Abstract
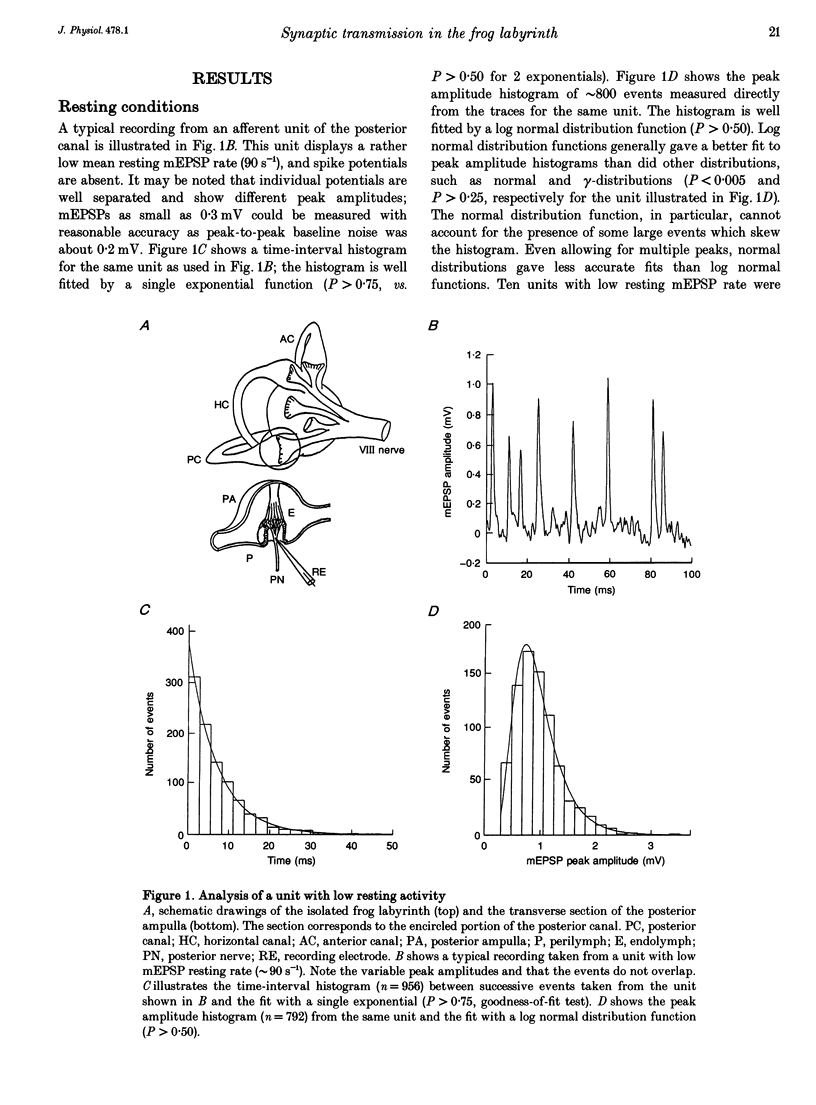
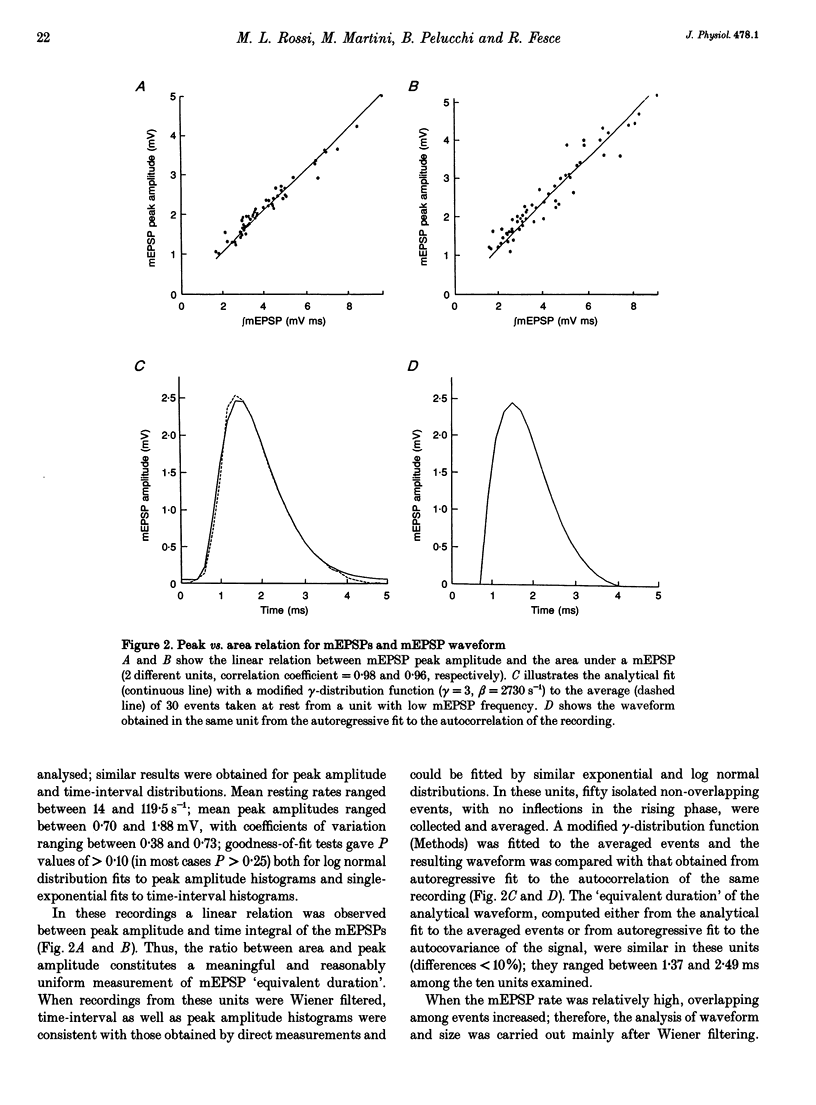
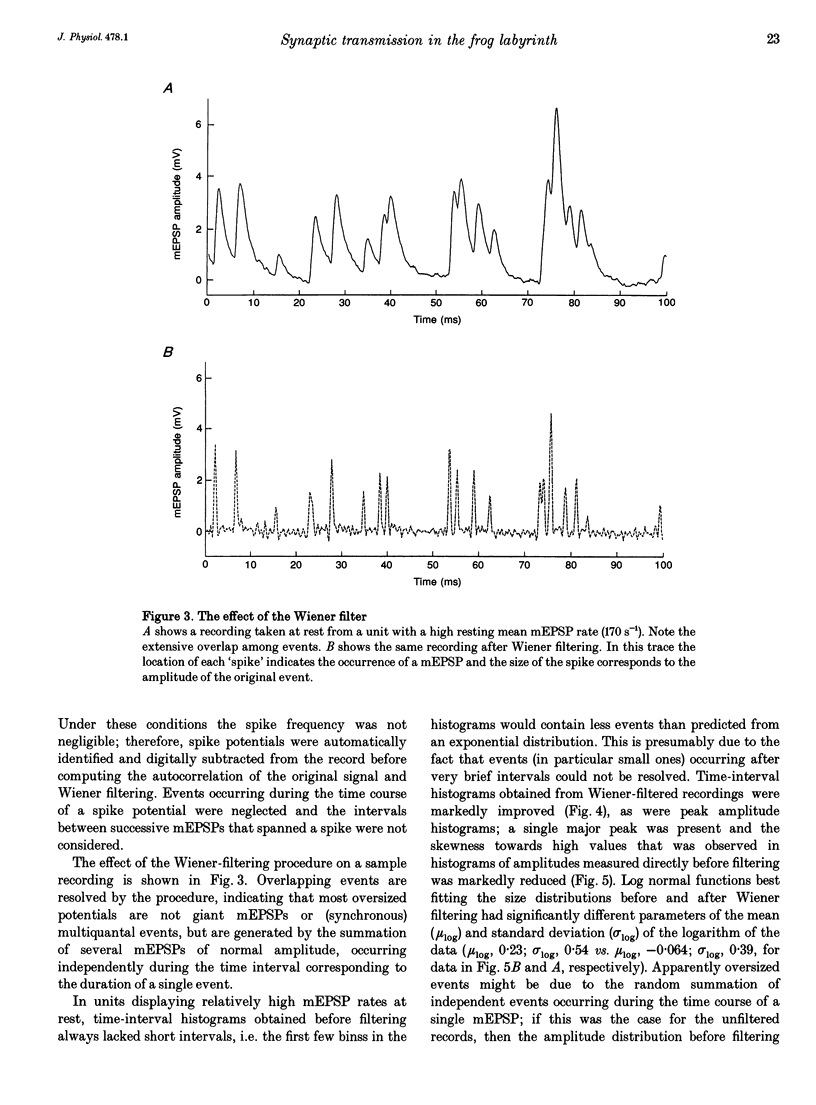
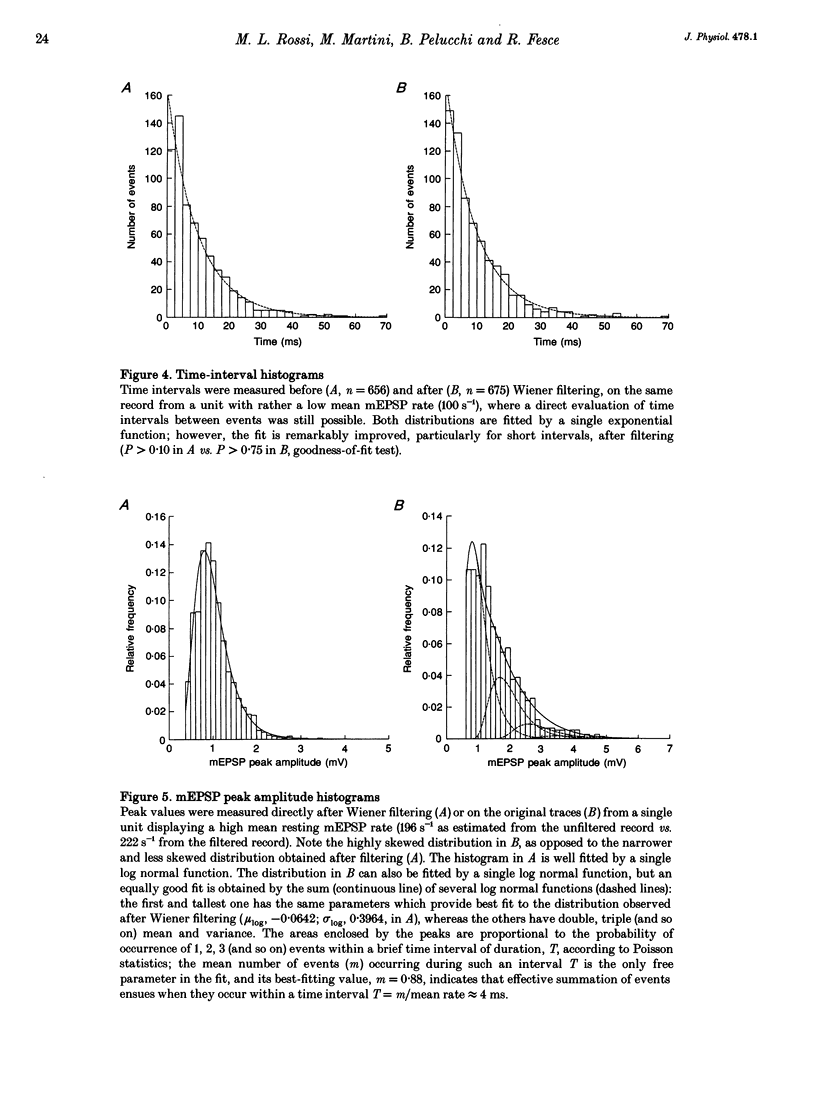
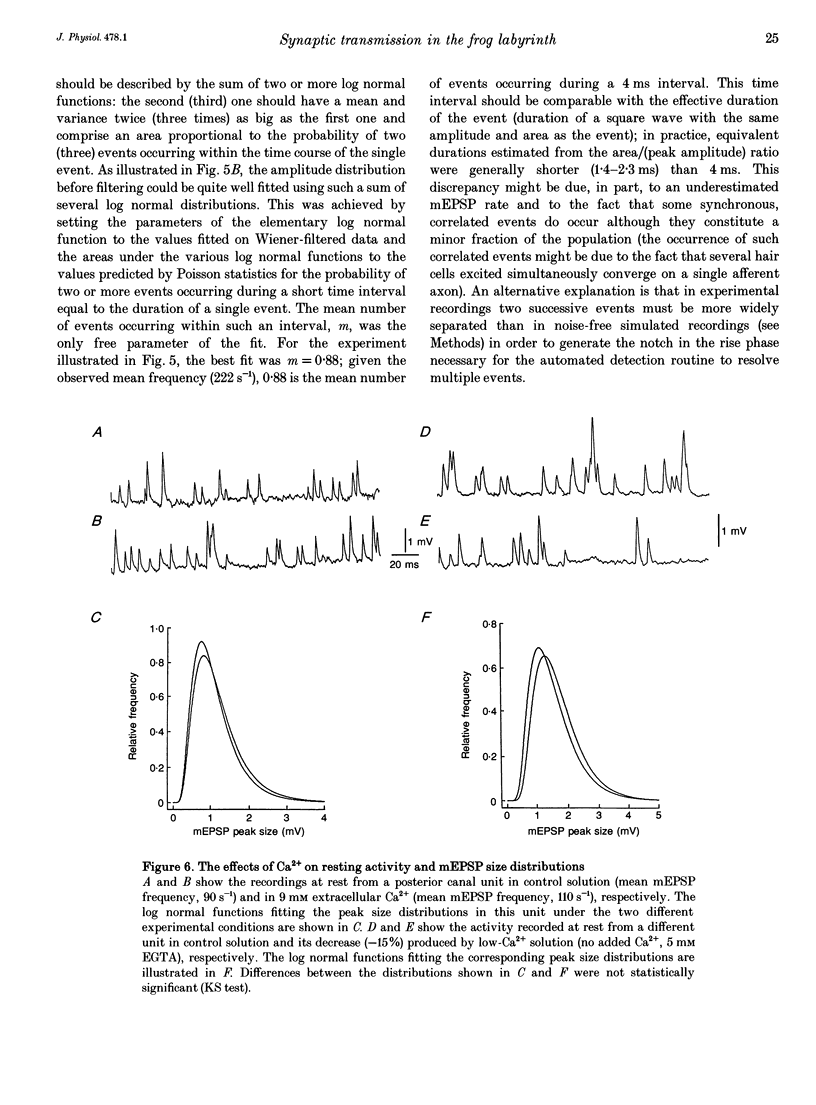
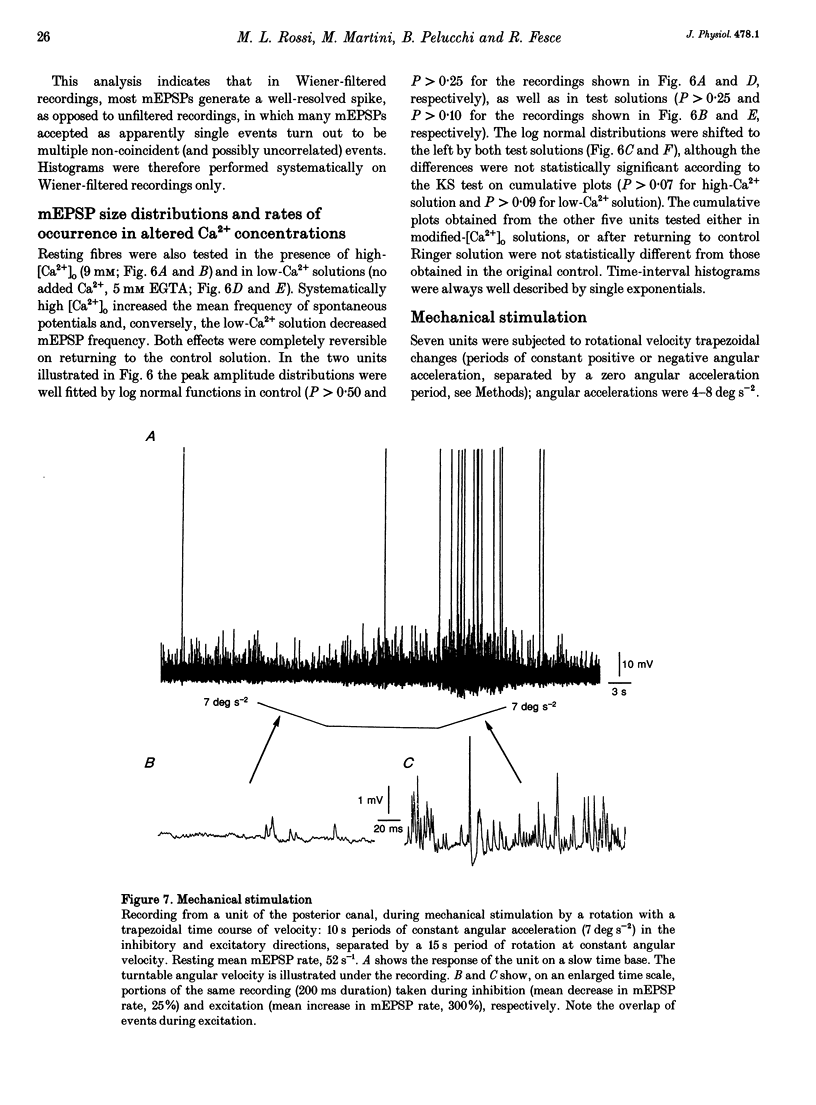
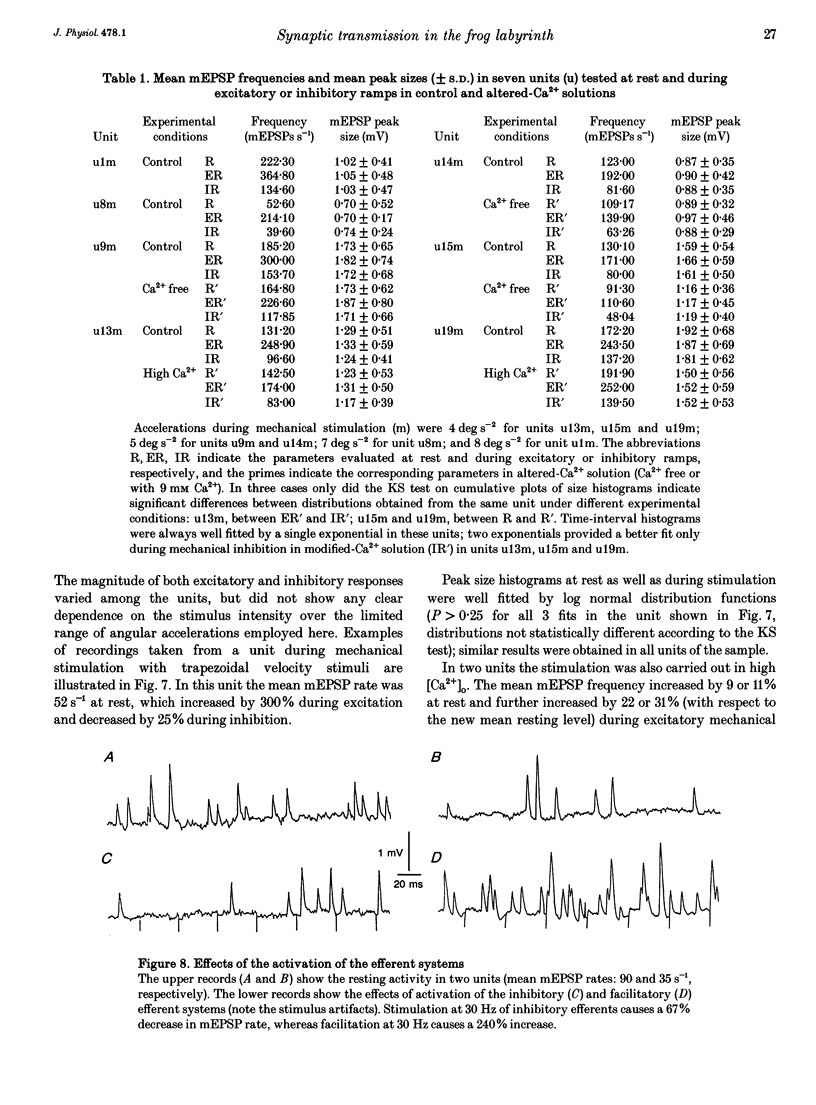
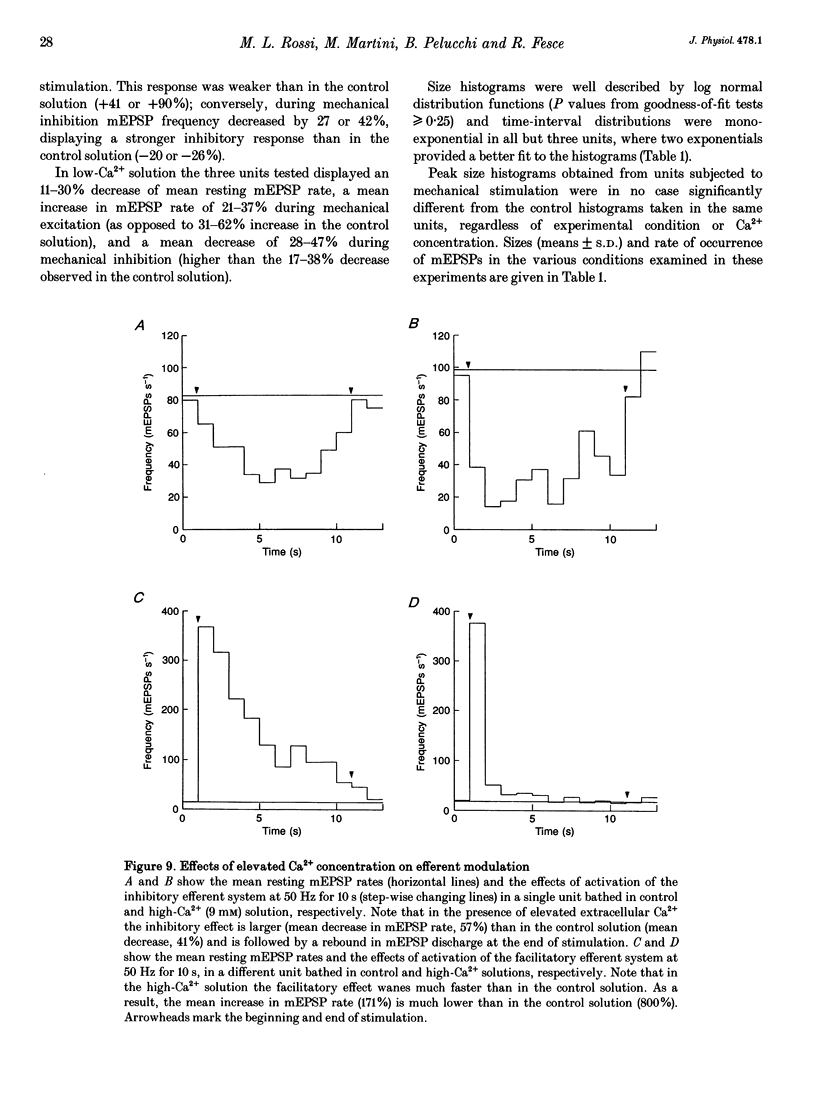
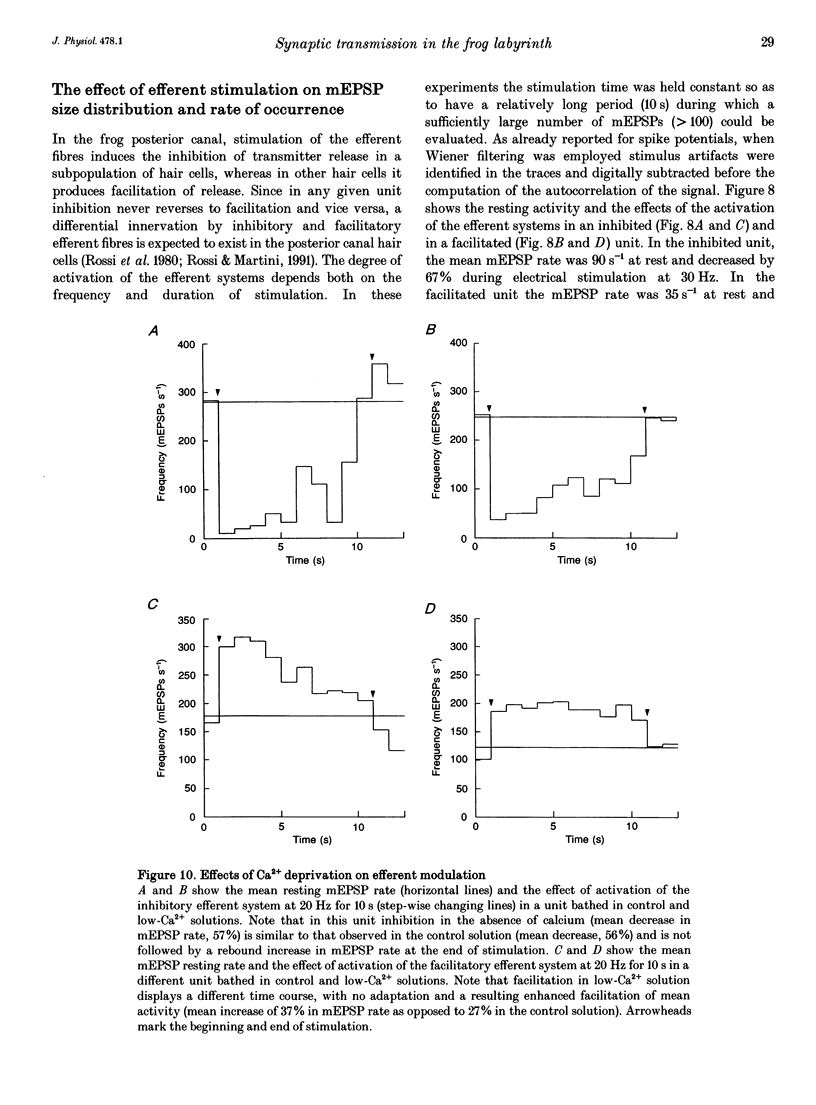
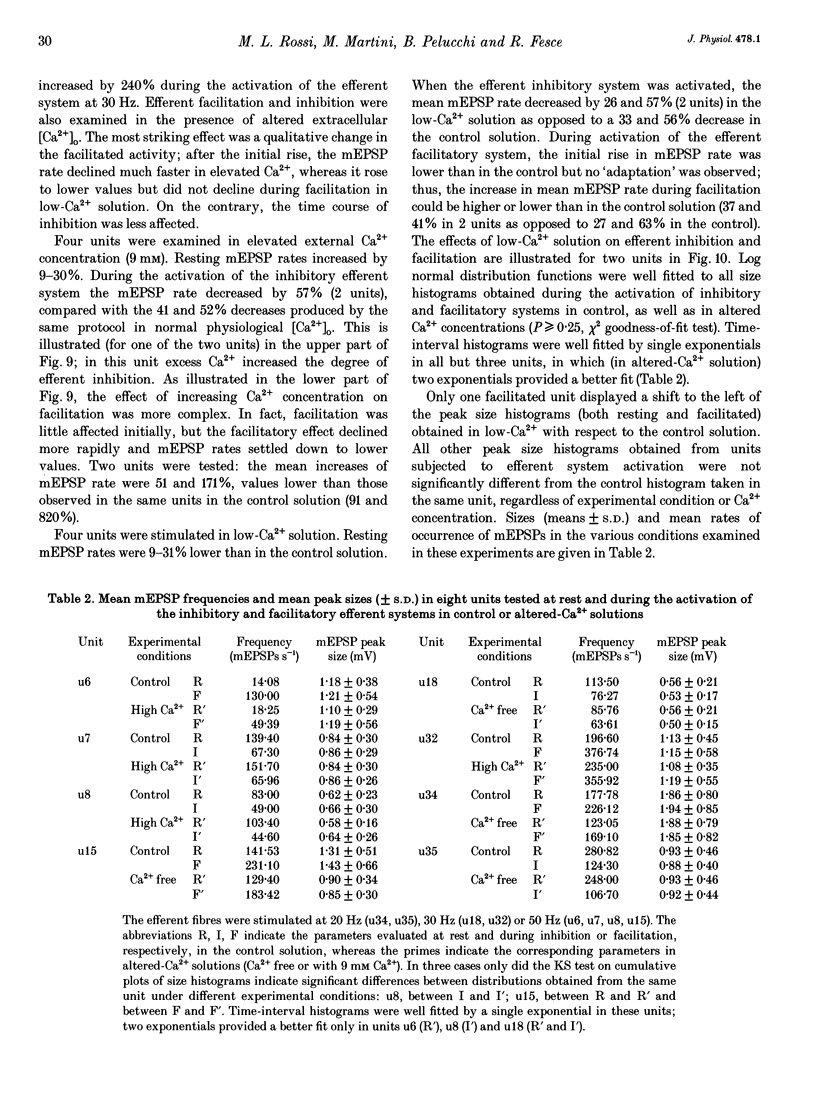
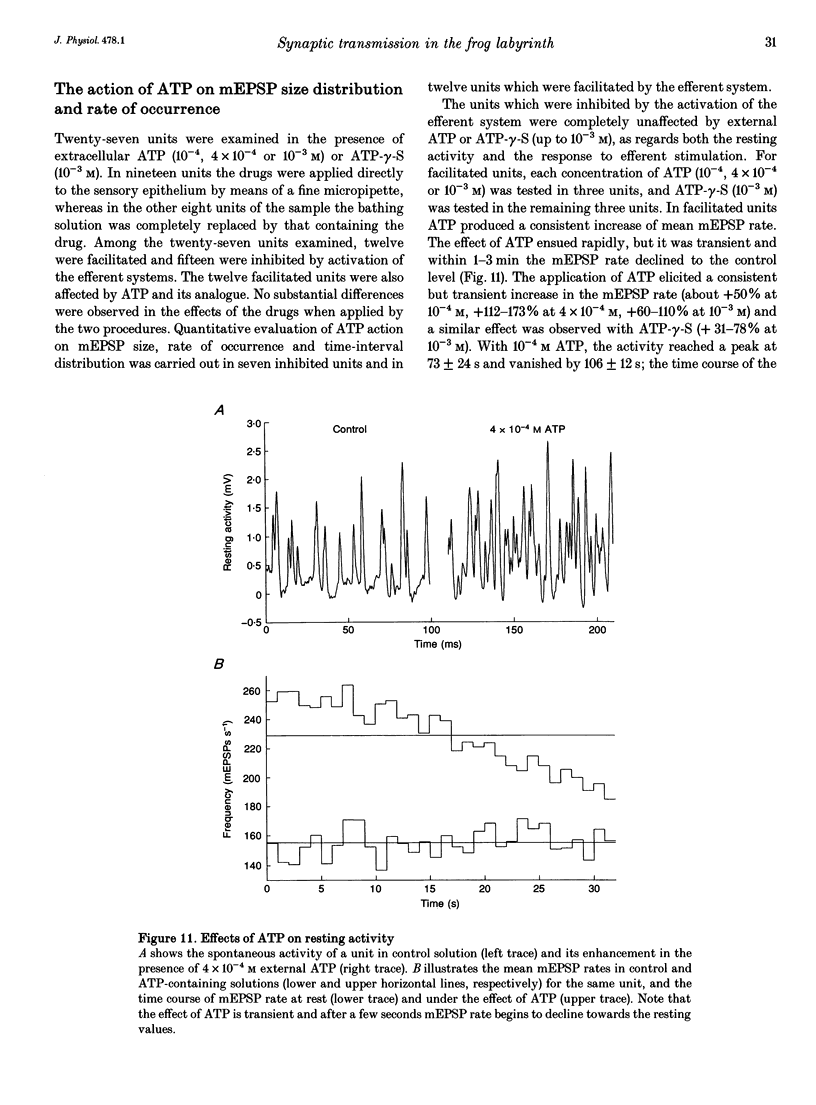
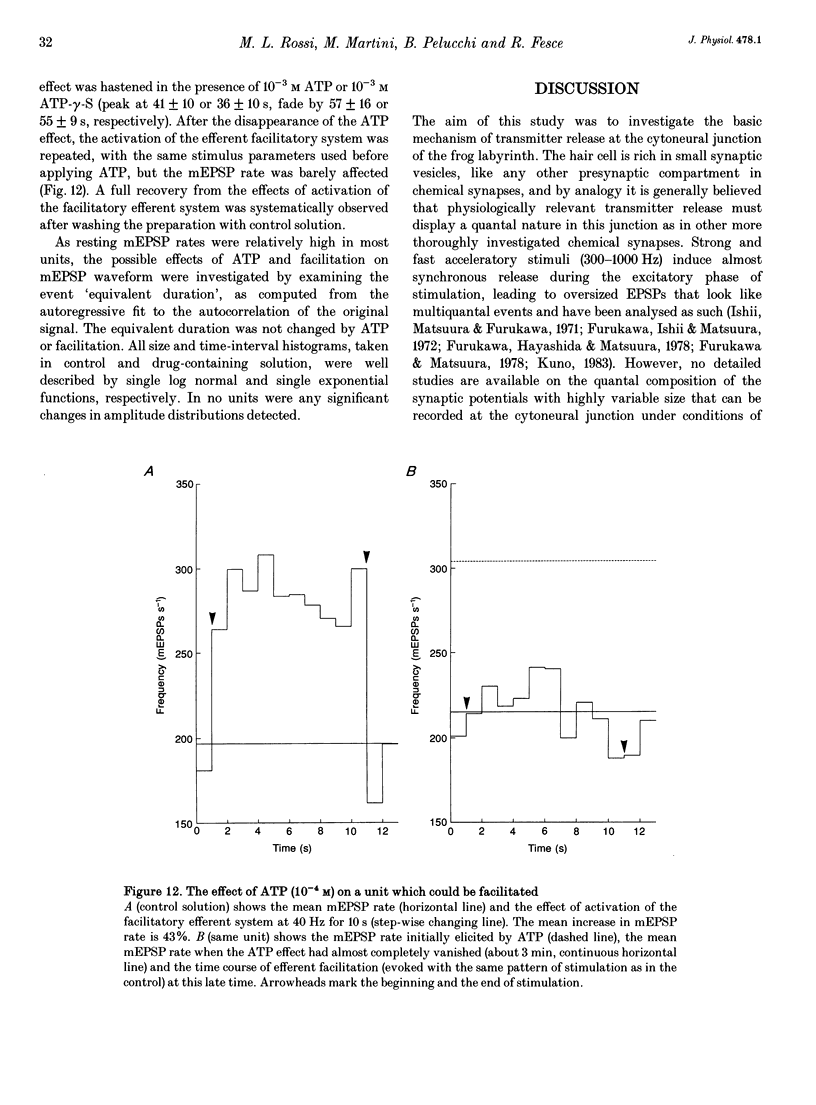
1. The mechanism of transmitter release at the cytoneural junction of the frog posterior canal was investigated by recording intracellularly subthreshold postsynaptic potentials (EPSPs), and performing a statistical analysis of time intervals and peak amplitudes. In single units EPSPs display highly variable size, so it is not clear whether they are generated by the release of single quanta of transmitter and whether large ones represent giant events, multiquantal events, or the random summation of independent unitary events. 2. In units with low resting EPSP rates, peak amplitudes and time intervals between EPSPs were measured directly. Peak amplitude histograms were continuous, unimodal and well fitted by log normal distributions. Time-interval histograms were well described by single exponentials. 3. At high EPSP rates (either at rest or during experimental treatments), where single events overlapped extensively, peak amplitude histograms were skewed markedly towards high values. Under these conditions, the EPSP waveform was estimated by autoregressive fit to the autocorrelation of the recorded signal. The fit was used to build a Wiener filter, for sharpening the original signal, before computing time-interval and peak amplitude histograms. This yielded consistent log normal peak amplitude distributions with no 'excess' skewness, similar to those obtained with low resting rates. 4. After sharpening by the Wiener filter, shoulders or small second peaks in amplitude distributions were observed only at the highest EPSP rates (> 300 s-1). The number of 'multiquantal' events was reduced by Wiener filtering, and was in general consistent with the expectation that more than one independent event occurred within the duration of the single event. This suggests that the events are uniquantal, random and independent, i.e. miniature EPSPs (mEPSPs). 5. In general, peak amplitude distributions obtained with modified external Ca2+ concentration ([Ca2+]o) and/or during mechanical stimulation or under efferent activation were not significantly altered with respect to those obtained in the same units at rest. Time-interval histograms were generally mono-exponential at rest as well as during mechanical or efferent stimulation, and irrespective of [Ca2+]o. Resting mEPSP rate was slightly increased by elevated [Ca2+]o and reduced by low [Ca2+]o. The increase in mEPSP rate produced by mechanical excitation was depressed by both high and low [Ca2+]o, whereas both conditions enhanced mechanical inhibition. Efferent inhibition was little affected. High [Ca2+]o hastened adaptation during efferent facilitation. Low [Ca2+]o reduced peak response during facilitation, but suppressed its warning. 6. In the presence of ATP a consistent though transient increase in resting mEPSP rate was observed in about 50% of units.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards F. A., Gibb A. J., Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992 Sep 10;359(6391):144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fesce R. Stochastic approaches to the study of synaptic function. Prog Neurobiol. 1990;35(2):85–133. doi: 10.1016/0301-0082(90)90019-d. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Hayashida Y., Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. J Physiol. 1978 Mar;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Ishii Y., Matsuura S. Synaptic delay and time course of postsynaptic potentials at the junction between hair cells and eighth nerve fibers in the goldfish. Jpn J Physiol. 1972 Dec;22(6):617–635. doi: 10.2170/jjphysiol.22.617. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Matsuura S. Adaptive rundown of excitatory post-synaptic potentials at synapses between hair cells and eight nerve fibres in the goldfish. J Physiol. 1978 Mar;276:193–209. doi: 10.1113/jphysiol.1978.sp012228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. M., Fernández C. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol. 1980 Apr;43(4):986–1025. doi: 10.1152/jn.1980.43.4.986. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Ashmore J. F. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc Biol Sci. 1991 May 22;244(1310):161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Greenwood D., Ashmore J. F. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci. 1992 Sep 22;249(1326):265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Matsuura S., Furukawa T. Quantal nature of transmission at the synapse between hair cells and eighth nerve fibers. Jpn J Physiol. 1971 Feb;21(1):79–89. doi: 10.2170/jjphysiol.21.79. [DOI] [PubMed] [Google Scholar]
- Kuno M. Adaptive changes in firing rates in goldfish auditory fibers as related to changes in mean amplitude of excitatory postsynaptic potentials. J Neurophysiol. 1983 Sep;50(3):573–581. doi: 10.1152/jn.1983.50.3.573. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Niedzielski A. S., Schacht J. P2 purinoceptors stimulate inositol phosphate release in the organ of Corti. Neuroreport. 1992 Mar;3(3):273–275. doi: 10.1097/00001756-199203000-00015. [DOI] [PubMed] [Google Scholar]
- Rossi M. L., Bonifazzi C., Martini M., Fesce R. Static and dynamic properties of synaptic transmission at the cyto-neural junction of frog labyrinth posterior canal. J Gen Physiol. 1989 Aug;94(2):303–327. doi: 10.1085/jgp.94.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M. L., Martini M. Efferent control of posterior canal afferent receptor discharge in the frog labyrinth. Brain Res. 1991 Jul 26;555(1):123–134. doi: 10.1016/0006-8993(91)90868-v. [DOI] [PubMed] [Google Scholar]
- Rossi M. L., Prigioni I., Valli P., Casella C. Activation of the efferent system in the isolated frog labyrinth: effects on the afferent EPSPs and spike discharge recorded from single fibres of the posterior nerve. Brain Res. 1980 Mar 3;185(1):125–137. doi: 10.1016/0006-8993(80)90677-0. [DOI] [PubMed] [Google Scholar]
- Rossi M. L., Valli P., Casella C. Post-synaptic potentials recorded from afferent nerve fibres of the posterior semicircular canal in the frog. Brain Res. 1977 Oct 21;135(1):67–75. doi: 10.1016/0006-8993(77)91052-6. [DOI] [PubMed] [Google Scholar]
- Valli P., Taglietti V., Rossi M. L. Effects of D-tubocurarine on the ampullar receptors of the frog. Acta Otolaryngol. 1974 Jul-Aug;78(1-2):51–58. doi: 10.3109/00016487409126325. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36(2):93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]


