Abstract
Purpose: To analyze changes in Google search volume after US Food and Drug Administration (FDA) approval and initiation of a direct-to-consumer marketing campaign for faricimab-svoa. Methods: Google Trends data between December 26, 2021, and June 17, 2023, were downloaded and searched for terms prominently featured in the marketing campaign, including “wet AMD”, “diabetic macular edema”, “Vabysmo”, and “faricimab-svoa”. Data were standardized to the week and the term with the highest search volume, resulting in weekly relative search volumes for each term. The mean relative search volume and percentage change in relative search volume were calculated for the time periods of interest. Results: The direct-to-consumer campaign was associated with an increase in relative search volume in the first month for the terms “Vabysmo” (2.5% to 18.0%) and “wet AMD” (3.0% to 77.3%) and was sustained in the second month (P < .01). No significant changes in relative search volume were seen for the terms “diabetic macular edema” or “faricimab-svoa”. After FDA approval, “Vabysmo” had the only significant increase in relative search volume (0.3% to 2.8%; P = .02). Conclusions: A direct-to-consumer advertising campaign for faricimab-svoa was associated with a surge in Google search volume of 620% and 2475% for “Vabysmo” and “wet AMD,” respectively (P < .01), without a corresponding increase for “diabetic macular edema”. Although FDA approval was associated with an increase in search volume for “Vabysmo” (P = .02), the marketing campaign was more influential at driving internet search behavior.
Keywords: wet AMD, neovascular age-related macular degeneration, DME, diabetic macular edema, faricimab-svoa, Vabysmo, direct-to-consumer campaign, Google Trends, retina
Introduction
Neovascular age-related macular degeneration (nAMD; also known as wet AMD) and diabetic macular edema (DME) are common retinal vascular diseases associated with vision loss. 1 Intravitreal antivascular endothelial growth factor agents are used as first-line treatments for these conditions.2,3 On January 28, 2022, faricimab-svoa (Vabysmo, Roche/Genentech) was approved by the US Food and Drug Administration (FDA) for the treatment of nAMD and DME. 4 On April 20, 2023, Genentech launched a direct-to-consumer campaign encompassing television, radio, digital, and print 5 to market Vabysmo for the treatment of wet AMD and DME. 3 To our knowledge, the effects of this advertising campaign on the public’s awareness of faricimab-svoa have not been published.
Google Trends is an analytical tool that provides data on internet search volume for specific terms over time. Previous studies used Google Trends to assess public interest and track disease outbreaks in myriad health phenomena.6 –8 In ophthalmology, studies have evaluated public interest in thyroid eye disease, 9 artificial intelligence, 10 and cataract surgery, 11 and detected conjunctivitis epidemics. 12
The aim of our study was to evaluate the influence of the direct-to-consumer campaign on information-seeking behavior as it relates to faricimab-svoa.
Methods
The terms prominently featured on Genentech’s “Open Up Your World” advertisement were identified. 13 Google Trends data were downloaded on June 22, 2023, for the following searches made in the US between December 26, 2021, and June 17, 2023, for the primary terms “wet AMD”, “diabetic macular edema”, “Vabysmo”, and “faricimab-svoa”. Data were normalized and scaled from 0 to 100 to the week with the highest search volume for “wet AMD” (April 23, 2023, to April 29, 2023) to provide a relative search volume for each term. Additional Google Trends data were downloaded on March 31, 2024, for the following searches made in the US between December 26, 2021, and June 17, 2023, for the secondary terms for retinal diseases, including “age-related macular degeneration”, “wet macular degeneration”, “dry macular degeneration”, “macular degeneration”, “geographic atrophy”, “diabetic retinopathy”, “retinal vein occlusion”, and “retinal arterial occlusion” and for the retinal therapeutics “Avastin”, “bevacizumab”, “Eylea”, “aflibercept”, “Lucentis”, “ranibizumab”, “faricimab”, “Syfovre”, and “pegcetacoplan”.
The mean weekly relative search volume values, percentage changes, and statistical analysis were calculated using Excel for Macintosh (version 16.73, Microsoft Corp) and presented descriptively for specific time periods of interest related to the FDA approval of Vabysmo and the direct-to-consumer campaign. Using the Student t test, P < .05 was found to be significant. All mean values are ± SD.
Results
“Vabysmo” is spoken 7 times and displayed 11 times in Genentech’s “Open Up Your World” advertisement, “wet AMD” is spoken 3 times and displayed 3 times, “faricimab-svoa” is displayed twice, and “diabetic macular edema” is displayed once. The terms “Vabysmo” and “faricimab-svoa” are also shown in the corner of the video advertisement for approximately 40 seconds of the 60-second advertisement.
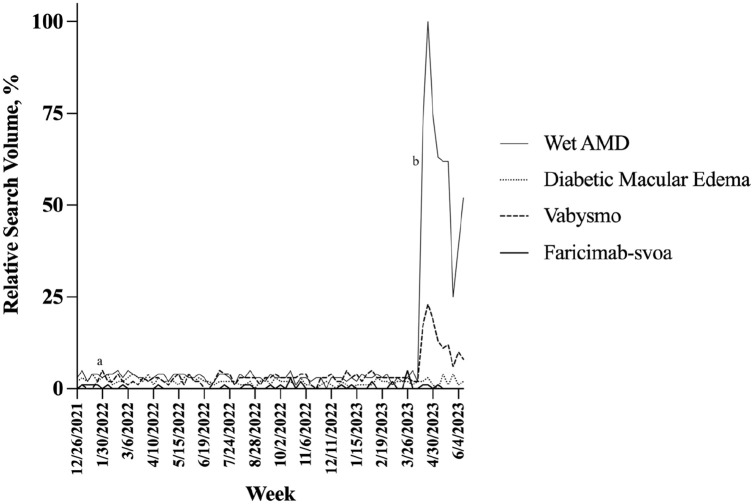
Figure 1 shows the trends in the weekly relative search volume for primary search terms. For the direct-to-consumer campaign, the mean relative search volume was determined for 3 time periods: a baseline 4-week period before the campaign, the first 4 weeks of the campaign, and the 4-week period after the initial 4 weeks of the campaign. Table 1 shows influence of the direct-to-consumer campaign on Google search volume. The change in relative search volume was statistically significant for the terms “wet AMD” and “Vabysmo” for the first month and second month relative to baseline (P < .01) but not significant for the terms “diabetic macular edema” or “faricimab-svoa” at either time period. For secondary search terms, the change in relative search volume was significant for “wet macular degeneration” for the first month and second month relative to baseline (P ≤ .03). There was no significant change in relative search volume for the remaining secondary search terms in the first month or second month relative to baseline for retinal diseases, including “age-related macular degeneration”, “dry macular degeneration”, “macular degeneration”, “geographic atrophy”, “diabetic retinopathy”, “retinal vein occlusion”, and “retinal arterial occlusion”, and for retinal therapeutics “Avastin”, “bevacizumab”, “Eylea”, “aflibercept”, “Lucentis”, “ranibizumab”, “faricimab”, “Syfovre”, and “pegcetacoplan”.
Figure 1.
Search volumes in the United States for “wet AMD”, “diabetic macular edema”, “Vabysmo”, and faricimab-svoa. (a) Faricimab-svoa receives US Food and Drug Administration approval on January 28, 2022. (b) Genentech initiates a direct-to-consumer campaign on April 20, 2023.
Abbreviation: AMD, age-related macular degeneration.
Table 1.
Influence of Direct-to-Consumer Campaign on Google Search Volume.
| Search Term | One Month Before Start of the DTC | First Month After Start of the DTC | Second Month After Start of the DTC | ||
|---|---|---|---|---|---|
| Mean RSV% ± SD | Mean RSV% ± SD | P Value a | Mean RSV% ± SD | P Value a | |
| Wet AMD | 3.0 ± 1.4 | 77.3 ± 16 | <.01 | 47.0 ± 18.2 | <.01 |
| Diabetic macular edema | 1.8 ± 0.5 | 1.5 ± 1.3 | .73 | 2.5 ± 1.7 | .44 |
| Vabysmo | 2.5 ± 0.6 | 18.0 ± 4.2 | <.01 | 9.8 ± 2.6 | <.01 |
| Faricimab-svoa | 1.3 ± 2.5 | 0.8 ± 0.5 | .71 | 0 ± 0 | .36 |
Abbreviations: AMD, age-related macular degeneration; DTC, direct-to-consumer campaign; RSV, relative search volume.
P values were calculated relative to the baseline RSV (1 month before start of the DTC).
For FDA approval, the mean relative search volume was determined for 3 time periods: a baseline 4-week period before FDA approval, the first 4 weeks after FDA approval, and weeks 4 to 8 after FDA approval. Table 2 shows the influence of FDA approval on Google search volume. The change in relative search volume was statistically significant for “Vabysmo” in both the first and second months (P = .02 and P = .03, respectively) but not significant for “wet AMD”, “diabetic macular edema”, or “faricimab-svoa” at either time period.
Table 2.
Influence of FDA Approval on Google Search Volume.
| Search Term | One Month Before FDA Approval | First Month After FDA Approval | Second Month After FDA Approval | ||
|---|---|---|---|---|---|
| Mean RSV% ± SD | Mean RSV% ± SD | P Value a | Mean RSV% ± SD | P Value a | |
| Wet AMD | 3.5 ± 1.3 | 3.8 ± 0.5 | .73 | 4.3 ± 1.0 | .39 |
| Diabetic macular edema | 2.8 ± 1.0 | 2.5 ± 1.3 | .77 | 2.8 ± 1.0 | 1.00 |
| Vabysmo | 0.3 ± 0.5 | 2.8 ± 1.5 | .02 | 2.3 ± 1.3 | .03 |
| Faricimab-svoa | 0.8 ± 0.5 | 0.5 ± 0.6 | .54 | 0.3 ± 0.5 | .21 |
Abbreviations: AMD, age-related macular degeneration; FDA, US Food and Drug Administration; RSV, relative search volume.
P values were calculated relative to the baseline RSV (1 month before start of FDA approval).
Conclusions
This study showed that a direct-to-consumer advertising campaign for faricimab-svoa was associated with a substantial increase in public interest in the drug and “wet AMD”, as measured by the relative Google search volume for the terms most prominently featured in the advertisement. The month after the campaign was initiated, search volume significantly increased by 620% for “Vabysmo” (2.5% to 18.0%) and 2475% for “wet AMD” (3.0% to 77.3%) (P < .01). This heightened interest was sustained in the second month (P < .01), with a 290% increase for “Vabysmo” (2.5% to 9.8%) and a 1467% increase for “wet AMD” (3.0% to 47.0%). Although faricimab-svoa is approved for both wet AMD (ie, nAMD) and DME, Genentech centered the direct-to-consumer efforts on wet AMD. 14 The relative search volume for “diabetic macular edema” was stagnant after the start of the campaign, which may be attributed to Genentech’s strategic focus on wet AMD.
The direct-to-consumer campaign was associated with a greater increase in Google searches for “wet AMD” than for “Vabysmo”. This finding is comparable to a study by Strawbridge et al 9 that analyzed the influence of pharmaceutical advertising on online searches for thyroid eye disease. In May 2021, a brand-name commercial for teprotumumab-trbw (Tepezza) aired on national television, resulting in a surge in Google searches for “thyroid eye disease” and “Tepezza”, with searches for “thyroid eye disease” being comparably higher than searches for “Tepezza”. The drivers underlying these phenomena are unclear. “Wet AMD” may be a more familiar and recallable term than “Vabysmo” and may be more likely to be used as a search term. Alternatively, individuals exposed to the marketing campaign may be less familiar with wet AMD and became interested in learning more before exploring Vabysmo as a treatment option.
Genentech’s marketing campaign had a substantially greater effect on public internet-search behavior than FDA drug approval. Although there was a significant increase (P = .02) in Google search volume for “Vabysmo” (0.3% to 2.8%) in the month after the drug’s FDA approval on January 28, 2022, the overall search volume remained relatively low and there was no significant change in search volume for the associated disease indications of “wet AMD” and “diabetic macular edema”. This finding was similar to that of teprotumumab-trbw, where the direct-to-consumer campaign elicited a surge in Google searches while FDA approval resulted in a relatively muted response. 9
The primary goal of our study was to assess whether Genentech’s marketing campaign was associated with an increase in Google search volume for the terms prominently featured in the advertisement. We also assessed the relative search volumes for other retinal drugs and diseases to assess the potential for confounding factors. Aside from a significant increase in search volume for the term “wet macular degeneration” after the initiation of the advertising campaign, there was no significant change in relative search volume for retinal diseases, including “age-related macular degeneration”, “dry macular degeneration”, “macular degeneration”, “geographic atrophy”, “diabetic retinopathy”, “retinal vein occlusion”, and “retinal arterial occlusion” or for retinal therapeutics “Avastin”, “bevacizumab”, “Eylea”, “aflibercept”, “Lucentis”, “ranibizumab”, “faricimab”, “Syfovre”, and “pegcetacoplan”. We chose not to assess search volume change for “DME” and “AMD” given the predominance of search results that included durable medical equipment and Advanced Micro Devices Inc.
The pharmaceutical industry is a major force in the advertising industry, with exponential growth in expenditures. Total spending on direct-to-consumer marketing was $12 million in 1980, $1.2 billion in 1998, 15 and $5 billion 2021. 16 This prolific growth was vitalized in the 1990s after a decision by the FDA to ease regulations on broadcast marketing, eliminating the requirement that advertisements disclose all risks detailed in a drug’s labeling and requiring disclosure only of “major risks” if the advertisement provides resources where consumers can access the full risk information, such as a website or a toll-free telephone number. 17
Pharmaceutical direct-to-consumer marketing is controversial and barred in all countries worldwide except the United States and New Zealand. Proponents cite studies showing that advertisements can educate and empower consumers, strengthen relationships with healthcare providers, and improve compliance with treatments.17,18 Critics point to data indicating that marketing efforts can lead to patient misinformation, overemphasize benefits and underemphasize risks, encourage overuse and inappropriate prescribing, and strain the patient–physician relationship. 15 A study by Bell et al 19 assessing patients’ responses to physician nonfulfillment of an advertising-induced prescription request found that 46% of patients felt disappointed, approximately 25% anticipated resorting to persuasion and seeking the prescription elsewhere, and 15% considered terminating their relationship with the physician. Not surprisingly, an FDA study 18 found that nearly half of physicians surveyed felt pressure to prescribe the medication advertised when prompted by patients.
The American Medical Association has campaigned to ban direct-to-consumer marketing, emphasizing that direct-to-consumer advertisements drive demand for costly medications, despite the clinical effectiveness of less costly alternatives. 20 Medicare Part B drug spending is principally driven by 3 medical specialties (oncology, ophthalmology, and rheumatology), which accounted for 21%, 11%, and 6% of expenditures in 2021, respectively. 21 Ophthalmology drug spending had the fastest growth rate relative to all other specialties from 2008 to 2021, expanding at an annual rate of 15%. The top 10 Medicare part B drug expenditures in 2021 included aflibercept (number 2) and ranibizumab (number 6), with costs of $2.7 billion and $0.8 billion, respectively.
This study has several limitations. Causal relationships cannot be inferred from the use of Google Trends data. In addition, analyzing Google Trends data through relative search traffic provides a measure of public interest rather than a direct reflection of public awareness regarding a particular subject. The population using the internet and Google for searches may not be representative of the population exposed to Genentech’s advertising campaign. It is not possible to discern the precise demographics of those performing internet searches or to determine whether volume is driven by retina patients, healthcare providers, etc. In addition, our study period coincided with the initiation of a marketing campaign for pegcetacoplan (Syfovre, Apellis Pharmaceuticals) for the treatment of geographic atrophy secondary to atrophic macular degeneration. This advertising campaign generated searches for “wet AMD” by individuals who became aware of macular degeneration and subsequently performed searches for the subtypes of macular degeneration, although the term “wet AMD” was not specifically used in the advertisement. Nonetheless, Google Trends remains a valuable tool for examining public engagement with specific topics.
In summary, this descriptive cross-sectional study found that the direct-to-consumer campaign for faricimab-svoa was associated with a surge in Google search volume for “wet AMD” and “Vabysmo” without a corresponding increase in search volume for “diabetic macular edema”. The public’s interest was markedly higher for “wet AMD” than for “Vabysmo”. FDA approval of faricimab-svoa resulted in only a small increase in absolute search volume for “Vabysmo”. Further studies assessing the impact of direct-to-consumer advertising on ophthalmology drug use and clinical disease management would be beneficial.
Footnotes
Ethical Approval: Ethical approval was not required for this study.
Statement of Informed Consent: Informed consent was waived for the present study.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Clarisa Marie P. Bloemhof  https://orcid.org/0009-0009-3708-1186
https://orcid.org/0009-0009-3708-1186
References
- 1. GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144-e160. doi: 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakri SJ, Thorne JE, Ho AC, et al. Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(1):55-63. doi: 10.1016/j.ophtha.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 3. Kim EJ, Lin WV, Rodriguez SM, Chen A, Loya A, Weng CY. Treatment of diabetic macular edema. Curr Diab Rep. 2019;19(9):68. doi: 10.1007/s11892-019-1188-4 [DOI] [PubMed] [Google Scholar]
- 4. Shirley M. Faricimab: first approval. Drugs. 2022;82(7):825-830. doi: 10.1007/s40265-022-01713-3 [DOI] [PubMed] [Google Scholar]
- 5. O’Brien J. Genentech opens up eye patients’ world with Vabysmo. April 20, 2023. Accessed June 21, 2023. https://www.mmm-online.com/home/channel/genentech-opens-up-eye-patients-world-with-vabysmo/
- 6. Yang SC, Weinberger JM, Shahinyan RH, Shahinyan GK, Mills JN, Eleswarapu SV. Regenerative therapies for erectile dysfunction: the influence of direct-to-consumer marketing on patient interest. Transl Androl Urol. 2023;12(4):586-593. doi: 10.21037/tau-22-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gazendam A, Nucci N, Ekhtiari S, et al. Trials and tribulations: so many potential treatments, so few answers. Int Orthop. 2020;44(8):1467-1471. doi: 10.1007/s00264-020-04625-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mavragani A, Ochoa G. Google trends in infodemiology and infoveillance: methodology framework. JMIR Public Health Surveill. 2019;5(2):e13439. doi: 10.2196/13439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strawbridge JC, Meer EA, Singh P, Rootman DB. Google searches for thyroid eye disease after regulatory approval of teprotumumab. JAMA Ophthalmol. 2022;140(6):639. doi: 10.1001/jamaophthalmol.2022.1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahuja AS, Rahimy E, Sridhar J. Tracking online interest in artificial intelligence in ophthalmology using google trends. Semin Ophthalmol. 2023;38(7):644-647. doi: 10.1080/08820538.2023.2204919 [DOI] [PubMed] [Google Scholar]
- 11. Skrzypczak T, Jany A, Michałowicz J, Hossa M, Bogusławska J, Targonska M. Public interest in cataract surgery: analysis and implications of google trends data from 14 European countries. Ophthalmic Epidemiol. 2022;29(1):108-115. doi: 10.1080/09286586.2021.1904513 [DOI] [PubMed] [Google Scholar]
- 12. Deiner MS, McLeod SD, Wong J, Chodosh J, Lietman TM, Porco TC. Google searches and detection of conjunctivitis epidemics worldwide. Ophthalmology. 2019;126(9):1219-1229. doi: 10.1016/j.ophtha.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vabysmo Commercial. YouTube. April 22, 2023. Accessed March 31, 2024. https://www.youtube.com/watch?v=f8h0iHg1wDg
- 14. Klahr Coey S. Roche’s Genentech opens up the world with new ad for Vabysmo. April 25, 2023. Accessed June 21, 2023. https://www.fiercepharma.com/marketing/roches-genentech-opens-world-new-ad-vabysmo
- 15. Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36(10):669-684. [PMC free article] [PubMed] [Google Scholar]
- 16. Adams B. Pharma bucks trend, shunning digital and sticking with TV when it comes to ads. May 24, 2022. Accessed June 21, 2023. https://www.fiercepharma.com/marketing/tv-and-diabetes-dominated-2021s-pharma-ad-spend-industrys-spending-growth-stayed-flat
- 17. Frosch DL, Grande D, Tarn DM, Kravitz RL. A decade of controversy: balancing policy with evidence in the regulation of prescription drug advertising. Am J Public Health. 2010;100(1):24-32. doi: 10.2105/AJPH.2008.153767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aikin KJ, Swasy JL, Braman AC. Patient and physician attitudes and behaviors associated with DTC promotion of prescription drugs—summary of FDA survey research results. November 19, 2004. Accessed June 22, 2023. https://www.fda.gov/media/112016/download
- 19. Bell RA, Wilkes MS, Kravitz RL. Advertisement-induced prescription drug requests: patients’ anticipated reactions to a physician who refuses. J Fam Pract. 1999;48(6):446-452. [PubMed] [Google Scholar]
- 20. American Medical Association. AMA calls for ban on DTC ads of prescription drugs and medical devices. November 17, 2015. Accessed June 21, 2023. https://www.ama-assn.org/press-center/press-releases/ama-calls-ban-dtc-ads-prescription-drugs-and-medical-devices
- 21. Nguyen NX, Olsen TA, Sheingold SH, De Lew N. Medicare Part B drugs: trends in spending and utilization, 2008-2021. June 9, 2023. Accessed June 21, 2023. https://aspe.hhs.gov/sites/default/files/documents/fb7f647e32d57ce4672320b61a0a1443/aspe-medicare-part-b-drug-pricing.pdf [PubMed]