior insula involvement. Peri-rolandic seizures are characterized by early motor (tonic, clonic, focal myoclonic) manifestations, sometimes with a Jacksonian march and/or sensory symptoms. Not uncommonly Todd's paresis may follow such a seizure. Distal involvement is more apparent in peri-rolandic seizures whereas proximal limb and trunk involvement is more often seen with supplementary motor area (SMA) seizures—this may be symmetric or asymmetric with tonic elevation of a limb being contralateral to side of ictal onset. Negative motor seizures occur with anterior SMA localization. Hyperkinetic seizures (HKS) are characteristic of mesial frontal lobe seizures; two subtypes have been described: type I HKS involve the anterior insula, temporal pole, orbitofrontal cortex and anterior cingulate whereas type II seizures engage primarily the ventral mesial premotor cortex. 5
Detailed semiologic analysis of frontal lobe seizures correlated with SEEG indicates elementary motor signs occur with precentral and premotor involvement whereas integrated behavior and distal stereotypies are seen with prefrontal involvement. Emotional and autonomic features are more pronounced with anterior cingulate and orbitofrontal involvement. 6 Seizure semiology has good lateralizing and localizing value, comparing favorably with scalp interictal and ictal EEG and magnetic resonance imaging (MRI). 7 In patients with nonlesional MRI, semiology plays an even larger role in formulating a hypothesis for presurgical evaluation.
Cognitive and Behavioral Complications of FLE: Symptoms, Assessment, and Therapy
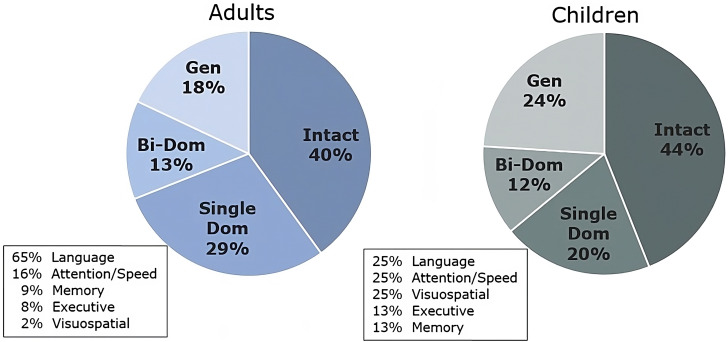
FLE is associated with various cognitive and behavioral symptoms, although considerable heterogeneity exists due to the complexity of frontal lobe functions. Approximately 60% of adults with pharmocoresistant FLE show some level of cognitive impairment, with language being the most common domain-specific impairment followed by attention, processing speed, executive function, and memory. Rates of cognitive impairment are similar in pediatric populations, although the most common domain-specific impairments appear to be more mixed (Figure 1).8,9 Research on postsurgical cognitive changes following frontal lobe surgery is somewhat limited, but, generally, about 40% to 60% of patients experience some level of cognitive decline, albeit usually mild.
Figure 1.
Congnitive phenotypes in adults and children with pharmacoresistant FLE based on IC-CoDE criteria. Note: Single Dom = Single Domain Impairment, Bi-Dom = Bi-Domain Impairment, Gen = Generalized Impairment..
Regarding behavioral symptoms in FLE, comorbidity of ADHD is quite high (30-57%), and autism spectrum disorder (20%) and intellectual disability (10-25%) also frequently co-occur. Patients with FLE commonly report symptoms of depression (20-40%) and anxiety (15-25%), though reported symptoms are typically in the mild range. 10 Preliminary research also suggests that certain psychiatric symptoms and personality traits are more prevalent in FLE, such as hyperactivity, obsessive and addiction traits, and internalizing behaviors, 10 as well as changes in social cognition (eg, increased difficulty with emotion recognition, faux pas, and perspective taking). 11
Cognitive and behavioral symptoms in FLE can significantly impact patient's quality of life (QOL), daily functioning, and ability to cope with their epilepsy. Thus, proper assessment of cognitive and behavioral symptoms is essential in the treatment of patients with FLE. Proper screening is often the first and most important step. Providing patients with early psychoeducation about the possibility of cognitive, behavioral, and emotional changes can encourage discussion and reduced stigma. A neuropsychological evaluation can help characterize cognitive difficulties, identify secondary contributors to cognitive inefficiency, and provide tailored strategies (including work and academic accommodations) to help compensate for cognitive changes. A psychiatric consult can help evaluate mood disorders and guide treatment recommendations, while social work can be useful in understanding other psychosocial factors that may be impacting the patient or the patient's care.
Treatment options include cognitive intervention, therapy, and medications. Although cognitive intervention in epilepsy is largely understudied, existing research has provided promising evidence that cognitive functions (primarily memory and attention) and QOL may be improved with cognitive rehabilitation. 12 For mental health symptoms, cognitive behavioral therapy has been shown to be quite effective for patients with epilepsy. 13 For children, particularly those who have behavioral symptoms or difficulties with social cognition, applied behavioral analysis or theory of mind, respectively, can be helpful. Psychotropic and stimulant medications are also common but must be considered in the context of the patient's medical history and current medication regime. Ultimately, comprehensive care requires a multidisciplinary approach to understanding, assessing, and treating the numerous cognitive and behavioral symptoms of FLE.
Genetics and the Frontal Lobe
Any gene with widespread brain expression, during and after development, can contribute to frontal lobe function. Given the large size of the frontal lobes relative to the other lobes of the brain, it is not surprising that dysfunction of a brain-expressed gene may lead to FLE and other consequences. Here we focus on genetic conditions affecting primarily the frontal lobes, some associated with cerebral malformations and some nonmalformation-associated.
Frontal lobe malformations associated with epilepsy: One of the earliest recognized frontal-lobe-predominant cerebral malformations is bilateral frontal polymicrogyria (BFP). Individuals with this pattern of malformation, visible on MRI, present with developmental delay, intellectual disability, mild spastic quadriparesis, and in 5/13 cases epilepsy. 14 Bilateral fronto-parietal polymicrogyria (BFPP) is another epilepsy-associated brain malformation, with T2 hyperintensities of the white matter; individuals also have intellectual disability and cerebellar dysfunction with a range of severity. 15 This BFPP pattern is associated with autosomal recessive (homozygous or compound heterozygous) variants in the gene GPR56, studies of which have established that the underlying pathobiology leads to a cobblestone malformation.16,17 More recently, de novo missense variants in the gene PANX1 have been identified in 3 individuals with extensive frontal-predominant polymicrogyria, 2 of whom had microcephaly. 18
Sleep related hypermotor (hyperkinetic) epilepsy and other focal epilepsies: There are a number of genes that are expressed across the brain, and when individuals have variants in these genes, focal epilepsy can be present in any region of the brain. This includes DEPDC5, which can affect any lobe of the brain and presents in families in an autosomal dominant fashion, with some individuals’ MRIs revealing small regions of what has been termed “bottom-of-sulcus” dysplasias.19,20 Another gene that has been associated with sleep-related hypermotor (hyperkinetic) epilepsy is KCNT1, which interestingly is also associated with a more severe condition called epilepsy of infancy with migrating focal seizures.21,22 Additional sleep related hypermotor (hyperkinetic) epilepsy genes, seen less frequently in the clinical setting, include CHRNA2, CHRNB2, and CHRNA4. 23 Given the emerging gene-based treatments that are under development for epilepsy caused by DEPDC5 and KCNT1, 24 among other genetic epilepsies, it is important to consider genetic testing for any individual with FLE.
The Role of Brain Imaging in FLE
There is clear evidence that identification of a focal structural or functional lesion on brain imaging correlates with improved surgical outcomes in focal epilepsy. Although structural brain MRI is standardly acquired in drug resistant epilepsy patients, roughly one-third of patients do not have a visible lesion identified across all focal epilepsy. In FLE the probability of visually identifying a lesion on structural MRI is lower, around 20%. Therefore, it is critical to utilize multimodal imaging to increase the probability of noninvasively identifying the seizure focus.
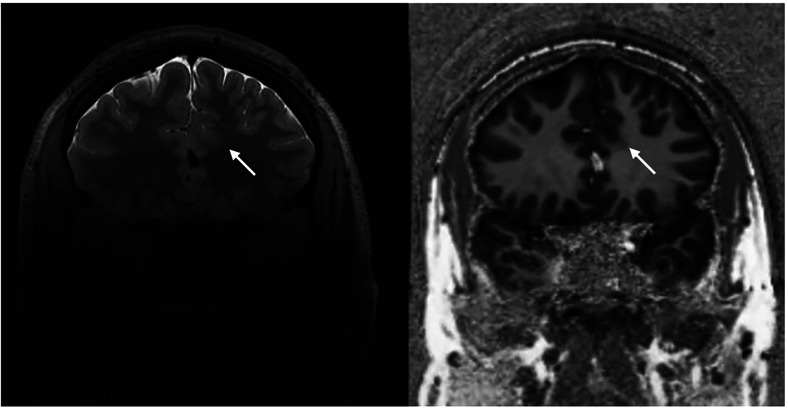
The most common lesions identified in FLE are caused by abnormal proliferation, migration and organization of the brain with cortical dysplasia as the primary etiology. However, even when a focal cortical dysplasia (FCD) is qualitatively detected there is concern that we are only seeing the “tip of the iceberg” and that the visible lesion has an unclear topographical relationship with the seizure onset zone. When evaluating structural imaging for FCD, cortical thickening with blurring of the gray–white junction or an abnormal gyral pattern may be evident. Specific to Type II (Taylor) FCD the “transmantle sign” representing abnormal glial fibers may be present with hyperintensity on T2-weight imaging and FLAIR and hypointensity on T1 weight imaging. Ultrahigh field 7 T MRI may increase the likelihood of detecting subtle lesions not easily seen on 3 T MRI by 20%. 25 Figure 2 demonstrates a subtle FCD detected at 7 T. Unfortunately, even with ultrahigh field MRI the majority of FLE patients remain MRI-negative using qualitative structural imaging.
Figure 2.
Left frontal FCD (white arrow) detected with 7T MRI.
Functional imaging may uncover lesions and improve understanding of the extent of the epilepsy network. Interictal 18F-FDG-PET and Ictal-Interictal SPECT are commonly acquired clinically. Hypometabolic areas on PET may indicate the seizure network and aid in identification of occult lesions and lateralization of the epileptogenic network. Coregistering PET with MRI can have additional benefit in detecting subtle lesions. Ictal SPECT may demonstrate increased tracer uptake in the epileptogenic network especially when subtracted from interictal SPECT but tracer timing remains a challenge. Magnetoencephalography (MEG) records magnetic fields produced by synchronized populations of neurons and provides localized brain activity. Tasks can be administered during MEG to map motor and language regions. MEG can assist in identification of the EZ in MRI-negative cases and refine the location of seizure onset within lesions.
There are very limited studies focused on imaging in FLE. Seminal work from Knowlton et al 26 in extra-TLE found that MEG localized 45% of patients, PET localized in about 20% and ictal SPECT localized in 25%. This paper concluded that each functional imaging modality has clinical value in detecting seizure foci and predicting surgical outcomes. Despite the scant literature substantiating the use of multimodal functional and structural imaging in epilepsy most surgical epilepsy centers rely heavily on imaging in decision making for patients with FLE.
There is a clear need for large multicenter studies focused on imaging in FLE. Fortunately, there are several worldwide efforts focused on creating “big data” in epilepsy imaging. Quantitative analytics of these larger datasets have already shown promise in detecting hidden FCDs with sensitivities of artificial intelligence-based algorithms of up to 85% detecting MRI-negative FCDs.27,28 As these efforts grow including well-curated multimodal imaging, EEG, and clinical data, we will have the opportunity to develop diagnostic tools specific to FLE and improve the clinical outcomes in our patients.
Surgical Approaches to FLE in Countries With Lower Resources
For over 4 decades it has been stressed that the impact of epilepsy is more than just seizure recurrence and the objectives of surgery must include avoiding comorbidities and worsening of quality of life. Factors acting as barriers to epilepsy surgery include biological (lack of biomarkers, microenvironment, epileptogenesis, epileptic networks) and sociodemographic-related factors (incomplete patient referral guidelines, reduced access to specialized centers, increased costs, insufficient social and medical training, defective information, pervasive myths). Arguably these barriers are more prominent in resource-limited countries. Specifically in Latin America, major factors include the lack of specialized training, fewer specialized epilepsy centers with low numbers of EEG machines that are concentrated in urban areas. Numerous initiatives to increase education and displacing myths about the surgical options have led to the establishment of surgical programs comparable to international standards. In a recent survey of 80 centers in 18 countries of Latin America (total number of surgeries 25 333), 27.1% of surgeries involved extratemporal resections. 29 Surgical programs should have cost-efficient presurgical evaluations, with individualized selection of surgical candidates to attain localization and delimitation of the EZ, the eloquent cortex (EC) and the relationship between them. The best outcomes are achieved when there is concordance of presurgical techniques revealing an EZ that is restricted (electrically and semiologically) and that can be completely resected (avoiding EC by electrocorticography), with seizure freedom comparable to temporal lobectomy (80-85% Class Engel I). However, the underlying etiology significantly influences the recurrence rate of seizures, with cortical dysplasias exhibiting a higher frequency of recurrences. Cases requiring invasive monitoring include when MRI shows more than one lesion, focal epilepsy originating in eloquent areas, focal epilepsy with incongruent studies or nonlesional focal epilepsy. Cortical mapping and intraoperative somato-sensory evoked potentials are valuable neurophysiological tools to preserve neural function. Mapping of eloquent areas has improved in recent years but there is considerable variability among individuals in their localization. Cortical dysplasias may cause displacement of functional cortex, making it more complex. Epileptogenic areas within the Rolandic cortex in patients with preserved motor function present considerable decision-making challenges. A lesionectomy, or resection of neighboring EZs, would be options in these cases. Nonlesional epilepsy, multilobar lesions and “catastrophic” epilepsies of infancy continue to require extensive resections. A large series 30 of SMA epilepsy resulted in 61% seizure-free patients with only 17% exhibiting transitory postsurgical neurological deficit with akinetic mutism and hemiparesis that resolved within 6 to 12 months. Laplane 31 described this as the SMA syndrome and argued as a deterrent of this surgical approach. Careful use of subpial/endopial surgical technique with neurophysiological guidance is needed in functional zones or networks.
To enhance the advantages of surgical alternatives in countries with lower resources, essential components include: (a) prompt referral to epilepsy surgery, patient-centered evaluations, meticulous surgical planning and approaches, along with comprehensive follow-up; (b) promoting educational opportunities and fostering international collaboration for a rational, cost-effective utilization of resources; and (c) advancing strategies to enhance surgical access and improve communication through adequate registries and databases regarding the effectiveness and safety of surgical procedures.
Outcome in FLE: The Good, the Bad and the Ugly
There have been controversies whether FLE surgery should be undertaken because of low chances of success as compared to performing surgery in drug-resistant temporal lobe epilepsy. Most studies have been retrospective and are of small sample size. In addition, seizure outcomes are difficult to quantify as a common pattern of seizure outcomes are prolonged periods of seizure freedom intermixed with recurrences in FLE. 32
A meta-analysis has pooled retrospective evidence and reports Engel class I outcomes at 45%. 33 The presence of a lesion, gross total resection of a lesion, and a localized frontal resection as compared to a total lobectomy were favorable factors. There is no reliable evidence that epilepsy surgery is more effective in one region of the frontal lobes versus another. Anecdotal evidence suggests more favorable outcomes in SMA epilepsy, as the seizure onset zone can be more easily identified due to distinct semiology. SMA resection can lead to a temporary deficit in motor function and speech (SMA syndrome), which needs to be discussed as a possible complication presurgically. Newer surgical techniques such as laser interstitial thermal therapy and radiofrequency ablation have been employed in small case series, which are difficult to evaluate due to sample size considerations.
The seizure onset zone is the treasure trove for the surgical epileptologist, which is difficult to identify in the frontal lobes. It is unclear whether the currently more widely used approach of SEEG or an approach with subdural grids is more advantageous and can be biased by patient selection. In addition, other outcomes, such as cognitive outcomes, depression, quality of life, and social determinants are underreported in the literature.
Intracranial stimulation approaches are an additional treatment option if surgical resection is not an option or would result in a significant postoperative deficit. Currently in the United States, responsive stimulation of the seizure onset zone and deep brain stimulation of the anterior nucleus of the thalamus are FDA approved. In a randomized controlled trial of responsive neurostimulation, there was a 70% seizure reduction after 6 years. 34 In the comparable study of anterior nucleus stimulation of the thalamus there was a 59% reduction in seizure after 5 years. 35 These outcome measures differ from the outcomes deployed in studies of resections which usually report on seizure free outcomes (Engel class I) and not on reductions of seizure frequency.
Thus, resection of the seizure onset zone remains the best option to achieve seizure freedom in drug-resistant FLE granted that the seizure onset zone can be identified and safely resected (The Good). If this approach fails or is not possible (The Bad), intracranial neuromodulation is an option to decrease seizure burden. In some patients, despite applying those approaches to the best of our knowledge, we are still unable to reduce the burden of their disease (The Ugly) and we need to concentrate our efforts to advance the field to find some viable treatment options in the future for patients with FLE.
Conclusions
Despite great advances in the areas of imaging, molecular genetics, surgery, and pharmacology, FLE continues to have higher refractory rates when compared to epilepsies arising from other areas of the brain. In the last century, these new technologies have given us a better understanding of the pathophysiology of epilepsy. In In the next century, we will learn how to apply these techniques with an emphasis to improve cognition and quality of life instead of just seizure control.
Individuals with FLE may benefit from personalized and innovative interventions, fostering a brighter outlook.
Acknowledgments
We would like to thank the AES staff including Eileen Murray, MM, CAE, Shawna Strickland, PhD, CAE, RRT, FAARC, Susan Oliver, MBA, Cristina Graham, JoLynn Amsden, and Amy Kephart, MPH, CAE for their support and all the committee members in charge of program planning of the Annual American Epilepsy Society Meeting.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Barbara C. Jobst https://orcid.org/0000-0001-9243-2238
Prakash Kotagal https://orcid.org/0000-0002-8978-2680
Ignacio Valencia https://orcid.org/0000-0003-2385-4933
References
- 1.Tyner FS, Knott JR, Mayer Jr WB. Fundamentals of EEG Technology: Basic Concepts and Methods. Vol 1. Lippincott Williams & Wilkins; 1983. [Google Scholar]
- 2.Lüders HO, Awad I. Conceptual considerations. In: Lüders HO, ed. Epilepsy surgery. Raven Press; 1993:51-62. [Google Scholar]
- 3.Pelliccia V, Avanzini P, Rizzi Met al. Association between semiology and anatomo-functional localization in patients with cingulate epilepsy: a cohort study. Neurology. 2022;98(22):e2211-e2223. doi: 10.1212/WNL.0000000000200145 [DOI] [PubMed] [Google Scholar]
- 4.Jobst BC, Siegel AM, Thadani VM, Roberts DW, Rhodes HC, Williamson PD. Intractable seizures of frontal lobe origin: clinical characteristics, localizing signs, and results of surgery. Epilepsia. 2000;41(9):1139-1152. [DOI] [PubMed] [Google Scholar]
- 5.Rheims S, Ryvlin P, Scherer Cet al. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia. 2008;49(12):2030-2040. [DOI] [PubMed] [Google Scholar]
- 6.Bonini F, McGonigal A, Trebuchon Aet al. et al. Frontal lobe seizures: from clinical semiology to localization. Epilepsia. 2014;55(2):264-277. [DOI] [PubMed] [Google Scholar]
- 7.Elwan S, Alexopoulos A, Silveira DC, Kotagal P. Lateralizing and localizing value of seizure semiology: comparison with scalp EEG, MRI and PET in patients successfully treated with resective epilepsy surgery. Seizure. 2018;61:203-208. [DOI] [PubMed] [Google Scholar]
- 8.Arrotta K, Swanson SJ, Janecek JKet al. Application of the international classification of cognitive disorders in epilepsy (IC-CoDE) to frontal lobe epilepsy using multicenter data. Epilepsy Behav. 2023;148:109471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson L, Arenivas A, Arrotta Ket al. Application of the International Classification of Cognitive Disorders in Epilepsy (IC-CoDE) to Children with Pharmacoresistant Epilepsy. American Epilepsy Society; 2023. [Google Scholar]
- 10.Helmstaedter C, Witt JA. Multifactorial etiology of interictal behavior in frontal and temporal lobe epilepsy. Epilepsia. 2012;53(10):1765-1773. [DOI] [PubMed] [Google Scholar]
- 11.Ziaei M, Arnold C, Thompson K, Reutens DC. Social cognition in temporal and frontal lobe epilepsy: systematic review, meta-analysis, and clinical recommendations. J Int Neuropsychol Soc. 2023;29(2):205-229. [DOI] [PubMed] [Google Scholar]
- 12.Farina E, Raglio A, Giovagnoli AR. Cognitive rehabilitation in epilepsy: an evidence-based review. Epilepsy Res. 2015;109:210-218. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary N, Kumar A, Sharma Vet al. Effectiveness of CBT for reducing depression and anxiety in people with epilepsy: a systematic review and meta-analysis of randomized controlled trials. Epilepsy Behav. 2024;151:109608. [DOI] [PubMed] [Google Scholar]
- 14.Guerrini R, Barkovich AJ, Sztriha L, Dobyns WB. Bilateral frontal polymicrogyria: a newly recognized brain malformation syndrome. Neurology. 2000;54(4):909-913. [DOI] [PubMed] [Google Scholar]
- 15.Chang BS, Piao X, Bodell Aet al. Bilateral frontoparietal polymicrogyria: clinical and radiological features in 10 families with linkage to chromosome 16. Ann Neurol. 2003;53(5):596-606. [DOI] [PubMed] [Google Scholar]
- 16.Bahi-Buisson N, Poirier K, Boddaert Net al. GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010;133(11):3194-3209. [DOI] [PubMed] [Google Scholar]
- 17.Piao X, Hill RS, Bodell Aet al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303(5666):2033-2036. [DOI] [PubMed] [Google Scholar]
- 18.Akula SK, Chen AY, Neil JEet al. Exome sequencing and the identification of new genes and shared mechanisms in polymicrogyria. JAMA Neurol. 2023;80(9):980-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baulac S, Ishida S, Marsan Eet al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77(4):675-683. [DOI] [PubMed] [Google Scholar]
- 20.Scheffer IE, Heron SE, Regan BMet al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann Neurol. 2014;75(5):782-787. [DOI] [PubMed] [Google Scholar]
- 21.Barcia G, Fleming MR, Deligniere Aet al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44(11):1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heron SE, Smith KR, Bahlo Met al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44(11):1188-1190. [DOI] [PubMed] [Google Scholar]
- 23.Steinlein OK. Genetic heterogeneity in familial nocturnal frontal lobe epilepsy. Prog Brain Res. 2014;213:1-15. doi: 10.1016/B978-0-444-63326-2.00001-6 [DOI] [PubMed] [Google Scholar]
- 24.Demarest ST, Brooks-Kayal A. From molecules to medicines: the dawn of targeted therapies for genetic epilepsies. Nat Rev Neurol. 2018;14(12):735-745. [DOI] [PubMed] [Google Scholar]
- 25.Wang I, Oh S, Blumcke Iet al. Value of 7 T MRI and post-processing in patients with nonlesional 3 T MRI undergoing epilepsy presurgical evaluation. Epilepsia. 2020;61(11):2509-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowlton RC, Elgavish RA, Bartolucci Aet al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann Neurol. 2008;64(1):35-41. [DOI] [PubMed] [Google Scholar]
- 27.Gill RS, Lee HM, Caldairou Bet al. Multicenter validation of a deep learning detection algorithm for focal cortical dysplasia. Neurology. 2021;97(16):e1571-e1582. doi: 10.1212/WNL.0000000000012698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer H, Ripart M, Whitaker Ket al. Interpretable surface-based detection of focal cortical dysplasias: a multi-centre epilepsy lesion detection study. Brain. 2022;145(11):3859-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos M, Alonso M, Cendes Fet al. A survey of the reality of epilepsy surgery in Latin America. In: International Epilepsy Congress. ILAE; 2023:113. [Google Scholar]
- 30.Alonso-Vanegas MA, San-Juan D, Buentello Garcia RMet al. Long-term surgical results of supplementary motor area epilepsy surgery. J Neurosurg. 2017;127(5):1153-1159. [DOI] [PubMed] [Google Scholar]
- 31.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34(3):301-314. [DOI] [PubMed] [Google Scholar]
- 32.Lazow SP, Thadani VM, Gilbert KLet al. Outcome of frontal lobe epilepsy surgery. Epilepsia. 2012;53(10):1746-1755. [DOI] [PubMed] [Google Scholar]
- 33.Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg. 2012;116(5):1042-1048. [DOI] [PubMed] [Google Scholar]
- 34.Jobst BC, Kapur R, Barkley GLet al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005-1014. [DOI] [PubMed] [Google Scholar]
- 35.Salanova V, Sperling MR, Gross REet al. et al. The SANTE study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021;62(6):1306-1317. [DOI] [PubMed] [Google Scholar]