Abstract
(1). Background.
Early childhood experiences have long-lasting effects on subsequent mental and physical health, education, and employment. Measurement of these effects relies on insensitive behavioral signs, subjective assessments by adult observers, neuroimaging or neurophysiological studies, or retrospective epidemiologic outcomes. Despite intensive search, the underlying mechanisms for these long-term changes in development and health status remain unknown.
(2). Methods.
We analyzed scalp hair from healthy children and their mothers using an unbiased proteomics platform using tandem mass spectrometry, ultra-performance liquid chromatography, and collision induced dissociation to reveal commonly observed hair proteins with spectral count of 3 or higher.
(3). Results.
We observed 1368 non-structural hair proteins in children, 1438 non-structural hair proteins in mothers, with 1288 proteins showing individual variability. Mothers showed higher numbers of peptide spectral matches and hair proteins compared to children, with important age-related differences between mothers and children. Age-related differences were also observed in children, with differential protein expression patterns between younger (2 years and below) and older children (3-5 years). We observed greater similarity in hair protein patterns between mothers and their biological children as compared to mothers and unrelated children. The top 5% proteins driving population variability represent biological pathways associated with brain development, immune signaling, and stress response regulation.
(4). Conclusion.
Non-structural proteins observed in scalp hair include promising biomarkers to investigate the long-term developmental changes and health status associated with early childhood experiences.
Keywords: hair biomarkers, proteomics, brain development, developmental psychology, preschool children, non-structural proteins
1. Introduction
Early human development remains exquisitely sensitive to parental, environmental, and societal influences that multiplex the history of each individual (via genetic and epigenetic factors) with their daily experiences. Variations in these factors, such as stress and social determinants of health, can singly or collectively introduce differences in developmental outcomes1–4. Such differences are then magnified in the higher-order cognitive and behavioral capacities of the human mind-brain-body connectome, which are built on a series of sequential or staggered developmental epochs that can enable or constrain their future potential, role(s) in society, as well as their mental and physical health4–8.
Objective assessment of social, emotional, or other environmental inputs across multiple timescales is challenging in early childhood. These challenges result from most subjects being pre-verbal, coming from unknown environments, or accompanied by unreliable, fearful, or distrusting historians1, 3, 9, 10. Developmental timescales can also range from milliseconds to minutes (e.g., affecting acute neuromodulatory tone, neuronal oscillations, neuroendocrine changes), days to weeks (e.g., affecting circadian rhythms, metabolic functions, memory and learning), or months to years (e.g., affecting brain growth and brain plasticity, or emerging cognitive, behavioral, or social capacities)4, 11. Neurophysiological, neuroimaging, and observational studies have attempted to describe and quantify early developmental changes, but there remains a need for non-invasive, objective biomarkers that can be measured serially across the months and years required for childhood development12–15.
Human scalp hair of preschool children, derived from the neuroectoderm and mesoderm, grows constantly at about 1 cm/month and evolves via prenatal lanugo, postnatal vellus, intermediate medullary, and terminal hair stages16. Hair contains 65-85% proteins, 15-35% water, 1-9% lipids, and 0.1-5% pigments like melanin and trace elements17. Constantly growing scalp hair incorporates both endogenous and exogenous proteins in a time-averaged chronological manner18, unlike any other biospecimens19. Therefore, it is used routinely to monitor drug exposures, heavy metals, and other environmental toxins20, or even reflect the social determinants of health3.
Developmentally regulated hair proteins could offer biomarker candidates for the mind-brain-body connectome with the potential to monitor health status in real-time during early childhood development. However, all published data on hair proteins are limited to adult subjects, include relatively small sample sizes, and focus mainly on structural hair proteins. Lee et al. reported 343 hair proteins from three adults, showing evidence for post-translational modifications21. Laatsch et al. analyzed hair from 18 males and 3 females, reporting ethnic differences in keratins and keratin-associated proteins (KAPs)22. Carlson et al. characterized hair proteins from one adult with limited sample availability23 whereas Wu et al. used hierarchical protein clustering to match 10 monozygotic twin pairs and differentiate them from unrelated individuals24. Parker et al. reported quantifiable measures21 of identity discrimination and racial ancestry by detecting genetically variant peptides in the structural hair proteins for forensic purposes25.
To fill the extant gaps in knowledge, we analyzed non-structural hair proteins using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and ELISA based validation studies conducted on a limited subset of the detected non-structural hair proteins present in preschool children and their mothers. Our subjects were not exposed to early life adversity, as evidenced by parental income, household structure, health insurance, and parent education5 or by their hair cortisol concentrations (HCC)4, 26.
2. Materials and Methods
After IRB approval and parental consent, mothers and children aged 1-6 years were enrolled from local preschool facilities. All children were developmentally appropriate, healthy, and belonged to stable nuclear families (Table 1). We excluded children with tinea capitis, alopecia areata, eczema, or other scalp conditions; those receiving any prescription or over-the-counter drugs; or steroid therapy in the past 3 months; or those with chronic medical conditions, developmental delay, or chemical exposures to hair prior to study entry. Hair samples from the posterior vertex (1 cm2 area) were trimmed at 0.1 mm from the scalp and stored in Ziploc® bags at 4°C.
Table 1.
Demographic Characteristics and Hair Protein Data
| Family Code | Subject | Age (months) | Age (years) | Gender | Race | Ethnicity | # of hair proteins | Peptide Spectral Matches (PSMs) |
|---|---|---|---|---|---|---|---|---|
| F107 | Mother | 450.6 | 37.6 | F | White | Non-Hispanic | 819 | 6533 |
| Child1 | 27.6 | 2.3 | F | White | Non-Hispanic | 568 | 3949 | |
| Child2 | 58 | 4.8 | M | White | Other | 464 | 2873 | |
| F123 | Mother | 447.1 | 37.3 | F | White | Non-Hispanic | 809 | 10370 |
| Child1 | 24 | 2 | F | White | Non-Hispanic | 499 | 3728 | |
| Child2 | 52.4 | 4.4 | M | White | Non-Hispanic | 573 | 5078 | |
| F134 | Mother | 431.8 | 35.9 | F | White | Missing | 684 | 5445 |
| Child1 | 20.9 | 1.74 | M | Mixed | Missing | 387 | 2760 | |
| Child2 | 67.6 | 5.6 | F | Mixed | Missing | 759 | 6872 | |
| F142 | Mother | 447.3 | 37.3 | F | Asian | Missing | 650 | 8370 |
| Child1 | 20.1 | 1.7 | M | Mixed | Missing | 581 | 6208 | |
| Child2 | 50.6 | 4.2 | M | Mixed | Missing | 226 | 2353 | |
| F183 | Mother | 530 | 44.2 | F | Asian | Non-Hispanic | 1090 | 10527 |
| Child1 | 8.5 | 0.7 | M | Asian | Non-Hispanic | 314 | 2331 | |
| Child2 | 44 | 3.7 | M | Asian | Non-Hispanic | 1010 | 8065 | |
| F218 | Mother | 504 | 42 | F | White | Hispanic | 609 | 4144 |
| Child1 | 58.5 | 4.9 | F | White | Hispanic | 524 | 3107 | |
| Child2 | 35.2 | 2.9 | F | White | Hispanic | 631 | 4475 | |
| F271 | Mother | 402.6 | 33.6 | F | White | Non-Hispanic | 769 | 7525 |
| Child1 | 15.1 | 1.3 | F | White | Non-Hispanic | 557 | 7161 | |
| Child2 | 42.5 | 3.5 | F | White | Non-Hispanic | 600 | 5615 | |
| F286 | Mother | 489.8 | 40.8 | F | White | Non-Hispanic | 616 | 9209 |
| Child1 | 22 | 1.8 | M | White | Non-Hispanic | 403 | 4727 | |
| Child2 | 52.5 | 4.4 | F | White | Non-Hispanic | 614 | 6061 | |
| F346 | Child | 50.3 | 4.2 | M | White | Missing | 475 | 3429 |
| F192 | Child | 38.1 | 3.2 | F | Other | Hispanic | 283 | 1731 |
| F132 | Child | 51.4 | 4.3 | F | White | Missing | 272 | 1892 |
| F363 | Child | 56.6 | 4.7 | M | Mixed | Non-Hispanic | 270 | 1914 |
| F281 | Child | 53.5 | 4.5 | M | Mixed | Mixed | 406 | 3192 |
| F173 | Child | 51.3 | 4.3 | F | Other | Other | 835 | 7168 |
| F380 | Child | 14.8 | 1.2 | M | Asian | Missing | 237 | 1814 |
| F159 | Child | 62.7 | 5.2 | F | White | Non-Hispanic | 485 | 3830 |
| F179 | Child | 53 | 4.4 | F | Asian | Other | 494 | 2926 |
| F149 | Child | 61.3 | 5.1 | F | Mixed | Non-Hispanic | 698 | 5733 |
| F106 | Child | 56.7 | 4.7 | F | Asian | Non-Hispanic | 668 | 6390 |
| F153 | Child | 57.1 | 4.8 | M | Asian | Missing | 275 | 2549 |
| F256 | Child | 55.8 | 4.7 | M | Mixed | Mixed | 638 | 7016 |
| F190 | Child | 31.5 | 2.6 | F | Asian | Other | 527 | 7460 |
| F104 | Child | 50.2 | 4.2 | M | White | Non-Hispanic | 672 | 8084 |
| F113 | Child | 17.9 | 1.5 | F | White | Non-Hispanic | 441 | 3640 |
Note. Demographic data, total number of proteins, and peptide spectral matches observed in all 40 individuals. Mothers’ hair (n=8) had significantly higher number of proteins (p=0.001) and protein spectral matches (p=0.0004) compared to children’s hair (n=32). Related children (n=16, Cyan) are grouped with their mothers and unrelated children are listed below (n=16, Orange).
2.1. Hair protein extraction
Proprietary methods were developed for the extraction of soluble protein components of human scalp hair.
2.2. Proteomics method
Protein pellets were resuspended in 50 mM ammonium bicarbonate in the presence of 0.0015% ProteaseMAX (Promega) and total protein amount was estimated with Pierce BCA assays (Thermo Fisher Scientific) for a consistent loading of all samples. Proteins were digested with 0.25 μg of Trypsin/LysC (Promega) at a 1:100 enzyme/substrate ratio overnight at 37°C. Proteolytic digestion was quenched with 1% formic acid; peptides were dried by speed vac before dissolving in 30μl of reconstitution buffer (2% acetonitrile + 0.1% Formic acid) to a concentration of 1 μg/μl; 2 μl of this solution was injected into the MS instrument.
Experiments were performed on the Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific) coupled with ACQUITY M-Class ultra-performance liquid chromatography (UPLC, Waters Corporation). For a typical LCMS experiment (Liquid Chromatography/Mass Spectrometry), a flow rate of 450 nL/min was used, where mobile phase A is 0.2% formic acid in water and mobile phase B is 0.2% formic acid in acetonitrile. Analytical columns were pulled using fused silica (I.D. 100 microns) and packed with Magic 1.8-micron 120Å UChrom C18 stationary phase (nanoLCMS Solutions) to a length of ~25 cm. Peptides were directly injected onto the analytical column using a gradient (2-45% B, followed by a high-B wash) of 80 minutes. The MS was operated in data-dependent fashion using CID (collision induced dissociation) for generating MS/MS spectra, collected in the ion trap with collisional energy set at 35.
The *.RAW data files were processed using Byonic v3.2.0 (ProteinMetrics) to infer protein isoforms using the Uniprot homo sapiens database. Proteolysis with Trypsin/LysC was assumed to be semi-specific allowing for N-ragged cleavage with up to 2 missed cleavage sites. Precursor mass accuracies were held within 12 ppm and 0.4 Da for MS/MS fragments. Proteins were held to a false discovery rate (FDR) of 1% or lower, using standard target-decoy approaches27, and only the proteins with >3 spectral counts were selected for further data processing; keratins and KAPs were removed at this stage.
2.3. Generation of age-associated proteomic libraries
Initially, the standard UPLC-MS/MS methods (section 2.2) were employed to identify non-structural hair shaft proteins, using protein purification to remove keratins and for establishing age-associated hair shaft proteomic libraries using pooled hair samples from 40 children of diverse race/ethnicity (Asian, White, Mixed, or Other races; Hispanic/non-Hispanic ethnicity), aged 1-5 years (mean/SD = 44.5 months±12.6 months), and 43 mothers also of diverse race/ethnicity (aged 39 years±5 years). Utilization of large numbers of individuals of diverse race and ethnicity favors our ability to detect representative patterns of non-structural proteins incorporated in the hair shaft. We observed 1368 non-structural hair proteins in children, 1438 non-structural hair proteins in mothers, with 1288 proteins showing individual variability. The total number of age-associated proteins discovered in these libraries were also detected in the analyses of 40 independent individual subjects that had not been used for generation of the libraries. Individual hair samples from 8 mothers with 16 biologically-related children and 16 unrelated children were analyzed against the pooled hair protein libraries to create a master library of hair proteins. These data were deposited through the PRIDE repository28 into the ProteomeXchange Consortium29, 30.
2.4. Human scalp hair shaft proteoforms validation studies
Surplus volumes of protein remaining after UPLC-MS/MS generated libraries, individual evaluations, and quantification of hair cortisol concentrations (HCC) were pooled based on low, intermediate, or high HCC values. Hair cortisol (HCC) assays were validated previously31. These pools were evaluated using commercially available ELISA kits, used according to manufacturer’s instructions: cortisol (ALPCO/11-CORHU-E01-SLV), arginine vasopressin (AVP, Enzo/ADI-900-017A), Cu/ZN superoxide dismutase (SOD1, Enzo/ALX-850-033), glial fibrillary acidic protein (GFAP, Bioatrik/EKU04380), and HtrA serine peptidase 2 (HTRA2, Thermoscientific/EHHTRA2).
2.5. Statistical analysis
Spectral counts were used to calculate Euclidean distances between individuals, and to determine hierarchical clustering. A correlation matrix with Spearman’s coefficient was also used for rank-based depiction of similarities between the individual hair proteomes.
Principal Component Analysis (PCA)32–34 was used to reduce dimensionality of this rich dataset. PCA is a widely used technique for analytical modeling of linear combinations of the original dimensions called principal components34. The largest proportion of data variance is captured by the first principal component, the second largest proportion of variance falls along the second principal component, and so on32. For the first five principal components from each PCA, we multiplied the loading scores of each protein by the percent variance explained by that corresponding principal component; these weighted scores were summed for each protein to give its Total Loading Score (TLS).
Based on their TLS values, top 5% proteins were selected as the main drivers of variability in hair protein expression.
Additionally, we used t-distributed stochastic neighboring embedding (tSNE), a non- linear probabilistic approach35, 36, to visualize proteins with non-linear similarity in high-dimensional space as neighbors in low-dimensional linear depictions. Unlike the reproducible PCA results, the probabilistic nature of tSNE can result in somewhat different results with each computation. To avoid serendipitous results, we ran each computation at least 10 times to ensure reproducibility. For each computation, the maximum number of iterations to converge was set to 1000, and perplexity set to the maximum permitted value. Statistical significance of tSNE clustering was calculated by how often a given statistic was reproduced in 1000 simulations of permuted versions of the dataset.
Boolean profiles of the hair proteins were also compared between the original dataset (each mother coupled with her own children) and 5000 simulated datasets, created by swapping mothers between families such that no mother was paired with her own children, but the two siblings remained together in all simulated datasets. Observed conservation in pairwise intra-family Manhattan distances from the original dataset could then be attributed to the similarities in hair protein expression between each mother and her children.
For the top 5% proteins in children (n=32), we averaged spectral counts for girls and boys separately, and divided the girls’ average by the boys’ average. Resulting values were converted to log-base 2. The same process was followed for spectral counts from mothers and children.
Log fold-change values of the top 5% proteins were used as input for Ingenuity Pathway Analysis (Qiagen: https://digitalinsights.qiagen.com/products/features/). We analyzed direct and indirect relationships between molecules based on experimentally observed data, restricted to human databases in the Ingenuity Knowledge Base. We used Random Forest (RF) models for both the classification (boy vs. girl, mother vs. child) and regression (age prediction) tasks, with protein concentrations as model features and individuals as samples37. In classification, the model output was the probability of an individual being female (sex classification) or being a mother (person classification). For regression (age prediction), the model output was the individual’s predicted age.
Results were based on a 10-fold cross-validation repeated 100 times. Members of the same family were included in the same set, i.e. either training or test sets, to avoid information leak due to familial similarities. For the age prediction, we evaluated results using the R2 coefficient of determination and the linear model p-value fitted on the predicted and observed data. For the classification tasks, we used area under the ROC curve (AUC) and the Wilcoxon-Mann-Whitney test, testing the null-hypothesis that one distribution is not stochastically greater than the other.
3. Results
3.1. Features of hair proteins
There were 3,124 proteoforms, representing the gene products of 2,278 genes. Expression of protein isoforms, alternative splicing of messenger RNA (mRNA), and post-translational modifications resulted in a higher number of hair proteins than their associated genes21, 38. Hair proteins observed in individual mothers and children contained 2,269 unique ‘proteoforms’ or protein isoforms; 1,438 proteins were commonly observed in mothers, 1,368 proteins were commonly observed in children, whereas 1,288 hair proteins showed individual variability among mothers and children. Higher spectral counts (p=0.0004) and higher numbers of proteins (p=0.001) were observed in mothers compared to children (Figure 1), perhaps reflecting a wider array of biological functions in adult females related to reproduction39–41, aging37, 42, or disease states43. These age differences were explored further in subsequent analyses.
Figure 1. Hair Proteins in Mothers and Children.
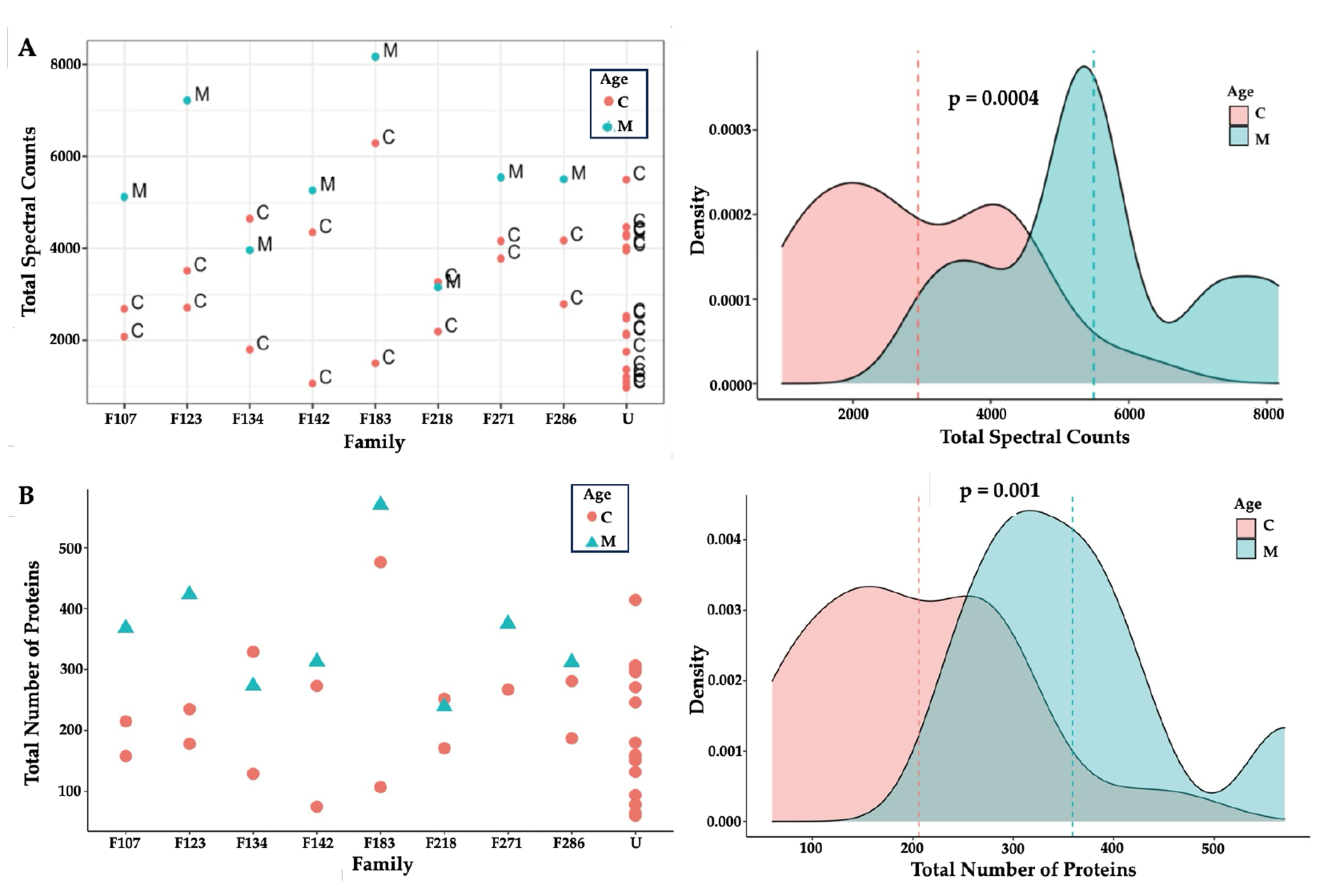
Note. (A) Protein spectral counts (p=0.0004) and (B) the numbers of proteins observed (with spectral counts >3) were consistently higher (p=0.001, Wilcoxon tests) in the mothers (M; Cyan) as compared to children (C; Pink). Mothers and their biological children (family labels: F107, F123, F134, F142, F183, F218, F271, F288) and Unrelated children (U) are identified on the X- axis: every mother except F134 and F218 had higher spectral counts and more hair proteins than her children.
3.2. Hair protein profiles in individuals and families
Peptide spectral matches for each protein were combined to compare protein expression for all individuals and assess Spearman rank correlations. Hair proteins from the mothers were closely correlated with each other, whereas hair proteins in children showed correlations based on age and sex (Figure 2A). Euclidean distances were calculated for pairwise comparisons between individuals (Figure 2B) and used for hierarchical clustering to identify subjects with similarities in the hair protein patterns (Figure 2C). Consistent with the correlation matrix, all mothers were clustered close together, younger children (0-2 years) were mostly located in one cluster, whereas older children were clustered with the mothers (Figure 2C). Boolean profiles of the hair proteins for each mother and her two biological children showed significantly shorter intra-family Manhattan distances (p<0.0002) as compared to 5000 ‘simulated’ families with mismatched mothers and children (Figure 2D), revealing hereditary vs. environmental conservation of hair protein profiles within each family.
Figure 2. Similarities in Hair Protein Profiles of Individuals and Families.
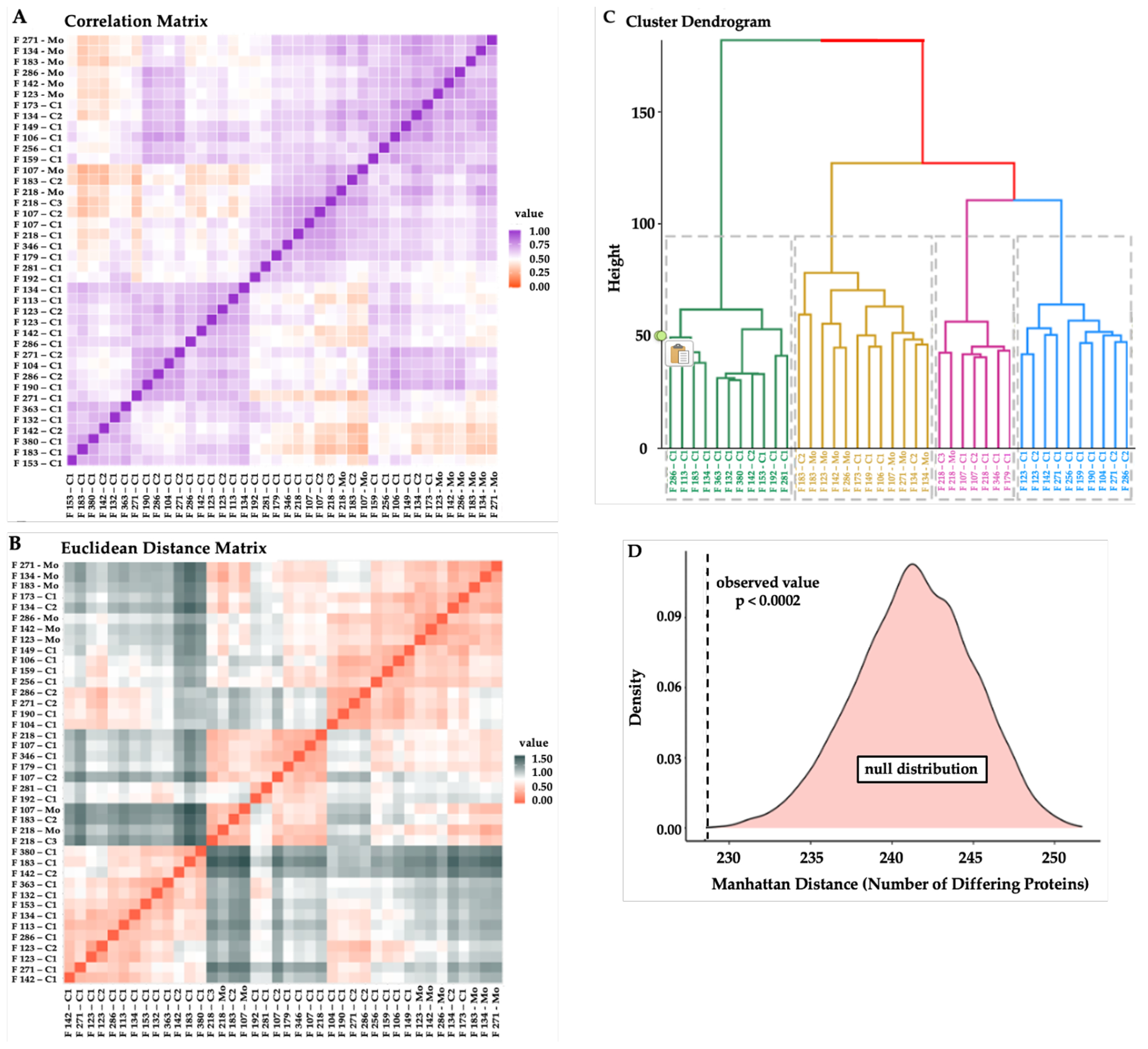
Note. (A) Spearman rank correlation matrix, with high (purple) to low (orange) correlation coefficients*; (B) Euclidean distances based on protein spectral counts, showing individuals more closely related (red) or more distant (grey) from each other*; (C) Hierarchical cluster dendrogram based on log spectral counts showing 7/8 mothers grouped in one cluster (mustard) with one mother in an adjacent cluster (pink); younger children (0-2 years) in one cluster (green) whereas older children dispersed in the other clusters*; (D) Intra-family Manhattan distances from Boolean hair protein profiles were shorter in mothers matched with their own children (p<0.0002) vs. 5000 simulated datasets created with mismatched mothers and children.
*Individuals are listed on the X- and Y-axes with their family identifier, with Mo for mother, C1 for the younger child, and C2 for the older child in each family.
3.3. Age- and sex-related differences in hair proteins
Both PCA32–34 and tSNE35, 36 were used to reduce the data dimensionality and to identify the major contributors of hair protein variability. Principal components 1-5 accounted for 61.6% of hair protein variability for all subjects, 57.5% for all children, 84.0% for all mothers, 60.8% for mothers and related children, and 62.3% for mothers and unrelated children.
Age differences were observed by plotting the first two principal components (PC1, PC2) and tSNE dimensions (Figure 3). We observed two separate clusters of the younger children and the mothers, with the older children dispersed across these groups (Figure 3A). Similar clusters were observed from the remaining principal components. The tSNE projections also showed mothers located separately from the children (Figure 3B). Proteins driving these differences showed higher spectral counts in mothers vs. children for SERPINB4 (serine protease inhibitor), POF1B (actin filament binder), PLEC (cytoskeleton binding protein), A2ML1 (α2-macroglobulin-like proteinase inhibitor), HIST1H3A (histone), UQCRQ (electron transfer from ubiquinol to cytochrome C), and AHCY (adenosylhomocysteine hydrolase). In contrast, mammaglobin-B (SCGB2A1), a heterodimerization protein that binds androgen and other steroids, was observed only in children (Table 2). Older children had higher spectral counts for PLEC (plectin), EIF3A (eukaryotic translation initiation factor 3), AHCY (adenosylhomocysteinase), HAL (histidine ammonia- lyase), and TUBA1C (Tubulin alpha 1c), whereas younger children had higher protein spectral counts for SCGB2A1 (secretoglobin 2A member 1) and CSN2 (casein beta) (Table 3).
Figure 3. Age and Sex-related Differences in Hair Proteins.
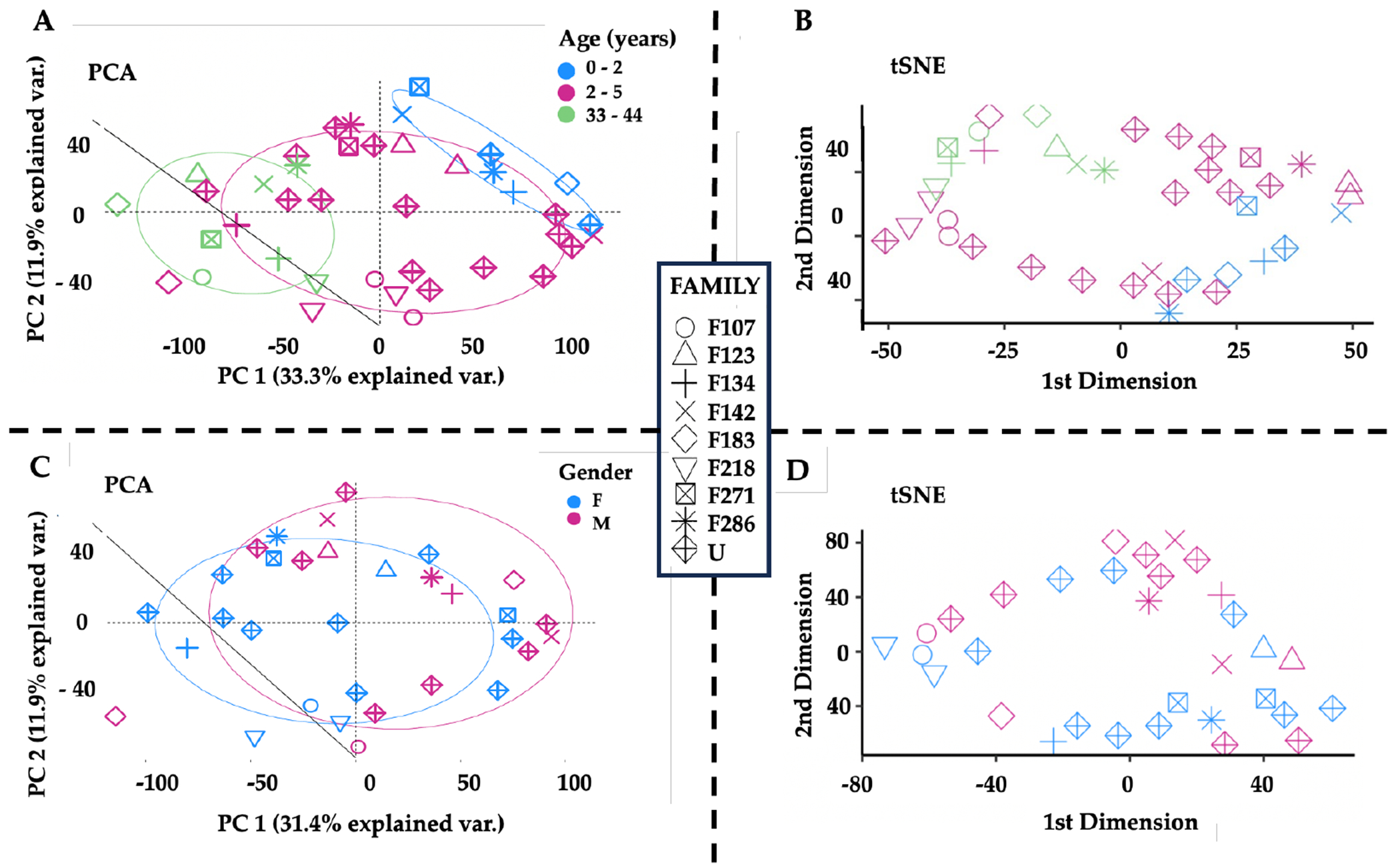
Note. (A) The first two principal components showing spatial separations by age, with children above 2 years (pink) located in between the children 0-2 years (blue, upper right) and the mothers (green, lower left). (B) The first two tSNE dimensions by age, showing mothers in the left upper quadrant separate from the children. Higher spectral counts for 7/17 hair proteins occurred in mothers (SERPINB4, POF1B, PLEC, A2ML1, HIST1H3A, UQCRQ, AHCY) and one protein (SCGB2A1) in children (Kruskal-Wallis ANOVA and post hoc Benjamini-Hochberg corrections. (C) PCA analyses of all children showing overlapping circles for girls (blue) and boys (pink). (D) tSNE dimensions by sex, showing overlap between boys and girls. Higher spectral counts were observed for CSN2 (Casein beta) in boys (p=0.0184) and ALMS1 (Alström syndrome protein 1) in girls (p=0.0214) (see Table 3).
Table 2.
Hair Proteins Mediating Differences between Mothers and Children
| Entrez Gene Name | Gene Symbol: human | Expr Log Ratio | P-value | Location | Type(s) |
|---|---|---|---|---|---|
| Involucrin | IVL | −2.85 | 0.0576 | Cytoplasm | other |
| Serpin family B member 4 | SERPINB4 | −2.452 | 0.0009*** | Cytoplasm | other |
| POF1B actin binding protein | POF1B | −2.097 | 0.0151* | Plasma Membrane | other |
| Plectin | PLEC | −1.886 | 0.0004*** | Cytoplasm | other |
| Alpha-2-macroglobulin like 1 | A2ML1 | −1.858 | 0.0042** | Cytoplasm | other |
| H3 clustered histone 1 | HIST1H3A | −1.743 | 0.0038** | Nucleus | other |
| Ubiquinol-cytochrome c reductase complex III subunit VII | UQCRQ | −1.716 | 0.0007*** | Cytoplasm | enzyme |
| Adenosylhomocysteinase | AHCY | −1.472 | 0.0040** | Cytoplasm | enzyme |
| Heat shock protein family A (Hsp70) member 1A | HSPA1A | −1.35 | 0.0569 | Cytoplasm | enzyme |
| H2B clustered histone 9 | HIST1H2BH | −1.17 | 0.507 | Nucleus | other |
| Histidine ammonia-lyase | HAL | −1.087 | 0.0851 | Cytoplasm | enzyme |
| COPI coat complex subunit zeta 1 | COPZ1 | −0.931 | 0.158 | Cytoplasm | transporter |
| Eukaryotic translation initiation factor 3 subunit A | EIF3A | −0.8 | 0.0567 | Cytoplasm | other |
| Tubulin alpha 1c | TUBA1C | −0.526 | 0.262 | Cytoplasm | other |
| Casein beta | CSN2 | −0.269 | 0.491 | Extracellular Space | kinase |
| ATP citrate lyase | ACLY | −0.249 | 0.0954 | Cytoplasm | enzyme |
| Protein disulfide isomerase family A member 3 | PDIA3 | −0.051 | 0.884 | Cytoplasm | peptidase |
| Scinderin | SCIN | 0.028 | 0.221 | Cytoplasm | other |
| Alström syndrome protein 1, centrosome and basal body associated protein | ALMS1 | 0.18 | 0.572 | Cytoplasm | other |
| Histone H3.4 | HIST3H3 | 0.64 | 0.153 | Nucleus | other |
| Myeloperoxidase | MPO | 0.925 | 0.886 | Cytoplasm | enzyme |
| Secretoglobin family 2A member 1 | SCGB2A1 | 5.32 | 0.0008*** | Extracellular Space | other |
Note. Mothers showed higher spectral counts than children for 7/17 hair proteins (shaded blue), although children had higher spectral counts for SCGB2A1 (shaded orange). Of these, SCGB2A1 showed the most prominent results, with >5-fold differences from the mothers. Significance was based on Kruskal-Wallis ANOVA with post hoc Benjamini Hochberg corrections for multiple comparisons (*p-value ≤ 0.05, **p-value ≤ 0.01, ***p-value ≤ 0.001).
Table 3.
Hair Proteins Mediating Differences between Preschool Boys and Girls
| Entrez Gene Name | Gene Symbol: human | Expr Log Ratio | P-value | Location | Type(s) |
|---|---|---|---|---|---|
| Casein beta | CSN2 | −3.046 | 0.0184* | Extracellular Space | kinase |
| Serpin family B member 4 | SERPINB4 | −1.303 | 0.391 | Cytoplasm | other |
| Secretoglobin family 2A member 1 | SCGB2A1 | −1.036 | 0.0513 | Extracellular Space | other |
| Protein disulfide isomerase family A member 3 | PDIA3 | −0.78 | 0.662 | Cytoplasm | peptidase |
| ATP citrate lyase | ACLY | −0.531 | 0.585 | Cytoplasm | enzyme |
| Myeloperoxidase | MPO | −0.493 | 0.581 | Cytoplasm | enzyme |
| Involucrin | IVL | −0.476 | 0.804 | Cytoplasm | other |
| Eukaryotic translation initiation factor 3 subunit A | EIF3A | −0.295 | 0.226 | Cytoplasm | other |
| Alpha-2-macroglobulin like 1 | A2ML1 | −0.254 | 0.923 | Cytoplasm | other |
| Scinderin | SCIN | −0.187 | 0.375 | Cytoplasm | other |
| Heat shock protein family A (Hsp70) member 1A | HSPA1A/HSPA1B | −0.122 | 0.573 | Cytoplasm | enzyme |
| POF1B actin binding protein | POF1B | 0.094 | 0.875 | Plasma Membrane | other |
| Histone H3.4 | H3-4 | 0.139 | 0.938 | Nucleus | other |
| Histidine ammonia-lyase | HAL | 0.175 | 0.522 | Cytoplasm | enzyme |
| COPI coat complex subunit zeta 1 | COPZ1 | 0.225 | 0.536 | Cytoplasm | transporter |
| Tubulin alpha 1c | TUBA1C | 0.245 | 0.314 | Cytoplasm | other |
| H3 clustered histone 1 | H3C1 | 0.249 | 0.562 | Nucleus | other |
| Adenosylhomocysteinase | AHCY | 0.333 | 0.202 | Cytoplasm | enzyme |
| Plectin | PLEC | 0.441 | 0.256 | Cytoplasm | other |
| Ubiquinol-cytochrome c reductase complex III subunit VII | UQCRQ | 1.415 | 0.0976 | Cytoplasm | enzyme |
| H2B clustered histone 9 | H2BC9 | 1.423 | 0.221 | Nucleus | other |
| Alström syndrome protein 1, centrosome and basal body associated protein | ALMS1 | 1.754 | 0.0214* | Cytoplasm | other |
Note. Girls showed higher spectral counts than boys for several proteins (shaded orange), whereas boys had higher spectral counts for other proteins (shaded blue). CSN2 was significantly higher in boys, whereas ALMS1 was significantly higher in girls. Significance was based on Kruskal-Wallis ANOVA with post hoc Benjamini Hochberg corrections for multiple comparisons(*p-value ≤ 0.05).
Sex differences showed slightly higher spectral counts in girls vs. boys (p=0.038) but no difference in the number of proteins (Table 1). PCA analyses and tSNE projections showed overlapping clusters of boys and girls (Figure 3C, 3D). When comparing individual proteins, higher spectral counts were observed for CSN2 (Casein beta, p = 0.0184) in boys and ALMS1 (Alström syndrome protein 1, p = 0.0214) in girls (Table 3).
To further characterize the effects of early childhood and adulthood on hair proteins, Random Forest regressions44 were used to predict the participants’ age from their hair protein profiles. This model predicted age differences in mothers and children (R2=0.37, Figure 4A), but the regression model improved (R2=0.45) when mothers were removed from this analysis and only children were included in this predictive model (Figure 4B). Random Forest classifier algorithms showed an acceptable mean accuracy for classifying mothers and children based on their predicted vs. observed age (mean area under the ROC curve = 0.93, Figure 4C; Wilcoxon test p=0.00011, Figure 4).
Figure 4. Machine Learning Algorithms Predict Age and Sex from Hair Proteins.
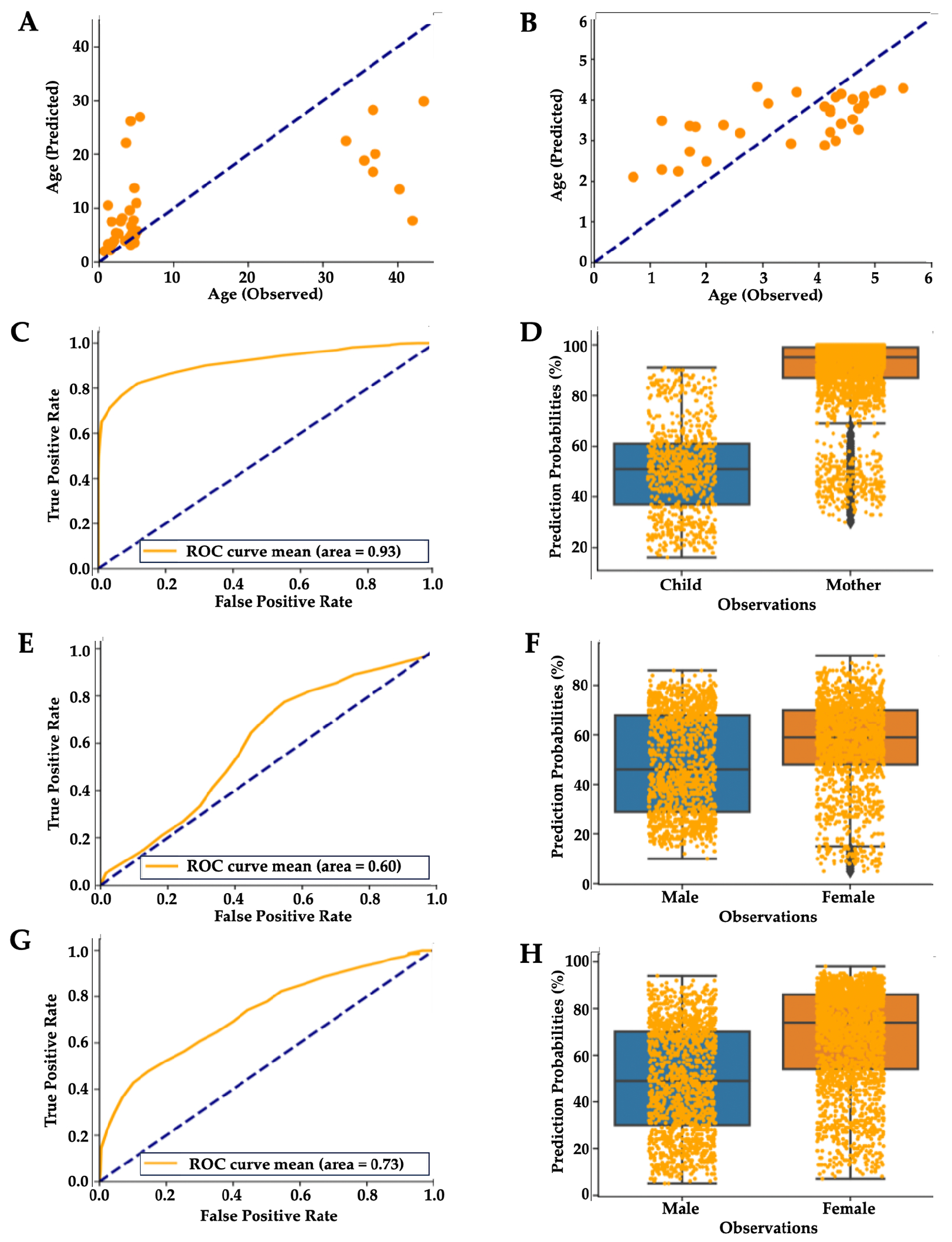
Note. Mean scatterplot from 100 runs of Random Forest regression showing (A) observed vs. predicted age for mothers and children (R2 0.37, p=0.00005) and (B) only for children (R2 0.45, p=0.00004). (C, D) Random Forest plot showing mean accuracy for classifying mothers and children based on hair proteins (mean area under the ROC curve = 0.93, Wilcoxon test p=0.00011). (E, F) Random Forest plot showing mean accuracy for classifying by sex based on hair proteins for children (mean area under the ROC curve = 0.60, Wilcoxon test p=0.1703). (G, H) Random Forest plot improved when classifying all participants including mothers and children (area under the ROC curve = 0.73, Wilcoxon test p = 0.00831).
A Random Forest classifier to predict sex from hair protein profiles in children could not reliably differentiate boys from girls (mean area under the ROC curve = 0.6, Figure 4E; Wilcoxon test p = 0.1703; Figure 4F), but predictions improved when classifying all participants including mothers and children (area under the ROC curve = 0.73, Figure 4G; Wilcoxon test p = 0.0083, Figure 4H). The latter result is likely due to the age-based distinction between mothers and children, although sample size-related effects cannot be ruled out (25 vs. 17 females).
3.4. Top contributors to hair protein variability
The top 5% proteins identified as the most prominent contributors, based on their total loading scores (TLS), explained 64.3% of hair protein variability in all individuals, 89.5% in all mothers, 57.5% in all children, 49.3% in mothers and related children, and 64.6% in mothers and unrelated children (Figure 5). Higher TLS indicates higher influence of that protein on total variability. Keratins and KAPs are structural components, but are usually considered as contaminants in most proteomics experiments, due to their high abundance in common lab analyses. We therefore performed PCA analyses for all individuals with (Figure 5A) and without excluding the keratins and KAPs (Figure 5B). Structural proteins contributed to hair protein variability but have limited biological significance. Separate PCA analyses performed to characterize the hair proteins observed in mothers (Figure 5C), children (Figure 5D), mothers and related children (Figure 5E), and mothers and unrelated children (Figure 5F) showed the same proteins as those ranked in all individuals and all children. Other than histones, no other proteins were common between mothers and children. TUBA1C, PLEC, SERPINB4, and UQCRQ were observed in multiple subgroups.
Figure 5. Top 5% Proteins Contributing to Hair Protein Variability.
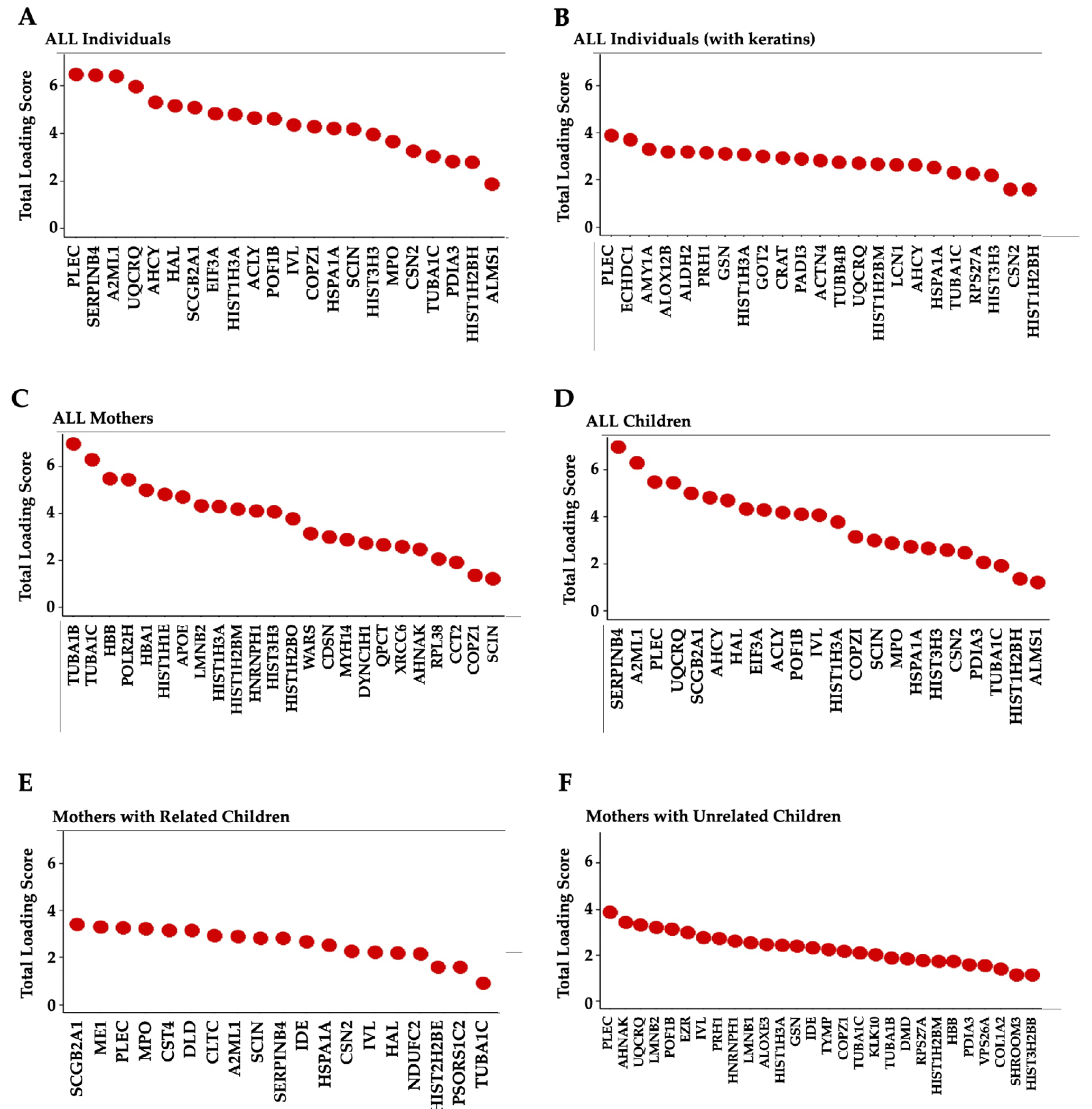
Note. The loading scores for each protein were weighted by the percent variance explained by the corresponding Principal Component and then summed to give the Total Loading Score (TLS) for each protein. The top 5% proteins based on their TLS were identified as the most prominent contributors in each group. (A) All individuals (N=40, 49% of hair protein variability), (B) All individuals including keratins and KAPs (N=40, 64.3% variability); (C) All mothers (n=8, 89.5% variability); (D) All children (n=32, 57.5% variability); (E) Mothers (n=8) and their biological children (n=16) (49.3% variability), and (F) Mothers (n=8) and unrelated children (n=16) (64.6% variability).
3.5. Biological role(s) of the strongest contributors to hair protein variability
Based on experimentally observed human data in the Ingenuity Knowledge Base, log- fold-change values of the top 5% proteins from our dataset were used to analyze direct and indirect relationships between protein molecules. Protein networks for the top 5% hair proteins contributing to age-related differences between mothers and children (Figure 6) and similar analyses for sex-related differences between girls and boys were examined (Figure 7). Using these molecular relationships as input for Ingenuity Pathway Analysis, we identified protein classes involved in cellular metabolism such as the protein ubiquitination pathway, Sirtuin signaling pathway, 14- 3-3 mediated signaling, Wnt-Ca++ pathway, histidine degradation, mitochondrial function, and oxidative phosphorylation (Figure 8). Other proteins were associated with immune responses, including phagosome maturation, IL-8 signaling, and regulation of macrophages, fibroblasts, and endothelial cells, or involved in the regulation of stress-related pathways, including corticotropin releasing hormone signaling, glucocorticoid receptor signaling, prolactin and aldosterone signaling. Finally, hair proteins associated with brain development including axonal guidance and gap junction signaling were also identified (Figure 8).
Figure 6. Protein Network for the Top 5% Hair Proteins Contributing to Age-related Differences between Mothers and Children.
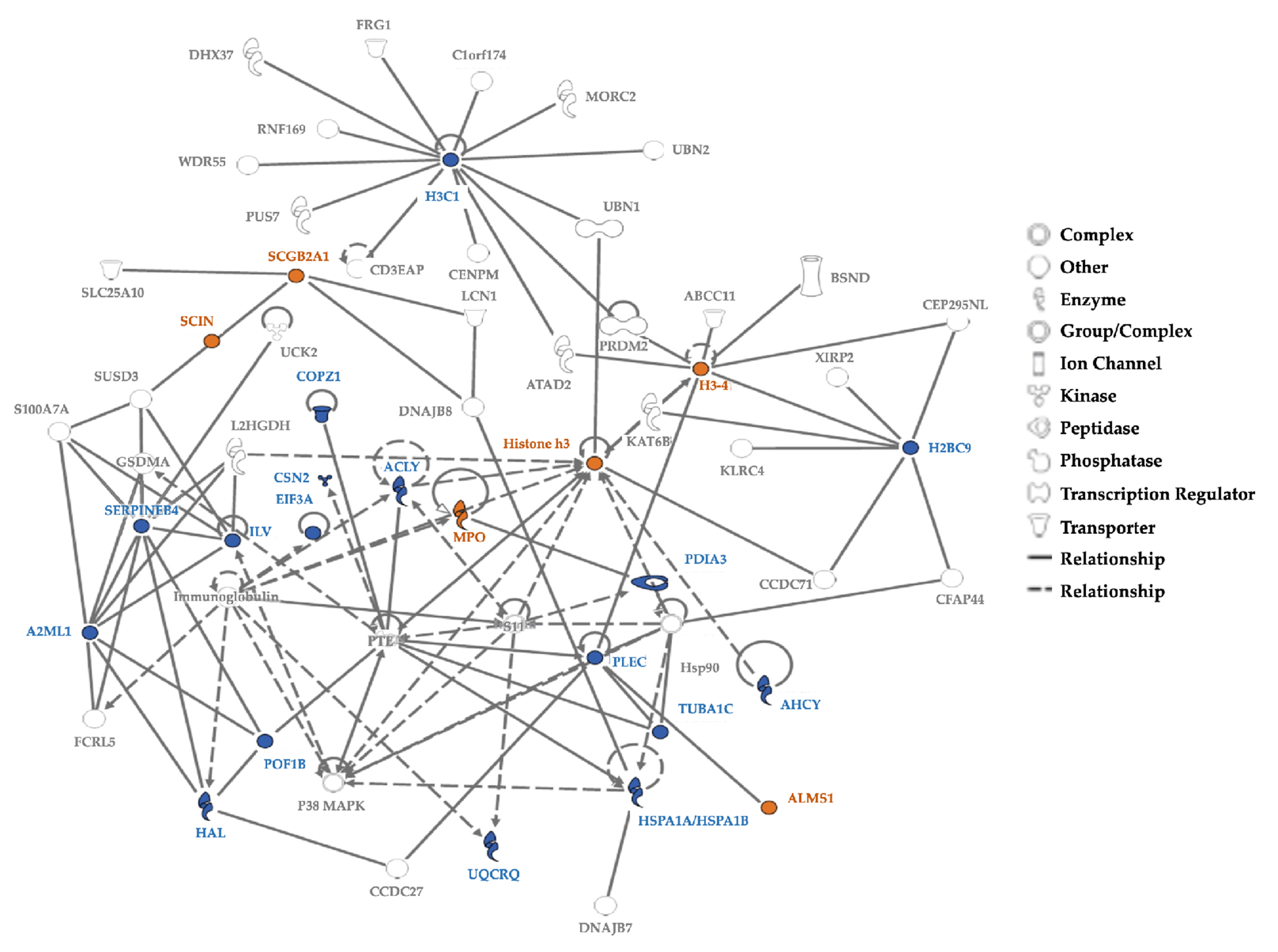
Note. Some hair proteins had higher spectral counts in children (Orange) and others had higher spectral counts in mothers (Blue); continuous lines show direct relationships and interrupted lines denote indirect relationships. Mothers show higher spectral counts mostly for ‘enzymes’ and ‘peptidases’ involved in cellular and metabolic processes, while proteins with higher spectral counts in children belong to the ‘other’ group involved in growth and biological maturation.
Figure 7. Protein Network for Top 5% Hair Proteins Contributing to Sex Differences between Boys and Girls.
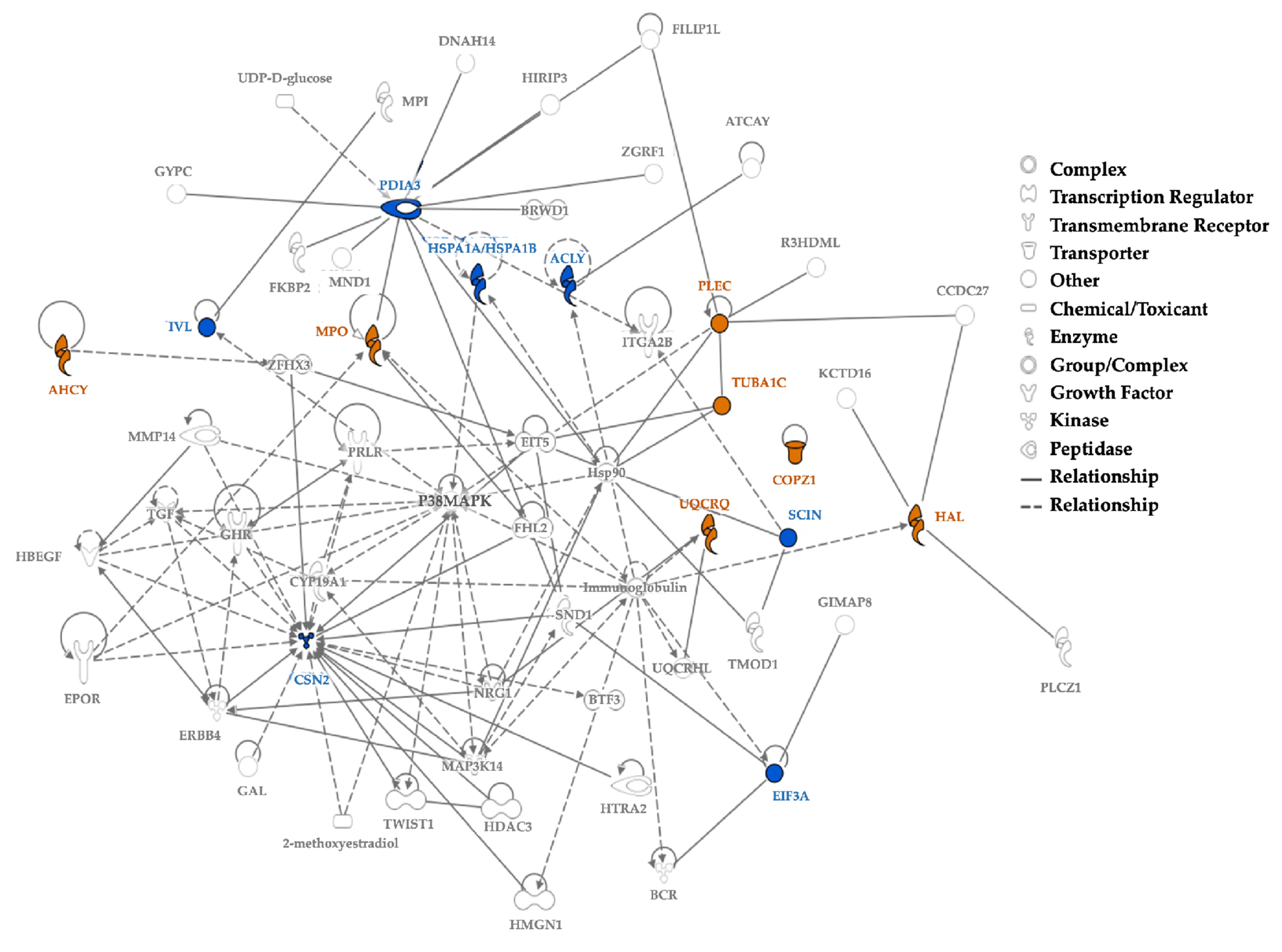
Note. Protein network for top 5% hair proteins contributing to sex differences between boys and girls. Some proteins had higher spectral counts in girls (Orange) and others had higher spectral counts in boys (Blue); continuous lines show direct relationships and interrupted lines denote indirect relationships. Girls show higher protein spectral counts mostly for ‘enzymes’ or ‘ransporters’ associated with cellular localization and metabolic processes. Proteins with higher spectral count in boys are ‘enzymes’ like ‘kinases’ or ‘peptidases’ associated with biological regulation of cellular and metabolic processes.
Figure 8. Canonical Pathways.
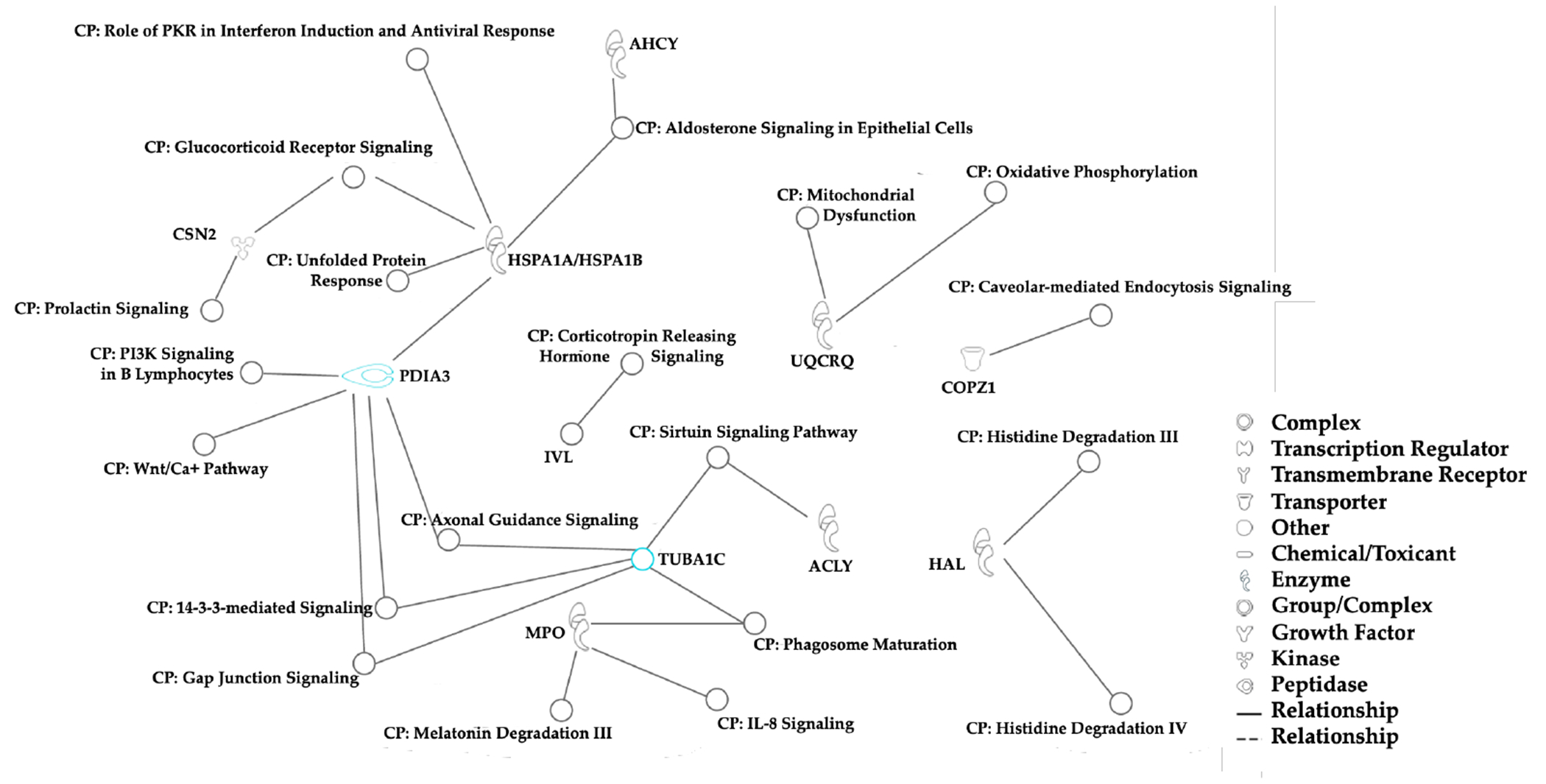
Note. Canonical pathways associated with biologically significant proteins from the top 5% variables in all individuals (n = 40) contributing to age- and sex-related differences were identified using the Ingenuity Pathway Analysis. Most of these proteins are involved in cellular metabolism, immune responses, brain development, and stress regulatory pathways.
3.6. ELISA validation of other non-structural hair proteins
Select proteins of interest detected via standard UPLC-MS/MS methods were validated and quantified using commercially available ELISA kits. The first portion of the surplus volumes of individual protein extracts remaining after UPLC-MS/MS and HCC measures were pooled based on low, intermediate, or high HCC values. Hair sample pools were used to quantify cortisol and arginine vasopressin (AVP), which potentiate hypothalamic release of corticotropin releasing hormone45, 46, Cu/Zn superoxide dismutase (SOD1), an important cellular defense against reactive oxygen species47, 48, HTrA serine peptidase 2 (HTRA2), a mitochondrial protease chaperone that regulates cellular proteostasis and cell-signaling events49; and glial fibrillary acid protein (GFAP), a protein responsible for the cytoskeletal structure of glial cells50, 51 (Table 4).
Table 4.
Validation and Correlates of Proteins Detected in Human Scalp Hair via UPLC- MS/MS
| Cortisol Level Based Sample Pools Std Method | Cortisol ng/ml | AVP pg/ml | Cu/Zn SOD ng/ml | HTRA2 ng/ml | GFAP ng/ml |
|---|---|---|---|---|---|
| Low Child pool cortisol (n = 72) | 40.84 | 14.81 | 0.25 | 7.54 | 0.00 |
| Moderate Child pool cortisol (n = 21) | 60.34 | 11.91 | 0.18 | 4.61 | 0.41 |
| High Child pool cortisol (n = 7) | 190.89 | 7.18 | 0.23 | 9.14 | n/a |
| Low Father pool cortisol (n = 13) | 22.39 | 8.36 | 0.63 | 9.65 | 2.64 |
| Low Mother pool cortisol (n = 39) | 17.24 | 7.88 | 0.49 | 7.71 | 1.45 |
| High Mother pool cortisol (n = 7) | 36.77 | 11.68 | n/a | n/a | n/a |
Note. Hair cortisol concentrations (HCC) were measured for 100 children and 49 parents. Groups of children and parents were determined based on low, moderate, or high HCC values. Each of the six pools of samples were loaded in duplicate on the respective ELISA plates for testing arginine vasopressin (AVP), Cu/Zn superoxide dismutase (SOD1), HTrA serine peptidase 2 (HTRA2), and glial fibrillary acid protein (GFAP). Each methods passed our criteria for low inter-assay (≤8% CV) and intra-assay (≤ 6%) variability.
4. Discussion
The chemical composition of hair17, 52–56 and its structural proteins (keratins, KAPs) are well-studied22–25, but minimal data exists on non-structural hair proteins. This study represents the first description of non-structural hair proteins in mothers and young children. We found 2,269 non-structural hair proteins with important differences between mothers and children, age- and sex-related differences among preschool children, and conserved hair protein profiles within families. Hair proteins driving variability in different populations were found to play vital roles in functions other than those of trichocytes in the hair follicle, including cellular metabolic pathways, brain development, immune signaling, and stress regulation.
We observed age-related hair protein profiles in children and mothers, with distinct patterns emerging in multiple analyses. Differences between mothers and children were largely driven by increased maternal expression of SERPINB4, PLEC, and UQCRQ. SERPINB4 is a granzyme inhibitor linked with squamous cell carcinomas and chronic liver disease57–59, Plectin mutations are linked with epidermolysis bullosa simplex and may be a susceptibility gene for testicular germ cell tumors60–62, and UQCRQ is a nuclear protein in the mitochondrial respiratory chain complex III essential for brain development63. Mammaglobin-B (SCGB2A1), which is linked with familial febrile seizures in preschool children64, 65 and chemoresistant cancers in adults66, was observed only in children’s hair.
We found minimal sex differences in early childhood, confirmed by Random Forest predictive models. Biological pathways for cellular metabolism and innate immunity appeared more prominent in girls, whereas brain development and stress regulation appeared more prominent in boys. Perhaps sex differences in hair proteins may be accentuated following the onset of puberty67. Although hair protein profiles were conserved in mothers and their biological children, future studies in mother-child dyads and monozygotic vs. dizygotic twins will be required to explore to gene × environment interactions responsible for hair protein profiles68.
From Ingenuity Pathway Analysis, we identified the hair proteins associated with axonal guidance69 and gap junction signaling70, both signifying important mechanisms in brain development. By cross-referencing the Uniprot database (https://www.uniprot.org/) with the Allen Brain Atlas (https://human.brain-map.org/static/brainexplorer) and the Human Brain Protein Atlas (https://www.proteinatlas.org/search/brain_category), we identified 191 hair proteins that are regionally enriched in the brain. Further studies will examine whether hair proteomics can complement neuroimaging and neurophysiological studies of early brain development12. A study from Nepal reported specific plasma proteins associated with higher non-verbal intelligence and pro-inflammatory proteins associated with lower intelligence in children71. This study, however, used an FDR of 5%, whereas the FDR threshold for our analyses was set at 1% or lower. Future developmental studies with large sample sizes could correlate hair proteins with cognitive or behavioral outcomes, thus investigating their role in brain development72. Thus, unbiased or targeted protein profiles from serial hair samples (or sequential hair segments in the same hair sample) could be used as probes for child development73, 74 or life-course studies43, 75, 76.
These findings must be interpreted in the light of three limitations. First, our sample size of 32 children was insufficient to examine developmental differences at each age in the preschool period. We selected healthy children from homogenous socioeconomic environments; they did not experience any adverse conditions and therefore, our data do not represent the full range of hair protein profiles present in the general population. Despite this, our sample size is larger than most other studies of hair proteomics in adults and it is the first to include mothers and children. Our study design also allowed us to investigate differences in hair protein profiles between related and unrelated individuals, as well as differences between adults and children.
Second, our proteomics platform relied on peptide spectral matches, which presented only semi- quantitative data on the abundance of hair proteins in individuals. Since this is the first study investigating non-structural proteins from hair in humans, we chose a ‘shot-gun’ proteomics approach rather than targeted and more quantitative approaches. We did, however, orthogonally confirm the presence of specific hair proteins using well-validated ELISA assays. Having established the first hair protein libraries in mothers and children, future studies can be designed for the quantitation of specific protein targets or protein groups. Lastly, we did not correlate hair proteins with the child’s developmental milestones or their cognitive and behavioral data. We feel that the sample size limitations at each age would preclude any generalizable conclusions from such analyses.
Despite these limitations, our initial findings reveal the potential importance of non-structural hair proteins as biomarkers for brain development, or other cellular regulatory pathways, providing a rich source of chronologically ordered information for life-course studies and early childhood development.
5. Conclusions
This research shows that exposures to family adversity, chronic stress, parenting and caregiving practices, and early attachment can be monitored by serial hair sampling to determine the child’s health status, brain development, physical and mental health. We found that hair protein profiles are related to age, sex, and family relationships. The top 5% contributors to variability in hair protein patterns are associated with regulation of: (a) immune pathways (for phagosome maturation, IL-8 signaling, PKR interferon induction, regulation of fibroblasts, macrophages, and endothelial cells); (b) stress signaling pathways (for corticotropin releasing hormone, glucocorticoid receptors, prolactin, and aldosterone); (c) brain development (axonal guidance, gap junction signaling); and (d) cellular metabolic pathways (for oxidative phosphorylation, mitochondrial dysfunction, histidine degradation, caveolar-mediated endocytosis, as well as the heat shock proteins, 14-3-3 protein, Sirtuin, and Wnt/Ca++ signaling pathways). When amalgamated with well-established methods for tracking changes in hair hormones, this approach may provide mechanistic explanations for the developmental sequences leading to HPA axis (dys)regulation in early life. The assessment of parent-child synchrony, the child’s circadian rhythms, or positive and negative attachments need not depend on subjective questionnaires, invasive blood sampling, or neuroimaging. We propose that non-invasive hair sampling and tandem mass spectrometry methods can be used to compare non-structural hair protein profiles in healthy, normal children against hair protein profiles in subpopulations of children with confirmed exposures to toxic stress and/or adverse living conditions. Future studies are designed to quantify and characterize panels of related hair proteins to probe changes in the immune system, stress regulation, brain development, and cellular metabolism to monitor environmental influences on the health status and development of children2.
6. Patents
Pursuant to the Patent Cooperation Treaty, an international patent was filed on November 10, 2022, identifiable in the United States Patent and Trademark Office by Application No. US2022/079619.
Acknowledgments:
The authors sincerely acknowledge the leadership of Santa Clara County Office of Education, the children, parents and staff at Bing Nursery School, Stanford Arboretum Children’s Center, Children’s Center of the Stanford Community, Stanford Madera Grove Children’s Center, McKinley, Rouleau, and Wool Creek Head Start Centers for their partnership. We also thank Drs. David K. Stevenson, Gary Shaw, and Timothy Cornell for their valuable suggestions on previous versions of this manuscript; Sahil Tembulkar and Jitka Hiscox for help with collecting or processing hair samples, Tanida Plamintr for administrative assistance, and Grant Padia for grant management.
Funding:
The Maternal & Child Health Research Institute (MCHRI) at Stanford, the Eunice Kennedy Shriver National Institute for Child Health & Human Development (R01 HD099296), the National Cancer Institute (P30 CA124435) for the Stanford Cancer Institute Proteomics/Mass Spectrometry Shared Resource, and the National Institute of General Medical Sciences (R35 GM138353) supported this research. Study sponsors had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, approval, or decision to publish this manuscript.
Footnotes
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Stanford University (eProtocol #41369; approved June 16, 2017).
Informed Consent Statement: Written informed consent was obtained from all subjects enrolled in the study.
Conflicts of Interest: The authors declare no conflict of interest. The authors received no honoraria, grants, or other payments for writing this manuscript and they report no relevant financial relationships and no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability Statement:
Minimal datasets related to results presented in this manuscript are available on request from the corresponding author. These data are not publicly available due to privacy, proprietary, and ethical considerations.
References
- 1.Bitsko RH, Holbrook JR, Robinson LR, Kaminski JW, Ghandour R, Smith C, Ed S, Peacock G. Health Care, Family, and Community Factors Associated with Mental, Behavioral, and Developmental Disorders in Early Childhood - United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2016;65(9):221–6. Epub 20160311. doi: 10.15585/mmwr.mm6509a1. [DOI] [PubMed] [Google Scholar]
- 2.Lopez M, Ruiz MO, Rovnaghi CR, Tam GK, Hiscox J, Gotlib IH, Barr DA, Carrion VG, Anand KJS. The social ecology of childhood and early life adversity. Pediatr Res. 2021;89(2):353–67. Epub 2021/01/20. doi: 10.1038/s41390-020-01264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubowitz H, Kressly SJ. Documenting Psychosocial Problems in Children’s Electronic Health Records. JAMA Pediatr. 2023;177(9):881–2. doi: 10.1001/jamapediatrics.2023.2380. [DOI] [PubMed] [Google Scholar]
- 4.Rovnaghi CR, Rigdon J, Roue JM, Ruiz MO, Carrion VG, Anand KJS. Longitudinal Trajectories of Hair Cortisol: Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Early Childhood. Front Pediatr. 2021;9:740343. Epub 2021/10/29. doi: 10.3389/fped.2021.740343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand KJS, Rigdon J, Rovnaghi CR, Qin F, Tembulkar S, Bush N, LeWinn K, Tylavsky FA, Davis R, Barr DA, Gotlib IH. Measuring socioeconomic adversity in early life. Acta Paediatr. 2019;108(7):1267–77. Epub 2019/01/08. doi: 10.1111/apa.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;371:m3048. Epub 2020/10/30. doi: 10.1136/bmj.m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y, Volgyi E, Rovnaghi CR, Moore A, Tran QT, Tylavsky FA. Early adversity, socioemotional development, and stress in urban 1-year-old children. J Pediatr. 2013;163(6):1733–9 e1. doi: 10.1016/j.jpeds.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Erema VV, Yakovchik AY, Kashtanova DA, Bochkaeva ZV, Ivanov MV, Sosin DV, Matkava LR, Yudin VS, Makarov VV, Keskinov AA, Kraevoy SA, Yudin SM. Biological Age Predictors: The Status Quo and Future Trends. Int J Mol Sci. 2022;23(23). Epub 20221201. doi: 10.3390/ijms232315103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand KJS, Rovnaghi CR, Rigdon J, Qin F, Tembulkar S, Murphy LE, Barr DA, Gotlib IH, Tylavsky FA. Demographic and psychosocial factors associated with hair cortisol concentrations in preschool children. Pediatr Res. 2020;87(6):1119–27. Epub 2019/12/04. doi: 10.1038/s41390-019-0691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmers I, Quaedflieg CWEM, Hsu C, Healthcote L, Rovnaghi CR, and Simons L The interaction between stress and chronic pain through the lens of threat learning. Neuroscience and Biobehavioral Reviews. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reh RK, Dias BG, Nelson CA 3rd, Kaufer D, Werker JF, Kolb B, Levine JD, Hensch TK. Critical period regulation across multiple timescales. Proc Natl Acad Sci U S A. 2020;117(38):23242–51. Epub 2020/06/07. doi: 10.1073/pnas.1820836117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TT, Jernigan TL. Brain development during the preschool years. Neuropsychol Rev. 2012;22(4):313–33. Epub 2012/09/26. doi: 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jernigan TL, Brown TT, Hagler DJ Jr., Akshoomoff N, Bartsch H, Newman E, Thompson WK, Bloss CS, Murray SS, Schork N, Kennedy DN, Kuperman JM, McCabe C, Chung Y, Libiger O, Maddox M, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Sowell ER, Kenet T, Kaufmann WE, Mostofsky S, Amaral DG, Dale AM, Pediatric Imaging N, Genetics S. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage. 2016;124(Pt B):1149–54. Epub 2015/05/06. doi: 10.1016/j.neuroimage.2015.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, Lagopoulos J, Hatton SN, Hickie IB, Carballedo A, Brooks SJ, Vuletic D, Uhlmann A, Veer IM, Walter H, Bulow R, Volzke H, Klinger-Konig J, Schnell K, Schoepf D, Grotegerd D, Opel N, Dannlowski U, Kugel H, Schramm E, Konrad C, Kircher T, Juksel D, Nenadic I, Krug A, Hahn T, Steinstrater O, Redlich R, Zaremba D, Zurowski B, Fu CHY, Dima D, Cole J, Grabe HJ, Connolly CG, Yang TT, Ho TC, LeWinn KZ, Li M, Groenewold NA, Salminen LE, Walter M, Simmons AN, van Erp TGM, Jahanshad N, Baune BT, van der Wee NJA, van Tol MJ, Penninx B, Hibar DP, Thompson PM, Veltman DJ, Schmaal L, Frodl T, for the E-MDDC. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50(6):1020–31. Epub 2019/05/16. doi: 10.1017/S003329171900093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warrier V, Stauffer EM, Huang QQ, Wigdor EM, Slob EAW, Seidlitz J, Ronan L, Valk SL, Mallard TT, Grotzinger AD, Romero-Garcia R, Baron-Cohen S, Geschwind DH, Lancaster MA, Murray GK, Gandal MJ, Alexander-Bloch A, Won H, Martin HC, Bullmore ET, Bethlehem RAI. Genetic insights into human cortical organization and development through genome-wide analyses of 2,347 neuroimaging phenotypes. Nat Genet. 2023. Epub 20230817. doi: 10.1038/s41588-023-01475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz MO, Rovnaghi CR, Tembulkar S, Qin F, Truong L, Shen S, Anand KJS. Linear hair growth rates in preschool children. Pediatr Res. 2023. Epub 20230904. doi: 10.1038/s41390-023-02791-z. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaides N, Rothman S. Studies on the chemical composition of human hair fat. II. The overall composition with regard to age, sex and race. J Invest Dermatol. 1953;21(1):9–14. [DOI] [PubMed] [Google Scholar]
- 18.Adeola HA, Van Wyk JC, Arowolo A, Ngwanya RM, Mkentane K, Khumalo NP. Emerging Diagnostic and Therapeutic Potentials of Human Hair Proteomics. Proteomics Clin Appl. 2018;12(2). Epub 2017/09/30. doi: 10.1002/prca.201700048. [DOI] [PubMed] [Google Scholar]
- 19.Tobin DJ. Chapter 2. In: Tobin DJ, editor. Hair in toxicology: an important bio-monitor. Cambridge: (Royal Society of Chemistry (U.K.); 2005. p. 34–56. [Google Scholar]
- 20.Villain M, Cirimele V, Kintz P. Hair analysis in toxicology. Clin Chem Lab Med. 2004;42(11):1265–72. Epub 2004/12/04. doi: 10.1515/CCLM.2004.247. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Rice RH, Lee YM. Proteome analysis of human hair shaft: from protein identification to posttranslational modification. Mol Cell Proteomics. 2006;5(5):789–800. Epub 2006/02/01. doi: 10.1074/mcp.M500278-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Laatsch CN, Durbin-Johnson BP, Rocke DM, Mukwana S, Newland AB, Flagler MJ, Davis MG, Eigenheer RA, Phinney BS, Rice RH. Human hair shaft proteomic profiling: individual differences, site specificity and cuticle analysis. PeerJ. 2014;2:e506. Epub 2014/08/29. doi: 10.7717/peerj.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson TL, Moini M, Eckenrode BA, Allred BM, Donfack J. Protein extraction from human anagen head hairs 1-millimeter or less in total length. Biotechniques. 2018;64(4):170–6. Epub 2018/04/18. doi: 10.2144/btn-2018-2004. [DOI] [PubMed] [Google Scholar]
- 24.Wu PW, Mason KE, Durbin-Johnson BP, Salemi M, Phinney BS, Rocke DM, Parker GJ, Rice RH. Proteomic analysis of hair shafts from monozygotic twins: Expression profiles and genetically variant peptides. Proteomics. 2017;17(13-14). Epub 2017/05/26. doi: 10.1002/pmic.201600462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker GJ, Leppert T, Anex DS, Hilmer JK, Matsunami N, Baird L, Stevens J, Parsawar K, Durbin-Johnson BP, Rocke DM, Nelson C, Fairbanks DJ, Wilson AS, Rice RH, Woodward SR, Bothner B, Hart BR, Leppert M. Demonstration of Protein-Based Human Identification Using the Hair Shaft Proteome. PLoS One. 2016;11(9):e0160653. Epub 2016/09/08. doi: 10.1371/journal.pone.0160653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tawfik DS, Rovnaghi C, Profit J, Cornell TT, Anand KJS. Prevalence of burnout and its relation to the neuroendocrine system among pediatric residents during the early Covid-19 pandemic: A pilot feasibility study. Compr Psychoneuroendocrinol. 2023;14:100174. Epub 20230201. doi: 10.1016/j.cpnec.2023.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4(3):207–14. Epub 2007/03/01. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, Perez E, Uszkoreit J, Pfeuffer J, Sachsenberg T, Yilmaz S, Tiwary S, Cox J, Audain E, Walzer M, Jarnuczak AF, Ternent T, Brazma A, Vizcaino JA. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47(D1):D442–D50. Epub 2018/11/06. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand KJS, Leib RD, Rovnaghi CR and Singhal K PRIDE Repository, ProteomeXchange. PRIDE Repository, ProteomeXchange, 2019. December 22, 2019. Report No. [Google Scholar]
- 30.Deutsch EW, Csordas A, Sun Z, Jarnuczak A, Perez-Riverol Y, Ternent T, Campbell DS, Bernal-Llinares M, Okuda S, Kawano S, Moritz RL, Carver JJ, Wang M, Ishihama Y, Bandeira N, Hermjakob H, Vizcaino JA. The ProteomeXchange consortium in 2017: supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017;45(D1):D1100–D6. Epub 20161018. doi: 10.1093/nar/gkw936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slominski R, Rovnaghi CR, Anand KJ. Methodological Considerations for Hair Cortisol Measurements in Children. Ther Drug Monit. 2015;37(6):812–20. doi: 10.1097/FTD.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdi HaW LJ . Principal component analysis. WIREs Comput Stat. 2010;2:433–59. [Google Scholar]
- 33.Hotelling H. Analysis of a complex of statistical variables into principal components. Journal of Educational Psychology 1933;24:417–41 [Google Scholar]
- 34.Jolliffe IT. Principal component analysis. New York, NY: Springer; 1986. [Google Scholar]
- 35.Linderman GC, Steinerberger S. Clustering with t-SNE, provably. SIAM J Math Data Sci. 2019;1(2):313–32. Epub 2019/01/01. doi: 10.1137/18m1216134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Maaten LaH G Visualizing data using t-SNE. Journal of Machine Learning Research. 2008;9:2579–605. [Google Scholar]
- 37.Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017;21:29–36. Epub 2017/04/12. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barthelemy NR, Bednarczyk A, Schaeffer-Reiss C, Jullien D, Van Dorsselaer A, Cavusoglu N. Proteomic tools for the investigation of human hair structural proteins and evidence of weakness sites on hair keratin coil segments. Anal Biochem. 2012;421(1):43–55. Epub 2011/11/08. doi: 10.1016/j.ab.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Gomes J, Au F, Basak A, Cakmak S, Vincent R, Kumarathasan P. Maternal blood biomarkers and adverse pregnancy outcomes: a systematic review and meta-analysis. Crit Rev Toxicol. 2019;49(6):461–78. Epub 2019/09/12. doi: 10.1080/10408444.2019.1629873. [DOI] [PubMed] [Google Scholar]
- 40.Handelman SK, Romero R, Tarca AL, Pacora P, Ingram B, Maymon E, Chaiworapongsa T, Hassan SS, Erez O. The plasma metabolome of women in early pregnancy differs from that of non-pregnant women. PLoS One. 2019;14(11):e0224682. Epub 2019/11/15. doi: 10.1371/journal.pone.0224682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Erez O, Maymon E, Chaemsaithong P, Xu Z, Pacora P, Chaiworapongsa T, Done B, Hassan SS, Tarca AL. The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study. Am J Obstet Gynecol. 2017;217(1):67 e1–e21. Epub 2017/03/07. doi: 10.1016/j.ajog.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lara J, Cooper R, Nissan J, Ginty AT, Khaw KT, Deary IJ, Lord JM, Kuh D, Mathers JC. A proposed panel of biomarkers of healthy ageing. BMC Med. 2015;13:222. Epub 2015/09/17. doi: 10.1186/s12916-015-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deelen J, Kettunen J, Fischer K, van der Spek A, Trompet S, Kastenmuller G, Boyd A, Zierer J, van den Akker EB, Ala-Korpela M, Amin N, Demirkan A, Ghanbari M, van Heemst D, Ikram MA, van Klinken JB, Mooijaart SP, Peters A, Salomaa V, Sattar N, Spector TD, Tiemeier H, Verhoeven A, Waldenberger M, Wurtz P, Davey Smith G, Metspalu A, Perola M, Menni C, Geleijnse JM, Drenos F, Beekman M, Jukema JW, van Duijn CM, Slagboom PE. A metabolic profile of all-cause mortality risk identified in an observational study of 44,168 individuals. Nat Commun. 2019;10(1):3346. Epub 2019/08/23. doi: 10.1038/s41467-019-11311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breiman L. Random Forest. Machine Learning 2001;45:5–32. [Google Scholar]
- 45.Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm Behav. 2012;61(3):359–79. Epub 20111229. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Muscogiuri G, Barrea L, Annunziata G, Vecchiarini M, Orio F, Di Somma C, Colao A, Savastano S. Water intake keeps type 2 diabetes away? Focus on copeptin. Endocrine. 2018;62(2):292–8. Epub 20180719. doi: 10.1007/s12020-018-1680-7. [DOI] [PubMed] [Google Scholar]
- 47.Chandrasekharan B, Montllor-Albalate C, Colin AE, Andersen JL, Jang YC, Reddi AR. Cu/Zn Superoxide Dismutase (Sod1) regulates the canonical Wnt signaling pathway. Biochem Biophys Res Commun. 2021;534:720–6. Epub 20201118. doi: 10.1016/j.bbrc.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Une M, Yamakawa M, Watanabe Y, Uchino K, Honda N, Adachi M, Nakanishi M, Umezawa A, Kawata Y, Nakashima K, Hanajima R. SOD1-interacting proteins: Roles of aggregation cores and protein degradation systems. Neurosci Res. 2021;170:295–305. Epub 20200726. doi: 10.1016/j.neures.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Nam MK, Seong Y, Jeong GH, Yoo SA, Rhim H. HtrA2 regulates alpha-Synuclein-mediated mitochondrial reactive oxygen species production in the mitochondria of microglia. Biochem Biophys Res Commun. 2023;638:84–93. Epub 20221118. doi: 10.1016/j.bbrc.2022.11.049. [DOI] [PubMed] [Google Scholar]
- 50.Ganne A, Balasubramaniam M, Griffin WST, Shmookler Reis RJ, Ayyadevara S. Glial Fibrillary Acidic Protein: A Biomarker and Drug Target for Alzheimer’s Disease. Pharmaceutics. 2022;14(7). Epub 20220626. doi: 10.3390/pharmaceutics14071354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin STG, Stanley LC, Yeralan O, Rovnaghi CR, and Marshak DR Cytokines in Human Neurodegenerative Disease. De Sousa EB, editor. San Diego: Academic Press, Inc.; 1993. 295 p. [Google Scholar]
- 52.Cruz CF, Fernandes MM, Gomes AC, Coderch L, Marti M, Mendez S, Gales L, Azoia NG, Shimanovich U, Cavaco-Paulo A. Keratins and lipids in ethnic hair. Int J Cosmet Sci. 2013;35(3):244–9. doi: 10.1111/ics.12035. [DOI] [PubMed] [Google Scholar]
- 53.Franbourg A, Hallegot P, Baltenneck F, Toutain C, Leroy F. Current research on ethnic hair. J Am Acad Dermatol. 2003;48(6 Suppl):S115–9. Epub 2003/06/06. doi: 10.1067/mjd.2003.277. [DOI] [PubMed] [Google Scholar]
- 54.Horvath AL. Solubility of structurally complicated materials: 3. Hair. ScientificWorldJournal. 2009;9:255–71. Epub 20090427. doi: 10.1100/tsw.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marti M, Barba C, Manich AM, Rubio L, Alonso C, Coderch L. The influence of hair lipids in ethnic hair properties. Int J Cosmet Sci. 2016;38(1):77–84. doi: 10.1111/ics.12261. [DOI] [PubMed] [Google Scholar]
- 56.Wilson ASaDJT. Aging Hair. Berlin, Germany: Springer-Verlag; 2010. p. 249–61. [Google Scholar]
- 57.Biasiolo A, Tono N, Ruvoletto M, Quarta S, Turato C, Villano G, Beneduce L, Fassina G, Merkel C, Gatta A, Pontisso P. IgM-linked SerpinB3 and SerpinB4 in sera of patients with chronic liver disease. PLoS One. 2012;7(7):e40658. Epub 2012/07/19. doi: 10.1371/journal.pone.0040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markovina S, Wang S, Henke LE, Luke CJ, Pak SC, DeWees T, Pfeifer JD, Schwarz JK, Liu W, Chen S, Mutch D, Wang X, Powell MA, Siegel BA, Dehdashti F, Silverman GA, Grigsby PW. Serum squamous cell carcinoma antigen as an early indicator of response during therapy of cervical cancer. Br J Cancer. 2018;118(1):72–8. Epub 2017/11/08. doi: 10.1038/bjc.2017.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Kempen PM, Noorlag R, Swartz JE, Bovenschen N, Braunius WW, Vermeulen JF, Van Cann EM, Grolman W, Willems SM. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol Immunother. 2016;65(5):575–85. Epub 2016/03/20. doi: 10.1007/s00262-016-1819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charlesworth A, Chiaverini C, Chevrant-Breton J, DelRio M, Diociaiuti A, Dupuis RP, El Hachem M, Le Fiblec B, Sankari-Ho AM, Valhquist A, Wierzbicka E, Lacour JP, Meneguzzi G. Epidermolysis bullosa simplex with PLEC mutations: new phenotypes and new mutations. Br J Dermatol. 2013;168(4):808–14. Epub 2013/01/08. doi: 10.1111/bjd.12202. [DOI] [PubMed] [Google Scholar]
- 61.Paumard-Hernandez B, Calvete O, Inglada Perez L, Tejero H, Al-Shahrour F, Pita G, Barroso A, Carlos Trivino J, Urioste M, Valverde C, Gonzalez Billalabeitia E, Quiroga V, Francisco Rodriguez Moreno J, Fernandez Aramburo A, Lopez C, Maroto P, Sastre J, Jose Juan Fita M, Duran I, Lorenzo-Lorenzo I, Iranzo P, Garcia Del Muro X, Ros S, Zambrana F, Maria Autran A, Benitez J. Whole exome sequencing identifies PLEC, EXO5 and DNAH7 as novel susceptibility genes in testicular cancer. Int J Cancer. 2018;143(8):1954–62. Epub 2018/05/16. doi: 10.1002/ijc.31604. [DOI] [PubMed] [Google Scholar]
- 62.Winter L, Turk M, Harter PN, Mittelbronn M, Kornblum C, Norwood F, Jungbluth H, Thiel CT, Schlotzer-Schrehardt U, Schroder R. Downstream effects of plectin mutations in epidermolysis bullosa simplex with muscular dystrophy. Acta Neuropathol Commun. 2016;4(1):44. Epub 2016/04/29. doi: 10.1186/s40478-016-0314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barel O, Shorer Z, Flusser H, Ofir R, Narkis G, Finer G, Shalev H, Nasasra A, Saada A, Birk OS. Mitochondrial complex III deficiency associated with a homozygous mutation in UQCRQ. Am J Hum Genet. 2008;82(5):1211–6. Epub 2008/04/29. doi: 10.1016/j.ajhg.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckhaus J, Lawrence KM, Helbig I, Bui M, Vadlamudi L, Hopper JL, Scheffer IE, Berkovic SF. Genetics of febrile seizure subtypes and syndromes: a twin study. Epilepsy Res. 2013;105(1-2):103–9. Epub 2013/03/26. doi: 10.1016/j.eplepsyres.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 65.Lagae L. Wha’s new in: “genetics in childhood epilepsy”. Eur J Pediatr. 2008;167(7):715–22. Epub 2008/03/06. doi: 10.1007/s00431-008-0690-5. [DOI] [PubMed] [Google Scholar]
- 66.Munakata K, Uemura M, Takemasa I, Ozaki M, Konno M, Nishimura J, Hata T, Mizushima T, Haraguchi N, Noura S, Ikenaga M, Okamura S, Fukunaga M, Murata K, Yamamoto H, Doki Y, Mori M. SCGB2A1 is a novel prognostic marker for colorectal cancer associated with chemoresistance and radioresistance. Int J Oncol. 2014;44(5):1521–8. Epub 2014/03/04. doi: 10.3892/ijo.2014.2316. [DOI] [PubMed] [Google Scholar]
- 67.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–82. Epub 2020/08/24. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright FA, Sullivan PF, Brooks AI, Zou F, Sun W, Xia K, Madar V, Jansen R, Chung W, Zhou YH, Abdellaoui A, Batista S, Butler C, Chen G, Chen TH, D’Ambrosio D, Gallins P, Ha MJ, Hottenga JJ, Huang S, Kattenberg M, Kochar J, Middeldorp CM, Qu A, Shabalin A, Tischfield J, Todd L, Tzeng JY, van Grootheest G, Vink JM, Wang Q, Wang W, Wang W, Willemsen G, Smit JH, de Geus EJ, Yin Z, Penninx BW, Boomsma DI. Heritability and genomics of gene expression in peripheral blood. Nat Genet. 2014;46(5):430–7. Epub 2014/04/15. doi: 10.1038/ng.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoeckli ET. Understanding axon guidance: are we nearly there yet? Development. 2018;145(10). Epub 2018/05/16. doi: 10.1242/dev.151415. [DOI] [PubMed] [Google Scholar]
- 70.Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719(1-2):59–68. Epub 2005/10/18. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Lee SE, West KP Jr., Cole RN, Schulze KJ, Wu LS, Yager JD, Groopman J, Christian P. General intelligence is associated with subclinical inflammation in Nepalese children: A population-based plasma proteomics study. Brain Behav Immun. 2016;56:253–63. Epub 2016/04/04. doi: 10.1016/j.bbi.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakshmi Priya MD, Geetha A. A biochemical study on the level of proteins and their percentage of nitration in the hair and nail of autistic children. Clin Chim Acta. 2011;412(11-12):1036–42. Epub 2011/02/23. doi: 10.1016/j.cca.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 73.Breen MS, Ozcan S, Ramsey JM, Wang Z, Ma’ayan A, Rustogi N, Gottschalk MG, Webster MJ, Weickert CS, Buxbaum JD, Bahn S. Temporal proteomic profiling of postnatal human cortical development. Transl Psychiatry. 2018;8(1):267. Epub 2018/12/07. doi: 10.1038/s41398-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlyle BC, Kitchen RR, Kanyo JE, Voss EZ, Pletikos M, Sousa AMM, Lam TT, Gerstein MB, Sestan N, Nairn AC. A multiregional proteomic survey of the postnatal human brain. Nat Neurosci. 2017;20(12):1787–95. Epub 2017/12/01. doi: 10.1038/s41593-017-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson AA, Shokhirev MN, Wyss-Coray T, Lehallier B. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res Rev. 2020;60:101070. Epub 2020/04/21. doi: 10.1016/j.arr.2020.101070. [DOI] [PubMed] [Google Scholar]
- 76.Pedlar CR, Newell J, Lewis NA. Blood Biomarker Profiling and Monitoring for High-Performance Physiology and Nutrition: Current Perspectives, Limitations and Recommendations. Sports Med. 2019;49(Suppl 2):185–98. Epub 2019/11/07. doi: 10.1007/s40279-019-01158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Minimal datasets related to results presented in this manuscript are available on request from the corresponding author. These data are not publicly available due to privacy, proprietary, and ethical considerations.


