Abstract
Introduction.Strongyloides stercoralis, the human threadworm, is a parasitic nematode with global distribution, estimated to infect over 600 million people. Chronic infection is often asymptomatic, but hyperinfection and dissemination syndromes can occur in the immunosuppressed with high case fatality rates. Whilst strongyloidiasis is endemic in Fiji, its prevalence in Fijian migrant groups in the UK is unknown.
Gap Statement. No previous studies have been conducted on the prevalence of Strongyloides and other gastrointestinal parasites (GIPs) in Fijian migrants to the UK.
Aim. We conducted a cross-sectional study of the prevalence of GIPs in a Fijian migrant population.
Methodology. Participants completed a questionnaire on residence, travel and clinical symptoms and were asked to provide a serum sample for S. stercoralis IgG ELISA, venous blood samples for eosinophil count and a faecal sample for charcoal culture, multiplex real-time PCR (rtPCR) and microscopy after formalin-ethyl acetate concentration. Sequencing was performed on pooled Strongyloides larvae for nuclear 18S rRNA hyper-variable regions (HVRs) I and IV.
Results. A total of 250 participants (94% male) with median (range) age 37 (20–51) years entered the study, 15 (1–24) years since leaving Fiji. S. stercoralis IgG ELISA was positive in 87/248 (35.1 %) and 14/74 (18.9 %) had a GIP detected in faeces. This included 7/74 (9.5 %) with Strongyloides and 5/74 (6.8 %) with hookworms. Dermatological symptoms were more common in those with Strongyloides, and eosinophilia (>0.5×109 cells per litre) was present in 55.6% of those with positive S. stercoralis IgG. rtPCR was the most sensitive faecal diagnostic test for Strongyloides and hookworms in faeces. Sequences of nuclear 18S rRNA for HVRs I and IV confirmed the presence of S. stercoralis.
Conclusion. This first cross-sectional study in Fijian migrants found a high rate of chronic infection with GIPs, particularly S. stercoralis. Faecal microscopy was insensitive compared to charcoal culture, rtPCR or serology, demonstrating the importance of specialist parasitological tests when investigating people with a suspected chronic infection. Our study highlights an overlooked burden of strongyloidiasis in the UK and has implications for screening and treatment programmes in Fiji and for migrants from Fiji.
Keywords: eosinophilia, Fiji, helminth, migrant, Strongyloides
Introduction
Strongyloides stercoralis, the human threadworm, is a parasitic nematode that is estimated to infect over 600 million people [1]. Until recently, the global burden of Strongyloides infection was underestimated, probably due to the dominant use of diagnostic tools, such as the Kato-Katz method, which have poor sensitivity [2]. Diagnosis remains a challenge, with no agreed ‘gold standard’ diagnostic test [3]. The growing attention on Strongyloides as a pathogen is now evidenced by its formal inclusion within the soil-transmitted helminth (STH) group, as part of the World Health Organization’s strategy for control of neglected tropical diseases [4].
S. stercoralis has an auto-infective nature to its life cycle and infection can last for decades. Many infected individuals are asymptomatic [5], but some may experience recurrent gastrointestinal, dermatological and respiratory symptoms. In those who are immunosuppressed, particularly by corticosteroid therapy, a life-threatening hyperinfection and dissemination syndrome [5] can occur with a case fatality of around 50% [6]. Strongyloides infection may therefore present a risk to those who have travelled from an endemic to a non-endemic area for many years after migration, and diagnostic awareness may not be high amongst clinicians.
Surveillance data on gastrointestinal parasites (GIPs) infection in migrants to the UK are limited; most studies are performed on patient populations who have presented to healthcare settings, often after a finding of eosinophilia [7,9]. Routine screening for GIPs has been offered to Nepalese Gurkhas, a migrant group within the UK Armed Forces, since 2012 [10]. A recent retrospective study of this group found GIPs in 20.5% and Strongyloides seropositivity in around 5% [11]. Fijian-born personnel represent the second largest group of migrants from a GIP-endemic nation in the UK Armed Forces. An estimated 1750 British military personnel had Fijian recorded as their nationality in 2021 [12].
There are a few formal surveillance data on the prevalence of Strongyloides in adults in Fiji or in the Pacific Islands in general, and data in any age group are extremely limited. Coincident with surveillance of STH, studies have focused on school-age children, and most are several decades old [13]. It is possible that the prevalence of STH in Fiji has been reduced by longitudinal lymphatic filariasis (LF) control programmes [14], although pockets of filariasis still persist [15,16] and might cause cross-reactivity in S. stercoralis serological tests. Data on Fijian migrants are also rare, although a recent retrospective study of patients with Strongyloides in Auckland, New Zealand, found that a high proportion came from Pacific islands including Fiji [17].
Following the incidental diagnoses of S. stercoralis infections in several Fijian-born UK Armed Forces personnel who had lived in the UK for several years [18], we postulated that there is an unrecognized burden of asymptomatic infection in the UK. We undertook the first cross-sectional study of GIP infection in a population of Fijian migrants to the UK, using a combination of eosinophil counts, serology and traditional and molecular faecal diagnostic techniques.
Methodology
Participant recruitment
We recruited Fijian-born personnel from throughout the UK Armed Forces by advertising through primary care centres, augmented by word of mouth through excellent social networks in this group. Study days were held at five locations throughout the UK for potential participants in 2021–2023. Participants were eligible if they were over the age of 18, were currently serving in the UK Armed Forces and had migrated to the UK from Fiji. Following the provision of written informed consent, participants were asked to fill in a brief questionnaire about prior residence and travel and relevant persistent symptoms, including diarrhoea, abdominal pain, blood in faeces, rashes, cough or shortness of breath and unexplained weight loss and fevers, and to provide venous blood samples and a faecal sample. For the research analysis, questionnaires and samples were anonymized with a participant number, linked to diagnostic samples for the same individual.
Data from the first blood and faecal tests are analysed in this report. All those with a positive/equivocal serological or faecal result or eosinophilia were invited for full clinical review and treatment as indicated, and limited diagnostic results following these visits are also discussed separately.
Differential white cell count
Samples of venous blood in EDTA were transported at room temperature within 24 h to the clinical haematology laboratory at the Royal Liverpool University Hospital, where differential white blood cell counts were performed as per standard protocols. Eosinophilia was defined as a peripheral blood eosinophil count >0.5×109 l−1 [19].
S. stercoralis IgG ELISA
Clotted blood samples were transported at room temperature within 24 h and refrigerated on arrival at the Clinical Diagnostic Parasitology Laboratory of the Liverpool School of Tropical Medicine. An aliquot of serum was collected from each sample. IgG antibodies to S. stercoralis were detected using a commercial S. stercoralis IgG ELISA (DRG Instruments, Marburg, Germany) and processed as described by Nevin et al. [11]. The threshold of positivity was defined as an optical density (OD) of ≥0.200. High negative values (−) were defined as an OD value between 0.185 and 0.199. Weak positives (+) were defined as an OD value between 0.200 and 0.249. Standard positives were defined as an OD value between 0.250 and 0.999 (++). A strong positive (+++) was defined as an OD value ≥1.000. This approach was informed by comparison with a previously designed in-house IgG ELISA and validated using known positives, known negatives, known cross-reactive samples and samples used in a national laboratory evaluation scheme [11].
Faecal analysis
Faecal samples were transported unrefrigerated without preservatives and were typically processed within 18–24 h of passage. Each sample was examined by light microscopy after formalin-ethyl acetate (FEA) concentration, and a faecal charcoal culture was performed as described by Nevin et al. [11].
Multiplex real-time PCR
Faecal samples underwent DNA extraction and real-time PCR (rtPCR) using the method described by Cunningham et al. [20]. Faecal samples were screened using a two-tube rtPCR assay, with Reaction 1 screening for Giardia duodenalis, Cryptosporidium spp., Entamoeba histolytica, Entamoeba dispar and the phocine herpesvirus (PhHV-1) internal positive control. Reaction 2 screened for Ascaris lumbricoides, Trichuris trichiura, Strongyloides spp., Schistosoma spp. and hookworms (both Ancylostoma duodenale and Necator americanus). These two assays used pre-published primers and probes [21] and were carried out in 20 µl reactions on the Rotor-Gene platform (Qiagen, Manchester, UK). For a single 20 µl, 2 µl of DNA template and 10 µl of iQ Supermix (Bio-Rad, Watford, UK) were used. The thermocycler conditions were as follows: hold at 95 °C for 15 min and then 50 cycles of 94 °C for 15 s, followed by 60 °C for 60 s. A cycle threshold (Ct) cut-off value of 38 was introduced and samples with a Ct of <38 were considered positive and those of ≥38 were considered equivocal. A negative test was defined as no amplification in the presence of an appropriate internal positive control reaction.
Molecular analyses
A sample of pooled Strongyloides larvae was harvested from the charcoal cultures of three participants. Each sample was centrifuged at 5000 r.p.m. for 5 min and a 10 µl pellet was taken. DNA was extracted by adding 48 µl of Tris-EDTA (TE) buffer and 2 µl of proteinase K (20 mg µl−1) to each sample. This was vortexed for 10–20 s and then centrifuged for 10–20 s at 7000 r.p.m., followed by incubation for 60 min at 56 °C. Samples were then vortexed and centrifuged again prior to further incubation for 15 min at 93 °C.
Hyper-variable regions (HVRs) I and IV of the nuclear 18S rRNA gene were targeted for sequencing, using published primers and the thermal PCR cycle from Barratt et al. [22] Primers are shown in Table S1 (available in the online version of this article).
Amplified DNA was separated by gel electrophoresis and suitable amplicons with clear visible bands were excised. The excised bands were centrifuged at 8000 r.p.m. for 5 min through a high-density polyethylene filter to separate the liquid DNA product, and the assessment of DNA concentration was performed using NanoDrop ND-1000. The product was purified using ExoSAP-IT (Thermo Fisher, Waltham, MA, USA).
Sanger sequencing was performed by Source BioScienceTM. Sequences were analysed and aligned using mega 11 software. Low-quality reads at the ends of chromatograms were trimmed and erroneous base calls were corrected manually. The nucleotide Basic Local Alignment Search Tool (blast) for nucleotides was used to find the most similar sequences from the National Center for Biotechnology Information non-curated core_nt reference database.
Statistical analysis
Prior residence in Fiji was classified by respondents as predominantly urban, rural or both. Participants were also stratified by reported residence in a province with a medium human development index (HDI) or one with a high HDI, according to the Subnational Human Development Database [23]. Data were analysed using SPSS V28 and GraphPad Prism 10 software. Odds ratios (ORs) were calculated using single and multiple logistic regression analyses, with the exception of the OR for detection of GIPs in those with non-pathogenic protozoa and OR for symptoms in participants with positive faecal tests for S. stercoralis, both of which were calculated via direct comparison of odds due to the small group sizes. For comparisons between populations, either a χ2 test was used or a Fisher’s exact test if any of the groups had an N of <5. For comparison of continuous variables, the Mann–Whitney U test or the Kruskal–Wallis test was used as appropriate due to the non-normal distribution of data.
Results
Demography and symptoms
Out of 250 participants recruited, 235 (94.0 %) were male; the median (range) age was 37 (20–51) years. Age was not normally distributed (Fig. S1). The country of birth was available for 244 participants, of whom 242 (99.2 %) were born in Fiji. All participants had migrated to the UK between 1998 and 2021, with a median (range) 15 (1–24) years since migration (interquartile range 3–20). The majority (160/244, 65.6%) also had a history of travel to another S. stercoralis endemic country. Of the 224 participants who provided further details about their previous residence in Fiji, 129 (52.9 %) had primarily lived in an urban environment, 100 (41.0 %) in a rural environment and 15 (6.1 %) split their time equally between a rural and an urban environment. Full details of previous residence in Fiji and other travel destinations are provided in Tables S5 and S6.
One or more persistent symptoms were reported by 94/244 [38.5 %, 95 % confidence interval (CI) 32.6–44.8] participants. This included 52 (21.3 %) with gastrointestinal symptoms, 42 (17.2 %) with rashes, 33 (13.5 %) with cough or shortness of breath, 42 (17.2 %) with subjective fevers and 4 (1.6%) with weight loss (Table S7). Associations between symptoms and diagnostic results are presented later.
Faecal results
Seventy-four participants provided a stool sample, of whom 14 (18.9 %) had at least one faecal test positive/equivocal for a GIP (Table 1). The most common pathogen was S. stercoralis, in seven cases (9.5%), followed by the hookworm spp. in five (6.8 %), E. histolytica and E. dispar co-infection in one (1.4%) and one equivocal rtPCR result for G. duodenalis. If rtPCR results above the clinical Ct cut-off value (≥38) are discounted, we identified GIP infection in eight participants (10.8%, 95 % CI 5.3–20.2); four S. stercoralis (5.4%), three hookworms (4.1%) and a single E. histolytica case. No multiple pathogen infections were found.
Table 1. Summary of positive/equivocal faecal tests in 14 study participants with GIPs.
| Organism | Participants with any positive/equivocal faecal result | rtPCR | FEA and microscopy | Charcoal culture | ||
| No. | Prevalence,% (95 % CI) | Positive | Equivocal | |||
| S. stercoralis | 7 | 9.5 (4.4–18.5) | 4 | 3 | 1 | 2 |
| Hookworm | 5 | 6.8 (2.6–15.2) | 3 | 2 | 0 | 1 |
| E. histolytica | 1 | 1.3 (0.0–8.0) | 1 | 0 | 1 | |
| G. duodenalis | 1 | 1.3 (0.0–8.0) | 0 | 1 | 0 | |
| Any | 14 | 18.9 (11.5–29.4) | 8 | 6 | 2 | |
CIConfidence intervalFEAFormalin-ethyl acetate
S. stercoralis
All seven participants with a positive/equivocal faecal test for S. stercoralis had DNA detected on rtPCR (4/7 positive, 3/7 equivocal); 2/7 were positive on charcoal culture and 1/7 was positive on FEA microscopy. All had a positive S. stercoralis IgG ELISA (median OD 1.604) and eosinophilia (Table 2). As such, all those with an equivocal S. stercoralis rtPCR were considered as likely true positive results for the purpose of further analyses discussed later. The median eosinophil count of 1.4×109 l−1 was significantly higher than that of participants with negative faecal results (median 0.2×109 l−1, P<0.001). The median (range) age of the seven participants was 38 (24–42) years and the median (range) time since migration to the UK was 16 (1–21) years; 3/7 participants had not travelled to any other S. stercoralis endemic country.
Table 2. Diagnostic test results in seven participants with positive/equivocal faecal rtPCR for S. Stercoralis.
For definitions of semi-quantitative results for faecal PCR and serological results, see text.
| Age (years) | Years since migration to UK | Eosinophil count (109 l−1) | FEA microscopy | Charcoal culture | rtPCR | S. stercoralis IgG ELISA OD | S. stercoralisIgG ELISA result |
| 38 | 16 | 0.7 | + | + | + | 1.037 | +++ |
| 42 | 20 | 1.7 | − | + | + | 2.642 | +++ |
| 29 | 1 | 2.5 | − | − | + | 0.626 | ++ |
| 24 | 2 | 3.0 | − | − | + | 0.351 | ++ |
| 25 | 3 | 1.3 | − | − | Equivocal | 1.929 | +++ |
| 29 | 21 | 1.4 | − | − | Equivocal | 1.825 | +++ |
| 41 | 19 | 0.7 | − | − | Equivocal | 1.604 | ++ |
Other GIPs
Five individuals had a positive or equivocal faecal test for hookworm species. The median age of these five individuals was 29 years. Three had eosinophilia and the median (range) eosinophil count was 0.5 (0.1–4.0) ×109 l−1, not significantly different from participants with negative faecal results. The longest time reported by a hookworm-positive participant since the last being in Fiji was 3 years. Details of those with a positive hookworm result can be found in Table S3.
One participant had infection with E. histolytica and one other had an equivocal PCR result for G. duodenalis. Protozoa that have disputed pathogenicity or are generally thought to be non-pathogenic were found in 6/74 (8.1 %) participants. The presence of non-pathogenic protozoa was not significantly associated with having a positive/equivocal faecal test for a pathogenic GIP (OR 0.85, 95 % CI 0.07–6.29, P=1.00).
S. stercoralis IgG ELISA
The S. stercoralis IgG ELISA was positive in 87/248 (35.1 %, 95 % CI 29.4–41.2) participants (Table S2). There was no difference in the median ages of those with a positive IgG ELISA (39 years) and those with a negative IgG ELISA (36 years, P=0.108).
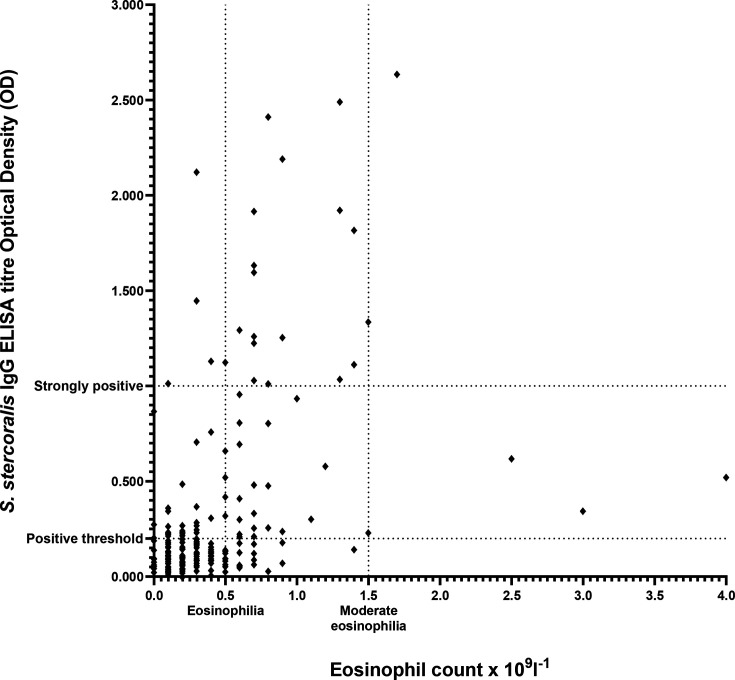
Overall, 68/229 (29.7 %, 95 % CI 24.1–35.9) participants had eosinophilia, which was more common in those with a positive S. stercoralis IgG ELISA (45/81, 55.6%, 95 % CI 44.7–65.9) compared to 23/148 (15.5 %, 95 % CI 10.52–22.3) with a negative ELISA (P<0.001). The median eosinophil count was significantly higher in those with a standard positive (0.5×109 l−1) or strongly positive (0.7×109 l−1) S. stercoralis IgG ELISA compared to those with negative or weakly positive results (both 0.2×109 l−1) (P<0.001, P=0.035, P<0.001 and P<0.001, respectively, Kruskal–Wallis test). There was no significant difference in median eosinophil counts between those that were IgG negative and those that were IgG weakly positive (P=1.00). There was a moderate positive correlation between increasing S. stercoralis IgG ELISA serological titre and increasing eosinophil count (Spearman’s rank-order correlation rs=0.461, P<0.001) (Fig. 1).
Fig. 1. Scatter plot of S. stercoralis IgG ELISA OD versus eosinophil count. Thresholds of S. stercoralis positivity (OD≥0.200) and strong positivity (≥1.000) are shown on the y-axis. Thresholds of eosinophilia (>0.5×109 l−1) and moderate eosinophilia (>1.5×109 l−1) are shown on the x-axis.
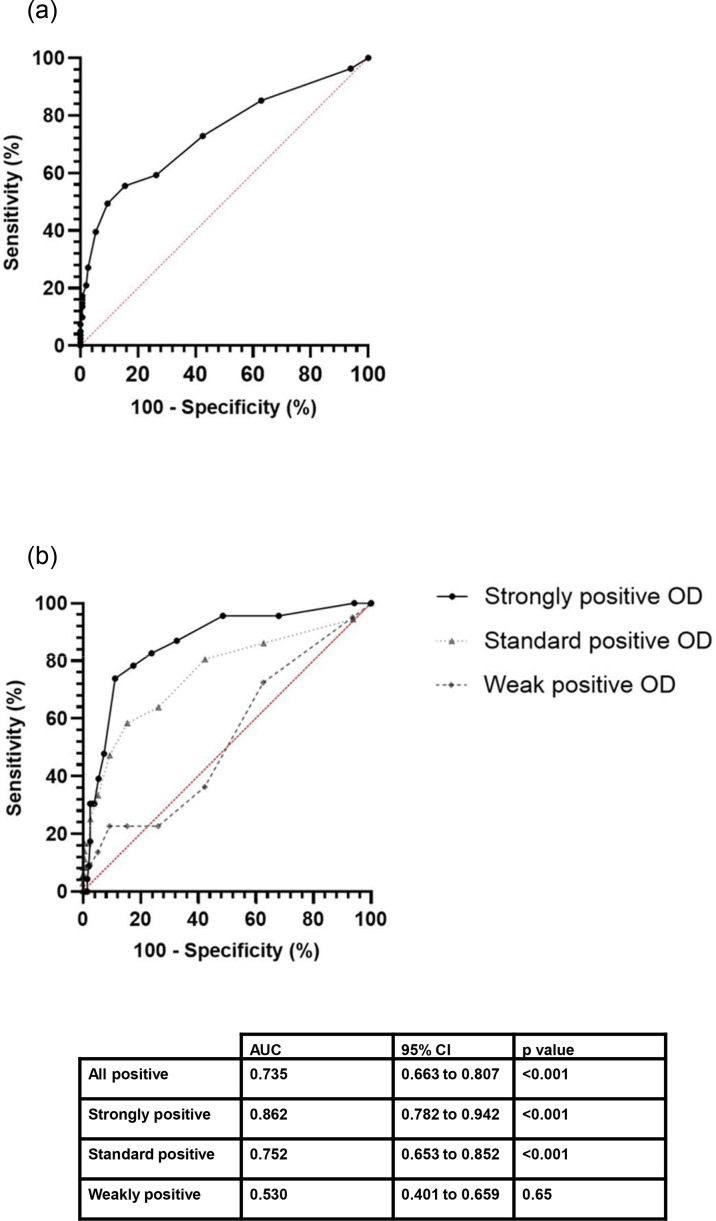
Receiver operating characteristic (ROC) curves were created to explore the sensitivity and specificity of eosinophil count as a diagnostic test to predict positivity of S. stercoralis IgG ELISA (Fig. 2]) if a diagnostic cut-off eosinophil count of 0.5×109 l−1 was used, sensitivity was 55.6% (95 % CI 44.7–65.9) and specificity was 84.5% (95 % CI 77.8–89.4).
Fig. 2. ROC curves for eosinophil count prediction for S. stercoralis IgG ELISA IgG positivity. All positive serological results are grouped together on graph A. Graph B stratifies serological results, by whether they were strongly positive, standard positive and weakly positive results (see text for definitions). AUC, area under the curve.
Comparison of Strongyloides diagnostic methods
The sensitivity and specificity of each diagnostic method for S. stercoralis were compared against two reference standards: positive/equivocal rtPCR and positive S. stercoralis IgG ELISA. All those with an equivocal S. stercoralis rtPCR were considered as positive results for the purpose of this analysis (Table 3).
Table 3. Sensitivity and specificity of diagnostic tests versus (i) rtPCR and (ii) S. stercoralis IgG ELISA.
Values used for the calculation of sensitivity and specificity can be found in Table S4.
| Versus rtPCR | Versus IgG ELISA | |||||
| Charcoal culture | FEA microscopy | IgG ELISA | Charcoal culture | FEA microscopy | rtPCR | |
| Sensitivity, % (95 % CI) | 28.6(3.7–71.0) | 14.3(0.4–57.9) | 100(59–100.0) | 5.9(0.7–19.7) | 2.9(0.07–15.3) | 20.6(8.7–37.9) |
| Specificity, % (95 % CI) | 100.0(94.6–100.0) | 100.0(94.6–100.0) | 58.5(45.6–70.6) | 100.0(90.8–100.0) | 100.0(90.8–100.0) | 100.0(90.8–100.0) |
ROC curves were created to explore the sensitivity and specificity of eosinophil count to predict positive/equivocal faecal tests for S. stercoralis. The area under the curve was 0.950 (95 % CI 0.897–1.000), suggesting that rising eosinophil count was a good predictor of faecal positivity for S. stercoralis (Fig. S3).
Migration and travel history
Logistic regression was performed to examine possible associations between a positive S. stercoralis IgG ELISA and travel to an endemic area, living in a rural environment or living in a medium HDI province. When analysed individually, both living in a rural environment and living in a medium HDI province were associated with seropositivity, but only living in a rural area remained significant in the multivariate analysis (OR 2.04, P=0.02). Full results of logistic regression can be found in Table S8. Of the participants who provided a faecal sample, 16 had no travel history apart from Fiji and the UK, and of these 3/16 had S. stercoralis. Of the participants who provided a serological sample, 84 had never travelled to another endemic area, and 23/84(27.4 %) had a positive S. stercoralis IgG result.
Symptomatology
One or more symptoms were reported by 94/244 (38.5 %, 95 % CI 32.6–44.8) participants overall, and by all 7 (100 %) with a positive/equivocal faecal test for S. stercoralis, compared to 27/65 (41.5 %) who did not (P=0.004). Of those with positive S. stercoralis IgG, 49/86 (57.0 %, 95 % CI 46.4–66.9) reported symptoms compared to 45/157 (28.7 %, 95 % CI 22.2–36.2) of those with negative serology. Although the presence of any symptoms, GI symptoms and rashes were all associated with positive S. stercoralis IgG ELISA seropositivity in univariate logistic regression analysis; the only significant association remaining after multivariate analysis was the presence of rashes (OR 3.44, P<0.01). Full results of logistic regression can be found in Table S9.
Two participants described classic larva currens rashes [24]. Both had strongly positive S. stercoralis IgG ELISA titres and eosinophilia. One was positive for S. stercoralis in all faecal tests and one had negative faecal examinations.
Molecular identification of species
18S rRNA HVR I
Each sample yielded identical 435 bp sequences. A nucleotide blast search most closely matched all three sequences with S. stercoralis, accession number LC537179.1 [25], with a 100% query cover and 100% identification. This sequence was obtained from Strongyloides taken from faecal samples of healthy volunteers in Myanmar. Strongyloides stercoralis sequences from a captive Sumatran orangutan (OM423625.1) [26] and environmental dog faeces (MK778085.1) in Australia [26] also had a 100% identification match (with 100 and 99% query cover, respectively). The most closely related genotype, according to the classification of Barratt et al. was II [22].
18S rRNA HVR IV
Each sample gave identical 255 bp sequences. Nucleotide blast found a high number of matches with S. stercoralis sequences with 100% query cover and 100.00 % identification. The top match was with accession number MN498130.1, sourced from faecal samples of dogs in Thailand, followed by MN498129.1, sourced from faecal samples of human agricultural workers in Thailand [27], and MK468664.1, sourced from environmental dog faeces in Australia [28]. The sequences corresponded to genotype variant A [29].
Discussion
We believe this to be the first cross-sectional study of GIP infection in Fijian migrants to any country, and it is the first study in any Fijian population to use a combination of traditional faecal diagnostics with molecular and serological investigations. We have demonstrated a high rate of infection, with 18.9% of participants having a positive or equivocal faecal test for a pathogenic GIP and 35.1% having a positive S. stercoralis IgG ELISA. These findings highlight the need for wider surveillance and treatment programmes for Strongyloides in Fiji and for diagnostic surveillance of strongyloidiasis in migrants to the UK.
There are limited data on the current prevalence of S. stercoralis within Fiji. A study of schoolchildren published in 2016 found a single positive case using the Baermann method. The same study examined 173 faecal samples using PCR, reporting a prevalence of 3.5% [30]. Whilst the authors of that paper did not describe a Ct cut-off, they used the same method described by Verweij et al., who included all values in their analyses [31]. Using the same primers, 7/74 (9.5 %) of our study population had positive/equivocal faecal results, including two with positive cultures and one positive on microscopy. Older reports demonstrated a higher prevalence of Strongyloides, with rates up to 50% depending on location [13,32, 33]. It is possible that rolling out mass drug administration (MDA) for LF from 2001 [34] may have reduced STH infection rates, as demonstrated in other countries [14].
Recent data for other STHs are also limited. Prevalence rates of about 10–20 % have been demonstrated using Kato-Katz and FEA microscopy [34,35], with older reports describing much higher rates of infection [32]. In contrast, we found a hookworm prevalence of 6.8%. One reason for the lower prevalence in our population may be that participants had no further exposure following migration to the UK and had cleared infections without treatment. Of those in our study population who had migrated to the UK within the last 10 years and provided a stool sample, 6/24 (25 %) had a positive/equivocal faecal result for STH (three each with hookworm or S. stercoralis). Our study should not, therefore, be taken as evidence of a low hookworm prevalence amongst adults in Fiji.
We found that reporting prior residence in a predominantly rural location was associated with having a positive Strongyloides serological test. However, STH infections in Fiji have been reported in both urban and rural areas [36,37].
Whilst we believe this is the first cross-sectional study on migrants from Fiji, Cutfield et al. performed a 10-year retrospective analysis of 691 Strongyloides patients in New Zealand, of whom 19% were born in Fiji. They described a similar rate of seropositivity (39%) to that found in our study. Whilst their work was on a retrospective patient cohort with a higher median age (63), it supports our findings of high rates of S. stercoralis in Fijian migrants. They also described a much higher rate of positive microscopy of 69/250 compared with 1/74 in our study. Their participants were a patient population, most of whom were immunosuppressed and so may have had higher faecal positivity rates [17]. Our positive microscopy rate (1.4%) was similar to that found in a global meta-analysis of Strongyloides infections in migrants (1.8%). However, we found a much higher seroprevalence (35.1%) versus the 17.3% previously reported in migrants from east Asia and the Pacific [38].
Previous studies in Fiji have largely relied on microscopic techniques, without access to culture or molecular methods. Microscopy has been reported to be insensitive for chronic Strongyloides [11,21, 39], consistent with our findings; only 1/7 case of S. stercoralis was found using FEA microscopy. The use of the Baermann technique or a culture method such as charcoal or agar can improve sensitivity [40,41], and we found further cases on charcoal culture compared with microscopy alone (2/7).
rtPCR was the most sensitive faecal test method and was also highly specific. For Strongyloides, all cases found via charcoal culture or microscopy were also detected by rtPCR. This was supported by findings at a clinical follow-up; faecal samples were provided by further 36 patients who had not yet given a sample. S. stercoralis was detected in 8/36 of them by PCR, of whom three had positive charcoal culture and one had positive FEA microscopy. Two hookworm positives were also found, both PCR positive, and one positive on charcoal culture and FEA. Whilst multiple different PCRs have been developed for Strongyloides, few have undergone validation [42]. However, the assay we used, developed by Verweij et al. [31], is one of the most widely utilized in both clinical and research settings [42]. Increased sensitivity using this assay compared with that using traditional faecal methods has been previously described[21,43]. However, a meta-analysis of molecular techniques reported that the sensitivity of PCR was only 56.5% when compared to Strongyloides serology or faecal methods [44].
Serological diagnosis has frequently been reported to have higher diagnostic sensitivity than other methods [11,45, 46]. It was the most common positive test in our cohort, with a sensitivity of 100% in patients with any positive faecal test, albeit in a small number of cases. Interpretation of the clinical relevance of seropositivity for S. stercoralis is difficult, especially in people with no symptoms. A positive result may represent resolved a past infection as sero-reversion may take months or even years following resolution [47]. Cross-reactions with other helminths, especially filariasis, are well documented [48]. LF caused by diurnally subperiodic Wuchereria bancrofti [49] was still highly endemic in Fiji in the early twenty-first century [50], and despite MDA remains endemic in some areas [15,16]. Norsyahida et al. analysed the sensitivity and specificity of a commercial assay in those with proven Strongyloides and found it to be 83.6 % when those with other helminths were included [51]. Ongoing investigations for potential serological cross-reactions in our cohort will be reported separately.
At least one symptom was present in 57% of those with positive serology and in all seven who had positive/equivocal faecal tests for S. stercoralis. Taking into consideration the wide CIs for the latter (59.6–100.0 %) due to small numbers, this is similar to the symptom rate described in other observational studies (62.6%) and a previous meta-analysis of 20 studies using faecal methods, which identified symptoms in 50.4% of cases [52].
As in the meta-analysis, we found that gastrointestinal and dermatological presentations were the most common symptoms in those with a positive test. The presence of rashes was the only symptom or symptom group significantly associated with a positive S. stercoralis IgG in multivariate logistic analyses in our study. Only two participants reported classic larva currens. Whilst supported by some literature [53], this contrasts with a previous study of chronic S. stercoralis infection in the UK that reported a 70% rate of larva currens [54]. However, the study population was much older (mean age 65 years) and many were admitted for intensive investigation, with increased opportunities to explore symptoms in detail.
We found no significant associations between serological positivity and respiratory symptoms, weight loss or subjective fevers in univariate or multivariate logistic regression models. Fevers are more common in Strongyloides hyperinfection, although they have been described in people who are immunocompetent, including a patient who had acquired S. stercoralis in Fiji [55,56].
Eosinophilia was present in all seven patients with positive faecal tests and 55.6% (45/81) of those with positive serology. Some authors, such as Naidu et al., have suggested that eosinophilia is a poor predictor for Strongyloides infection in migrants, finding a raised count in only 25% of their serologically positive cohort [57]. This contrasts with a study of migrants to Spain, where 95% of patients with positive serology had eosinophilia [58], and a large group of chronically infected former prisoners of war of whom 66% had eosinophilia [54]. The meta-analysis by Buonfrate et al. reported eosinophilia in 69.3% [52] of patients, and our results support the assertion that the majority of patients with S. stercoralis infection will have eosinophilia.
A rising eosinophil count was a good predictor that a patient would have a positive faecal result for S. stercoralis, as well as predictive of a positive S. stercoralis IgG ELISA. However, we found that a diagnostic cut-off eosinophil count of 0.5×109 l−1 had a sensitivity of only 55.6%, with over 40% (36/81) of serologically positive participants having a normal eosinophil count. We also note that at the clinical follow-up, three individuals with a faecal positive test had eosinophil counts that fluctuated between normal and high levels. Fluctuations in eosinophil counts with strongyloidiasis have been described by previous authors [59,61]. Therefore, whilst an important clue, a normal eosinophil count does not exclude the diagnosis of strongyloidiasis.
The rtPCR used in this study has been found to be accurate to the genus level, with potential detection of Strongyloides species other than S. stercoralis [62]. Few studies on Strongyloides have used molecular methods to identify the infecting species and most have assumed it to be S. stercoralis. Different species have implications with regard to possible zoonotic transmission, clinical features and potential for autoinfection [42]. Other human infections have been reported in the western Pacific area, such as Strongyloides fuelleborni kellyi in Papua New Guinea [63] and Strongyloides fuelleborni fuelleborni in southeast Asia [64]. Using 18S rRNA HVR I and HVR IV, our samples matched most closely with S. stercoralis haplotype II A [22], which has been described in humans and dogs [28]. Whilst large numbers of stray dogs are present in Fiji, we are not aware of any recent studies that have identified Strongyloides in the canine population. Further studies on the potential for zoonotic transmission in Fiji are required.
Our study had several limitations. Although all serving Fijian personnel were eligible and invited to attend, it is possible that those with symptoms or concerns about S. stercoralis would have been more likely to take part, affecting the generalizability of our findings to all Fijian personnel. The study included predominantly adult males who had lived in the UK for a median of 15 years since leaving Fiji, and results cannot be extrapolated directly to the general population in Fiji. For our sensitivity and specificity calculations, the number of positives was low, and as a result, CIs were large. We recognize the low numbers of faecal samples provided by participants compared with serum and blood samples. Efforts were made to mitigate this, such as providing stool collection instructions and kits in advance and offering collection and postage of samples. However, these did not result in a large increase in samples. The reasons for the lack of sample provision may be complex and multifactorial and should be investigated to aid future studies in this population. Given the further cases of S. stercoralis found at the clinical follow-up, it is likely that additional stool sampling would have identified more faecal positive cases.
Details of prior residence, travel and persistent symptoms were all self-reported in an unsupervised questionnaire that had not been rigorously validated; answers were not verified further. This may have skewed the significance of any potential associations between travel and the prevalence of infection. All participants were serving Armed Forces personnel and many could have undertaken occupational activities in endemic areas that involved soil exposure and hence potential infection. However, individuals should have access to good quality footwear and clothing during military travel, potentially reducing infection risk. Furthermore, positivity rates for STH infections were similar even in those who had never been to any endemic area other than Fiji when compared to those in the whole group. The only significant residential risk factor for Strongyloides infection in multivariate analysis was rural residence in Fiji, and many study participants reported at the clinical follow-up that they were frequently barefoot whilst in Fiji. As infections with S. stercoralis may persist for decades, these could have been acquired in Fiji at any time.
This analysis focuses on the results of one set of blood and faecal test, which is recognized to be less likely to identify infection than more intensive investigation [11,65]. As in other studies, PCR was the most sensitive faecal test modality [21,43]. The detection of DNA alone may not necessarily indicate active infection, although PCR has been evaluated as having a high specificity for S. stercoralis in meta-analysis [44]. Possible causes of a false positive rtPCR result include laboratory contamination. However, our samples were often run with other clinical samples, and there was no increase in positivity rates to suggest this. Furthermore, the diagnostic laboratory undergoes regular quality assurance. The interpretation of equivocal rtPCR results may be difficult, but in this study, all participants with equivocal results had both eosinophilia and positive S. stercoralis serology, suggesting they had true infections. All these cases were offered clinical follow-up and treatment.
Our findings suggest that there is a proportion of the adult population in Fiji with undiagnosed chronic S. stercoralis infection. Further study is required to confirm this and to evaluate the impact of LF MDA campaigns since 2001 that used albendazole. In 2017, the World Health Organization recommended the addition of ivermectin in areas that have not met the epidemiological targets for elimination of LF [66]. This may have the additional benefit of reducing S. stercoralis prevalence in these areas. A single dose of 200 mg/kg ivermectin has a cure rate of around 86% against S. stercoralis [67], higher than that after multiple doses of albendazole [68].
In conclusion, our findings have demonstrated for the first time that chronic S. stercoralis infection is present in a number of Fijian migrants to the UK. Sequencing confirmed the presence of S. stercoralis strains, known to be prevalent in the Pacific region. Infection was more common in those with a history of rashes or with eosinophilia but was also present without either of these indicators. As in other studies, diagnostic tests confirmed greater sensitivity of rtPCR than faecal microscopy or charcoal culture to detect STH in single faecal samples from a migrant population. rtPCR has the advantage over culture methods of increased speed of processing and does not require as much contact with potentially infective material [69]. However, it remains a relatively expensive technique that requires significant resources, and further study is required on its utility over traditional methods in a low-resource environment.
These results suggest that some adult male migrants from Fiji have chronic infections with potential for severe complications later in life and some may have no indicator symptoms or peripheral eosinophilia. Whilst the UK Migrant Health guide states that up to 20% of migrants from endemic countries may have helminth infections on arrival to the UK (https://www.gov.uk/guidance/helminth-infections-migrant-health-guide) [70], we have demonstrated it to be higher in certain populations. Programmes should be designed to identify and screen Fijian migrants to the UK for STH and to treat those with positive results or to treat all such migrants presumptively [71,74]. The application and success of such policies should also be studied in women and children arriving from Fiji. Systematic evaluation of the prevalence and risk factors for S. stercoralis infection in Fiji should employ modern diagnostic techniques to inform the need for changes to mass screening and treatment programmes for STHs, especially S. stercoralis.
supplementary material
Acknowledgements
The authors thank all members of the UK Armed Forces Fijian community, medical staff of all primary healthcare centres involved in the study and all members of the military units involved.
Abbreviations
- AUC
area under the curve
- blast
Basic Local Alignment Search Tool
- bp
base pair
- CI
confidence interval
- Ct
cycle threshold
- DNA
deoxyribonucleic acid
- ELISA
enzyme-linked immunosorbent assay
- FEA
formalin-ethyl acetate
- GIP
gastrointestinal parasite
- HDI
human development index
- HVR
hyper-variable region
- IgG
immunoglobulin G
- LF
lymphatic filariasis
- MDA
mass drug administration
- OD
optical density
- OR
odds ratio
- PhHV-1
phocine herpesvirus
- ROC
receiver operating characteristic
- rRNA
ribosomal ribonucleic acid
- rtPCR
real time polymerase chain reaction
- STH
soil-transmitted helminth
- TE
tris-EDTA
- UK
United Kingdom
Footnotes
Funding: This work was funded by the Defence Medical Services Research Steering Group, Defence Medical Services, Ministry of Defence, United Kingdom, Award 701 578 872, following an application by William Nevin. No one employed by the funders, other than the authors, played any role in the study, preparation of the article or decision to publish.
Ethical statement: Prospective approval for this study was provided by the Ministry of Defence Research Ethics Committee, protocol 2048/MODREC/21.
Author contributions: Funding acquisition was done by W.D.N. Conceptualization and methodology design were performed by W.D.N., J.J., L.C., L.L., M.K.O., N.J.B. and T.E.F. Recruitment of participants into the study and participant sampling (investigation) were performed by W.D.N., J.M., R.T., M.R., L.S., T.D.T., A.F., C.H., S.F., C.Hughes, M.Riley and S.R. Sample processing (investigation) was performed by W.D.N., J.J. and L.C. Data curation and formal analysis were performed by W.D.N. with advice from J.D. Clinical follow-up of (investigation) of study participants was performed by W.D.N., S.D.W., T.E.F. and N.J.B. Writing – original draft was done by W.D.N. Writing – review and editing was performed by all authors. Supervision was performed by L.L., M.K.O., N.J.B. and T.E.F.
Contributor Information
William D. Nevin, Email: william.nevin@lstmed.ac.uk.
Jake Melhuish, Email: jake.melhuish102@mod.gov.uk.
Jayne Jones, Email: jayne.jones@lstmed.ac.uk.
Lucas Cunningham, Email: lucas.cunningham@lstmed.ac.uk.
James Dodd, Email: james.dodd@lstmed.ac.uk.
Romeo Toriro, Email: romeo.toriro@lstmed.ac.uk.
Matthew Routledge, Email: matthew.routledge@nhs.net.
Luke Swithenbank, Email: luke.swithenbank1@nhs.net.
Thomas D. Troth, Email: tom.troth@nhs.net.
Stephen D. Woolley, Email: stephen.woolley@lstmed.ac.uk.
Angela Fountain, Email: angela.fountain101@mod.gov.uk.
Claire Hennessy, Email: claire.hennessy104@mod.gov.uk.
Simon A. Foster, Email: simon.foster792@mod.gov.uk.
Charlotte Hughes, Email: charlotte.hughes324@mod.gov.uk.
Mark R. Riley, Email: mark.robert.riley@gmail.com.
Simran Rai, Email: simran.rai207@mod.gov.uk.
Russell Stothard, Email: russell.stothard@lstmed.ac.uk.
Edward D. Nicol, Email: e.nicol@nhs.net.
Mark Dermont, Email: mark.dermont240@mod.gov.uk.
Duncan Wilson, Email: duncan.wilson651@mod.gov.uk.
David Woods, Email: doctordrwoods@aol.com.
Lucy Lamb, Email: lucylamb@nhs.net.
Matthew K. O'Shea, Email: m.k.oshea@bham.ac.uk.
Nicholas J. Beeching, Email: nicholas.beeching@lstmed.ac.uk.
Thomas Fletcher, Email: tom.fletcher@lstmed.ac.uk.
References
- 1.Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:468. doi: 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21:543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Al-Jawabreh R, Anderson R, Atkinson LE, Bickford-Smith J, Bradbury RS, et al. Strongyloides questions—a research agenda for the future. Phil Trans R Soc B. 2024;379:20230004. doi: 10.1098/rstb.2023.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Geneva: World Health Organization; 2020. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030.https://www.who.int/publications/i/item/9789240010352 [Google Scholar]
- 5.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10:105–110. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas OC, Montoya AM, Villanueva-Lozano H, Carrion-Alvarez D. Severe strongyloidiasis: a systematic review and meta-analysis of 339 cases. Trans R Soc Trop Med Hyg. 2023;117:682–696. doi: 10.1093/trstmh/trad032. [DOI] [PubMed] [Google Scholar]
- 7.Barrett J, Warrell CE, Macpherson L, Watson J, Lowe P, et al. The changing aetiology of eosinophilia in migrants and returning travellers in the Hospital for Tropical Diseases, London 2002-2015: an observational study. J Infect. 2017;75:301–308. doi: 10.1016/j.jinf.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 8.McGuire E, Welch C, Melzer M. Is Strongyloides seropositivity associated with diabetes mellitus? A retrospective case-control study in an East London NHS Trust. Trans R Soc Trop Med Hyg. 2019;113:189–194. doi: 10.1093/trstmh/try132. [DOI] [PubMed] [Google Scholar]
- 9.Smith PJ, Theis B, McCartney S, Brown M. Helminths: an unrecognised disease burden prevalent among migrants in the gastroenterology clinic. Frontline Gastroenterol. 2011;2:124–129. doi: 10.1136/fg.2010.003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shea MK, Fletcher TE, Tupper D, Ross D, Wilson D. Screening for latent tuberculosis and gastrointestinal parasite infections in Gurkha recruits: research driving policy change. J R Army Med Corps. 2014;160:180–182. doi: 10.1136/jramc-2014-000259. [DOI] [PubMed] [Google Scholar]
- 11.Nevin WD, Jones J, Tupper D, Dunbar JAT, Wilson D, et al. Gastrointestinal parasite infections in Nepalese Gurkha recruits arriving in the United Kingdom from 2012-2020. PLoS Negl Trop Dis. 2024;18:e0011931. doi: 10.1371/journal.pntd.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministry of Defence: MOD Main Building, Whitehall, London, United Kindom; 2021. Freedom of Information Request FOl2021/05260, Ministry of Defence. [Google Scholar]
- 13.Jansen AAJ, Parkinson S, Robertson A. Food and Nutrition in Fiji: A Historical Review. editorips@ usp.ac.fj. 1990. [Google Scholar]
- 14.Drabo F, Ouedraogo H, Bougma R, Bougouma C, Bamba I, et al. Successful control of soil-transmitted Helminthiasis in school age children in Burkina Faso and an example of community-based assessment via Lymphatic filariasis transmission assessment survey. PLoS Negl Trop Dis. 2016;10:e0004707. doi: 10.1371/journal.pntd.0004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy M, Samuela J, Kama M, Tuicakau M, Romani L, et al. Individual efficacy and community impact of ivermectin, diethylcarbamazine, and albendazole mass drug administration for lymphatic filariasis control in Fiji: a cluster randomized trial. Clin Infect Dis. 2021;73:994–1002. doi: 10.1093/cid/ciab202. [DOI] [PubMed] [Google Scholar]
- 16.Yajima A, Ichimori K. Progress in the elimination of lymphatic filariasis in the Western Pacific Region: successes and challenges. Int Health. 2020;13:S10–S16. doi: 10.1093/inthealth/ihaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutfield T, Motuhifonua SK, Blakiston M, Bhally H, Duffy E, et al. Strongyloidiasis in Auckland: a ten-year retrospective study of diagnosis, treatment and outcomes of a predominantly Polynesian and Fijian migrant cohort. PLoS Negl Trop Dis. 2024;18:e0012045. doi: 10.1371/journal.pntd.0012045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kain M. Submitted BMJ Military Health. 2024. An illustrative case-series of eosinophilia in UK armed forces personnel. [Google Scholar]
- 19.Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol. 2019;94:1149–1167. doi: 10.1002/ajh.25617. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham LJ, Odoom J, Pratt D, Boatemaa L, Asante-Ntim N, et al. Expanding molecular diagnostics of helminthiasis: piloting use of the GPLN platform for surveillance of soil transmitted helminthiasis and schistosomiasis in Ghana. PLoS Negl Trop Dis. 2018;12:e0006129. doi: 10.1371/journal.pntd.0006129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham L, Nevin WD, Mason J, Adams ER, Jones JJ, et al. A comparative study of traditional and molecular diagnostic methods for detection of gastrointestinal parasites in Nepalese migrants to the UK. J Infect. 2024:106324. doi: 10.1016/j.jinf.2024.106324. [DOI] [PubMed] [Google Scholar]
- 22.Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Negl Trop Dis. 2019;13:e0007609. doi: 10.1371/journal.pntd.0007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits J, Permanyer I. The subnational human development database. Sci Data. 2019;6:190038. doi: 10.1038/sdata.2019.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JD, Goette DK, Odom RB, currens L. Larva currens. Cutaneous strongyloidiasis. Arch Dermatol. 1976;112:1161–1163. [PubMed] [Google Scholar]
- 25.Ko PP, Suzuki K, Canales-Ramos M, Aung MPPTHH, Htike WW, et al. Phylogenetic relationships of Strongyloides species in carnivore hosts. Parasitol Int. 2020;78:102151. doi: 10.1016/j.parint.2020.102151. [DOI] [PubMed] [Google Scholar]
- 26.Nosková E, Modrý D, Baláž V, Červená B, Jirků-Pomajbíková K, et al. Identification of potentially zoonotic parasites in captive orangutans and semi-captive mandrills: phylogeny and morphological comparison. Am J Primatol. 2023;85:e23475. doi: 10.1002/ajp.23475. [DOI] [PubMed] [Google Scholar]
- 27.Sanpool O, Intapan PM, Rodpai R, Laoraksawong P, Sadaow L, et al. Dogs are reservoir hosts for possible transmission of human strongyloidiasis in Thailand: molecular identification and genetic diversity of causative parasite species. J Helminthol. 2019;94:e110. doi: 10.1017/S0022149X1900107X. [DOI] [PubMed] [Google Scholar]
- 28.Beknazarova M, Barratt JLN, Bradbury RS, Lane M, Whiley H, et al. Detection of classic and cryptic Strongyloides genotypes by deep amplicon sequencing: a preliminary survey of dog and human specimens collected from remote Australian communities. PLoS Negl Trop Dis. 2019;13:e0007241. doi: 10.1371/journal.pntd.0007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11:e0005752. doi: 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SH, Rinamalo M, Rainima-Qaniuci M, Talemaitoga N, Kama M, et al. Short communication: epidemiological assessment of Strongyloides stercoralis in Fijian children. Parasite Epidemiol Control. 2016;1:263–267. doi: 10.1016/j.parepi.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verweij JJ, Canales M, Polman K, Ziem J, Brienen EAT, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:342–346. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Mathai E, Goneyali S, Volavola I. Prevalence of Intestinal Helminth Infection in Fiji. 1998. [Google Scholar]
- 33.Lott DA. A survey of intestinal nematodes in children of a primary school in Ovalau. South Pacific Journal of Natural Science. 1980;1:22–25. vol. [Google Scholar]
- 34.Kim SH, Stothard JR, Rinamalo M, Rainima-Qaniuci M, Talemaitoga N, et al. A first nation-wide assessment of soil-transmitted helminthiasis in Fijian primary schools, and factors associated with the infection, using a lymphatic filariasis transmission assessment survey as surveillance platform. PLoS Negl Trop Dis. 2020;14:e0008511. doi: 10.1371/journal.pntd.0008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy M, Samuela J, Kama M, Tuicakau M, Romani L, et al. The safety of combined triple drug therapy with ivermectin, diethylcarbamazine and albendazole in the neglected tropical diseases co-endemic setting of Fiji: a cluster randomised trial. PLoS Negl Trop Dis. 2020;14:e0008106. doi: 10.1371/journal.pntd.0008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes RG, Sharp DS, Hughes MC, Akau’ola S, Heinsbroek P, et al. Environmental influences on helminthiasis and nutritional status among Pacific schoolchildren. Int J Environ Health Res. 2004;14:163–177. doi: 10.1080/0960312042000218589. [DOI] [PubMed] [Google Scholar]
- 37.Thomas M, Woodfield G, Moses C, Amos G. Soil-transmitted helminth infection, skin infection, anaemia, and growth retardation in schoolchildren of Taveuni Island, Fiji. N Z Med J. 2005;118:U1492. [PubMed] [Google Scholar]
- 38.Asundi A, Beliavsky A, Liu XJ, Akaberi A, Schwarzer G, et al. Prevalence of strongyloidiasis and schistosomiasis among migrants: a systematic review and meta-analysis. Lancet Glob Health. 2019;7:e236–e248. doi: 10.1016/S2214-109X(18)30490-X. [DOI] [PubMed] [Google Scholar]
- 39.Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, et al. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis. 2010;4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Kaminsky RG. Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J Parasitol. 1993;79:277–280. [PubMed] [Google Scholar]
- 41.Koga K, Kasuya S, Khamboonruang C, Sukavat K, Nakamura Y, et al. An evaluation of the agar plate method for the detection of Strongyloides stercoralis in Northern Thailand. J Trop Med Hyg. 1990;93:183–188. [PubMed] [Google Scholar]
- 42.Buonfrate D, Tamarozzi F, Paradies P, Watts MR, Bradbury RS, et al. The diagnosis of human and companion animal Strongyloides stercoralis infection: challenges and solutions. A scoping review. Adv Parasitol. 2022;118:1–84. doi: 10.1016/bs.apar.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Wammes LJ, van Asten SAV, van Lieshout L, Wessels E, Verweij JJ. Real-time PCR for diagnosing and monitoring treatment effect of Strongyloides stercoralis infection in a non-endemic setting. Front Parasitol. 2023;2:1277372. doi: 10.3389/fpara.2023.1277372. [DOI] [Google Scholar]
- 44.Buonfrate D, Requena-Mendez A, Angheben A, Cinquini M, Cruciani M, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection-A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006229. doi: 10.1371/journal.pntd.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arifin N, Hanafiah KM, Ahmad H, Noordin R. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect. 2019;52:371–378. doi: 10.1016/j.jmii.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buonfrate D, Bradbury RS, Watts MR, Bisoffi Z. Human strongyloidiasis: complexities and pathways forward. Clin Microbiol Rev. 2023;36:e0003323. doi: 10.1128/cmr.00033-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muck AE, Pires ML, Lammie PJ. Influence of infection with non-filarial helminths on the specificity of serological assays for antifilarial immunoglobulin G4. Trans R Soc Trop Med Hyg. 2003;97:88–90. doi: 10.1016/s0035-9203(03)90033-2. [DOI] [PubMed] [Google Scholar]
- 49.Organization WH. Geneva: WHO; 2013. Report No.: 9241505648; 2019. Lymphatic filariasis: a handbook of practical entomology for national lymphatic filariasis elimination programmes. [Google Scholar]
- 50.Manolas RK, Kama M, Rainima-Qaniuci M, Bechu VD, Tuibeqa S, et al. Lymphatic filariasis in Fiji: progress towards elimination, 1997-2007. Trop Med Health. 2020;48:88. doi: 10.1186/s41182-020-00245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norsyahida A, Riazi M, Sadjjadi SM, Muhammad Hafiznur Y, Low HC, et al. Laboratory detection of strongyloidiasis: IgG-, IgG4 - and IgE-ELISAs and cross-reactivity with lymphatic filariasis. Parasite Immunol. 2013;35:174–179. doi: 10.1111/pim.12029. [DOI] [PubMed] [Google Scholar]
- 52.Buonfrate D, Fittipaldo A, Vlieghe E, Bottieau E. Clinical and laboratory features of Strongyloides stercoralis infection at diagnosis and after treatment: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:1621–1628. doi: 10.1016/j.cmi.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Tian Y, Monsel G, Paris L, Danis M, Caumes E. Larva currens: report of seven cases and literature review. Am J Trop Med Hyg. 2023;108:340–345. doi: 10.4269/ajtmh.21-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gill GV, Welch E, Bailey JW, Bell DR, Beeching NJ. Chronic Strongyloides stercoralis infection in former British Far East prisoners of war. QJM. 2004;97:789–795. doi: 10.1093/qjmed/hch133. [DOI] [PubMed] [Google Scholar]
- 55.Agyemang EA, Virk A. 57-Year-old man with fever, rash, chronic watery diarrhea, cough, and sweats. Mayo Clin Proc. 2012;87:e83–6. doi: 10.1016/j.mayocp.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neumann I, Ritter R, Mounsey A. Strongyloides as a cause of fever of unknown origin. J Am Board Fam Med. 2012;25:390–393. doi: 10.3122/jabfm.2012.03.110101. [DOI] [PubMed] [Google Scholar]
- 57.Naidu P, Yanow SK, Kowalewska-Grochowska KT. Eosinophilia: a poor predictor of Strongyloides infection in refugees. Can J Infect Dis Med Microbiol. 2013;24:93–96. doi: 10.1155/2013/290814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dopico E, Rando-Matos Y, Solsona L, Almeda J, Santos FLN, et al. Infection by Strongyloides stercoralis in immigrants with Chagas disease: evaluation of eosinophilia as screening method in primary care. Trop Med Int Health. 2020;25:467–474. doi: 10.1111/tmi.13363. [DOI] [PubMed] [Google Scholar]
- 59.JONES CA. Clinical studies in human strongyloidiasis. I Semeiology. Gastroenterology . 1950;16:743–756. [PubMed] [Google Scholar]
- 60.Milder JE, Walzer PD, Kilgore G, Rutherford I, Klein M. Clinical features of Strongyloides stercoralis infection in an endemic area of the United States. Gastroenterology. 1981;80:1481–1488. [PubMed] [Google Scholar]
- 61.Zaidi A, Natarajan N, Sharma VR. Eosinophilia as a marker of Strongyloides infection. Blood. 2011;118:4932. doi: 10.1182/blood.V118.21.4932.4932. [DOI] [Google Scholar]
- 62.Saugar JM, Merino FJ, Martín-Rabadán P, Fernández-Soto P, Ortega S, et al. Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop. 2015;142:20–25. doi: 10.1016/j.actatropica.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Ashford RW, Barnish G, Viney ME. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol Today . 1992;8:314–318. doi: 10.1016/0169-4758(92)90106-c. [DOI] [PubMed] [Google Scholar]
- 64.Janwan P, Rodpai R, Intapan PM, Sanpool O, Tourtip S, et al. Possible transmission of Strongyloides fuelleborni between working Southern pig-tailed macaques (Macaca nemestrina) and their owners in Southern Thailand: Molecular identification and diversity. Infect Genet Evol. 2020;85:104516. doi: 10.1016/j.meegid.2020.104516. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen PB, Mojon M. Improved diagnosis of Strongyloides stercoralis by seven consecutive stool specimens. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;263:616–618. doi: 10.1016/s0176-6724(87)80207-9. [DOI] [PubMed] [Google Scholar]
- 66.WHO Guidelines Approved by the Guidelines Review Committee . In Guideline: Alternative Mass Drug Administration Regimens to Eliminate Lymphatic Filariasis. World Health Organization; 2017. [PubMed] [Google Scholar]
- 67.Buonfrate D, Salas-Coronas J, Muñoz J, Maruri BT, Rodari P, et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): a multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect Dis. 2019;19:1181–1190. doi: 10.1016/S1473-3099(19)30289-0. [DOI] [PubMed] [Google Scholar]
- 68.Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;2016:CD007745. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, et al. A modified agar plate method for detection of Strongyloides stercoralis. Am J Trop Med Hyg. 1991;45:518–521. doi: 10.4269/ajtmh.1991.45.518. [DOI] [PubMed] [Google Scholar]
- 70.Office for Health Improvement and Disparities . London: Office for Health Improvement and Disparities; 2021. Helminth infections: migrant health guide.https://www.gov.uk/guidance/helminth-infections-migrant-health-guide [Google Scholar]
- 71.Chaves NJ, Paxton GA, Biggs B-A, Thambiran A, Gardiner J, et al. The Australasian Society for Infectious Diseases and Refugee Health Network of Australia recommendations for health assessment for people from refugee-like backgrounds: an abridged outline. Med J Aust. 2017;206:310–315. doi: 10.5694/mja16.00826. [DOI] [PubMed] [Google Scholar]
- 72.Maskery B, Coleman MS, Weinberg M, Zhou W, Rotz L, et al. Economic analysis of the impact of overseas and domestic treatment and screening options for intestinal helminth infection among US-Bound Refugees from Asia. PLoS Negl Trop Dis. 2016;10:e0004910. doi: 10.1371/journal.pntd.0004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agbata EN, Morton RL, Bisoffi Z, Bottieau E, Greenaway C, et al. Effectiveness of screening and treatment approaches for Schistosomiasis and Strongyloidiasis in newly-arrived migrants from endemic countries in the EU/EEA: a systematic review. IJERPH. 2019;16:11. doi: 10.3390/ijerph16010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wikman-Jorgensen PE, Llenas-Garcia J, Shedrawy J, Gascon J, Muñoz J, et al. Cost-effectiveness of different strategies for screening and treatment of Strongyloides stercoralis in migrants from endemic countries to the European Union. BMJ Glob Health. 2020;5:e002321. doi: 10.1136/bmjgh-2020-002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.