Abstract
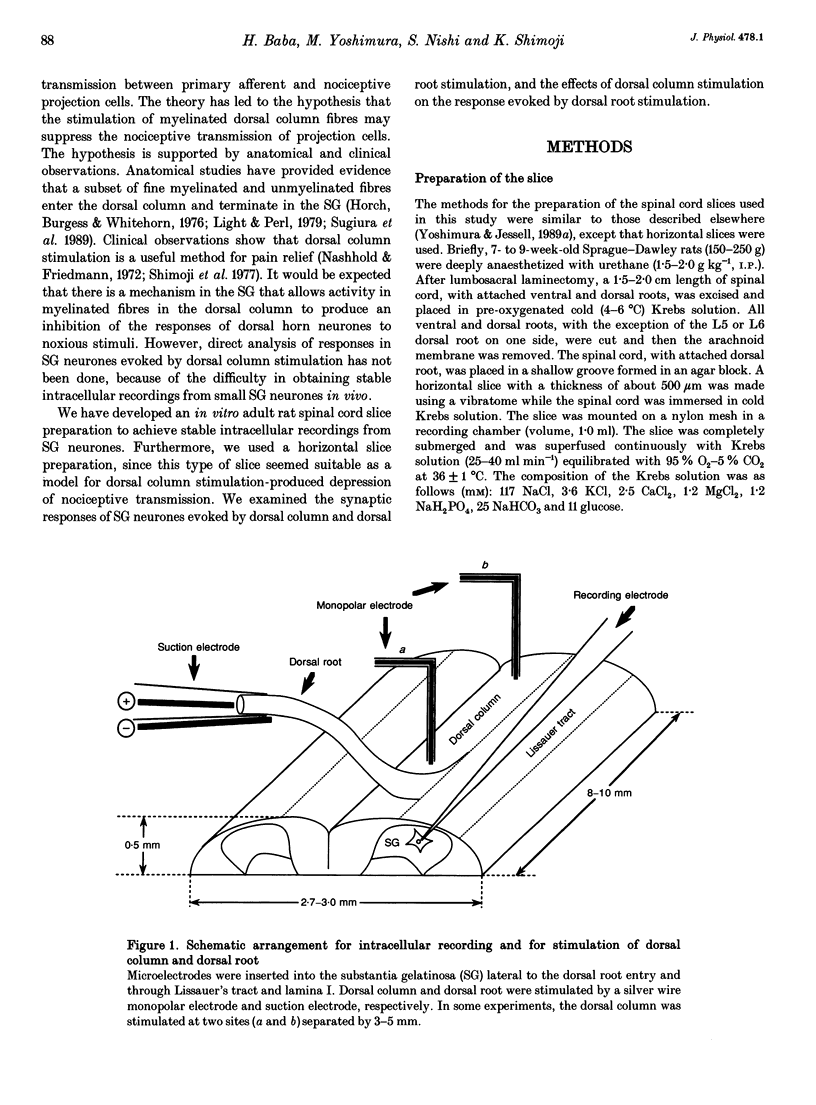
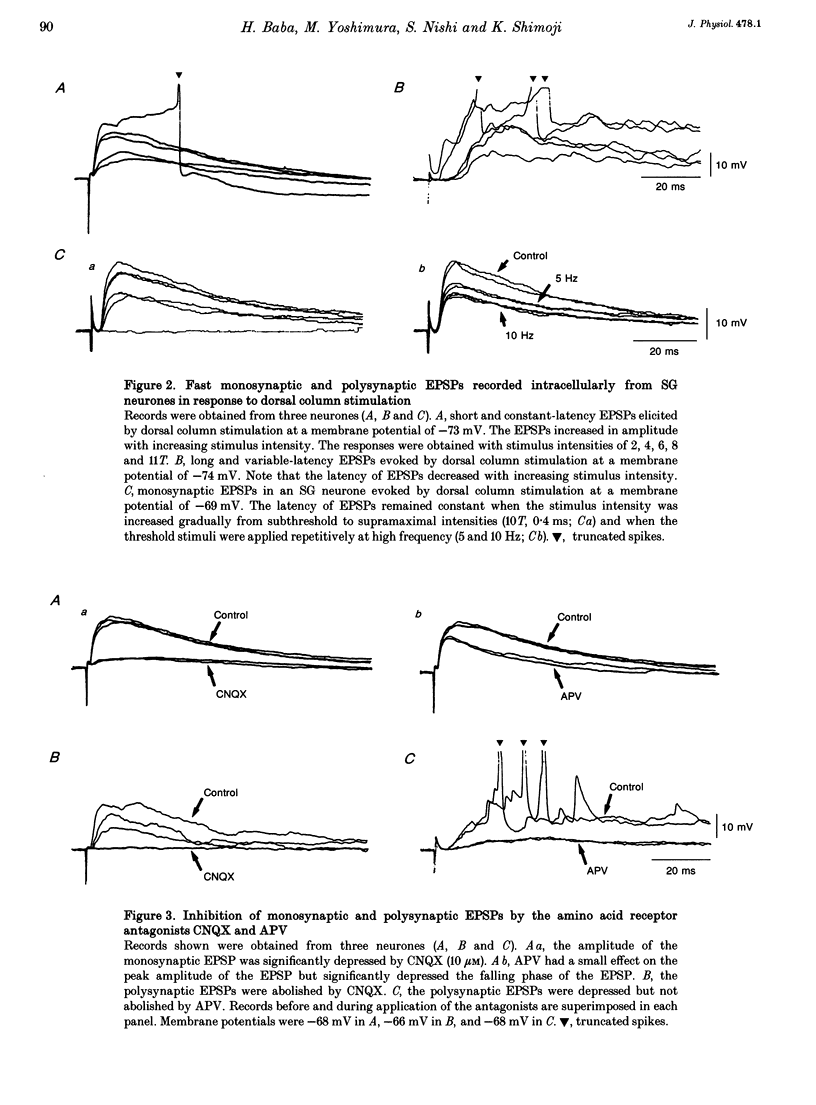
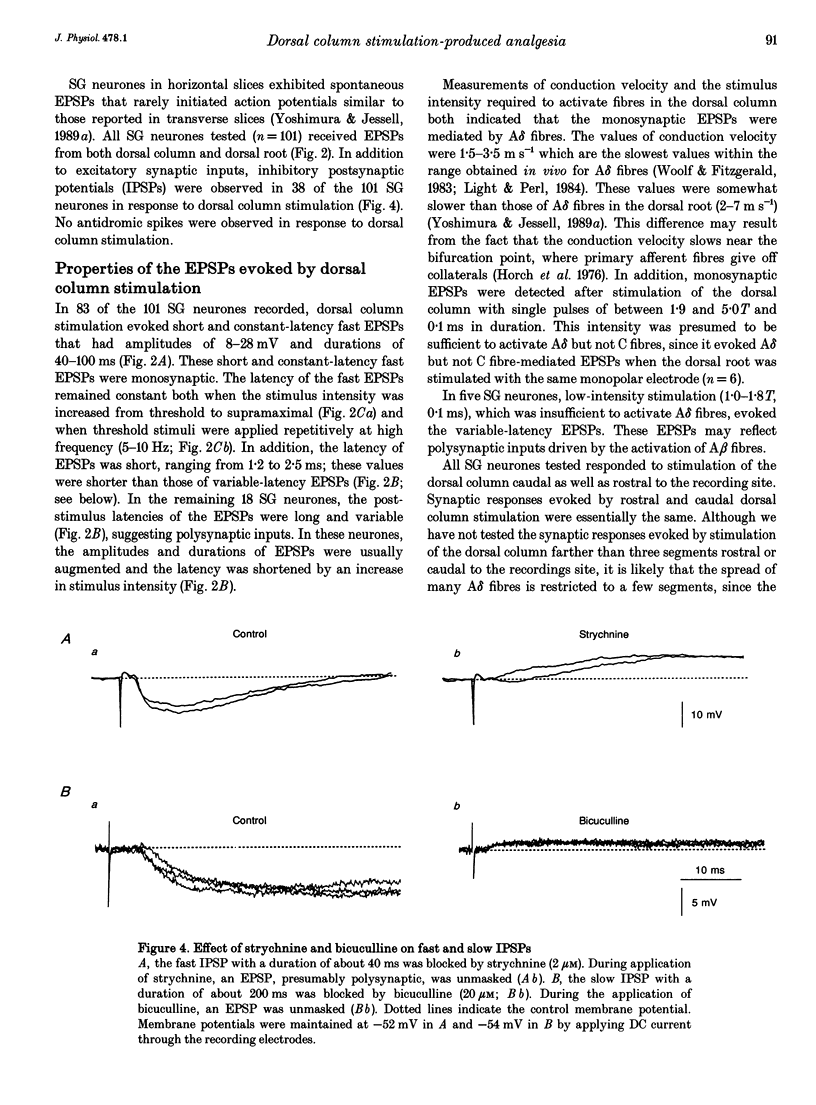
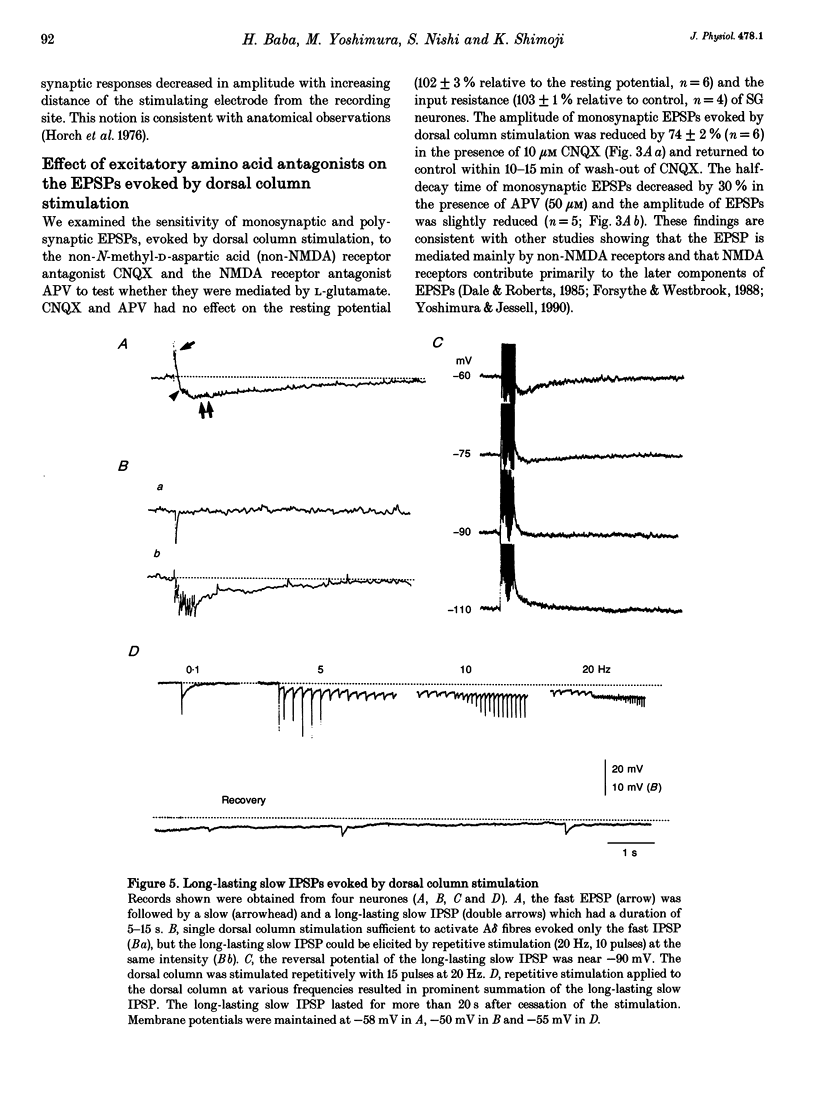
1. To study the mechanism of dorsal column stimulation-induced depression of nociceptive transmission in the spinal cord, synaptic responses evoked in dorsal horn neurones by dorsal column and dorsal root stimulations were examined in a horizontal spinal cord slice of the adult rat. Intracellular recordings were made from substantia gelatinosa (SG) neurones. 2. All SG neurones examined received monosynaptic inputs and/or polysynpatic inputs from both dorsal column and dorsal root. A delta fibres were probably responsible for the synaptic responses. The responses evoked by dorsal column stimulation were similar to those evoked by primary afferent A delta fibre stimulation. 3. Monosynaptic excitatory postsynaptic potentials (EPSPs) evoked by dorsal column A delta fibres were depressed by 6-cyano-7-nitroquinoxaline-2,3-dione, suggesting that these fibres released L-glutamate or a related amino acid as a transmitter. 4. In 38 of 101 SG neurones, dorsal column stimulation evoked an initial EPSP followed by fast and/or slow inhibitory postsynaptic potentials (IPSPs). These IPSPs reversed polarity at a membrane potential of -73 +/- 2 mV. The fast IPSPs observed in 16 of the SG neurones (42%) that received inhibitory inputs were depressed by strychnine, while the slow IPSPs observed in 22 SG neurones were depressed by bicuculline. In a few cells, a long-lasting slow IPSP with a much slower time course was detected; this IPSP was insensitive to strychnine and bicuculline, and reversed polarity at a membrane potential near -90 mV. 5. Repetitive stimulation of the dorsal column depressed the amplitude of monosynaptic EPSPs evoked by dorsal root stimulation. 6. The responses of SG neurones to dorsal column stimulation had configurations and durations similar to responses to dorsal root stimulation, and may be mediated largely by the same A delta fibres. However, a C fibre-mediated response could not be detected in SG neurones from dorsal column stimulation, although dorsal root stimulation could evoke C fibre-mediated monosynaptic EPSPs in 18 of 88 SG neurones (20%). 7. These observations suggest that SG neurones receive abundant A delta but not C fibre inputs from the dorsal column and that dorsal column stimulation inhibits primary afferent transmission in the spinal cord both by reducing transmitter release from primary A delta fibres and by hyperpolarizing SG neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber R. P., Vaughn J. E., Saito K., McLaughlin B. J., Roberts E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978 Feb 3;141(1):35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- Briner R. P., Carlton S. M., Coggeshall R. E., Chung K. S. Evidence for unmyelinated sensory fibres in the posterior columns in man. Brain. 1988 Oct;111(Pt 5):999–1007. doi: 10.1093/brain/111.5.999. [DOI] [PubMed] [Google Scholar]
- Carstens E., Gilly H., Schreiber H., Zimmermann M. Effects of midbrain stimulation and iontophoretic application of serotonin, noradrenaline, morphine and GABA on electrical thresholds of afferent C- and A-fibre terminals in cat spinal cord. Neuroscience. 1987 May;21(2):395–406. doi: 10.1016/0306-4522(87)90130-8. [DOI] [PubMed] [Google Scholar]
- Cervero F., Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980 Dec;103(4):717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- Chung K. S., Coggeshall R. E. Unmyelinated primary afferent fibers in dorsal funiculi of cat sacral spinal cord. J Comp Neurol. 1985 Aug 15;238(3):365–369. doi: 10.1002/cne.902380310. [DOI] [PubMed] [Google Scholar]
- Chung K., Coggeshall R. E. Numbers of axons in lateral and ventral funiculi of rat sacral spinal cord. J Comp Neurol. 1983 Feb 10;214(1):72–78. doi: 10.1002/cne.902140107. [DOI] [PubMed] [Google Scholar]
- Chung K., Sharma J., Coggeshall R. E. Numbers of myelinated and unmyelinated axons in the dorsal, lateral, and ventral funiculi of the white matter of the S2 segment of cat spinal cord. J Comp Neurol. 1985 Apr 1;234(1):117–121. doi: 10.1002/cne.902340109. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch K. W., Burgess P. R., Whitehorn D. Ascending collaterals of cutaneous neurons in the fasciculus gracilis of the cat. Brain Res. 1976 Nov 19;117(1):1–17. doi: 10.1016/0006-8993(76)90552-7. [DOI] [PubMed] [Google Scholar]
- Hori Y., Endo K., Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol. 1992 May;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. E., Thompson S. W., Urban L., Woolf C. J. The responses recorded in vitro of deep dorsal horn neurons to direct and orthodromic stimulation in the young rat spinal cord. Neuroscience. 1988 Oct;27(1):231–242. doi: 10.1016/0306-4522(88)90233-3. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978 Feb 1;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Light A. R., Perl E. R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979 Jul 15;186(2):133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P. D. Pain mechanisms: a new theory. Science. 1965 Nov 19;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold B. S., Jr, Friedman H. Dorsal column stimulation for control of pain. Preliminary report on 30 patients. J Neurosurg. 1972 May;36(5):590–597. doi: 10.3171/jns.1972.36.5.0590. [DOI] [PubMed] [Google Scholar]
- Nielson K. D., Adams J. E., Hosobuchi Y. Phantom limb pain. Treatment with dorsal column stimulation. J Neurosurg. 1975 Mar;42(3):301–307. doi: 10.3171/jns.1975.42.3.0301. [DOI] [PubMed] [Google Scholar]
- North R. A., Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984 Apr;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. W., Wilkin G. P., Turnbull M. J., Bowery N. G. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984 Jan 5;307(5946):71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Schneider S. P., Perl E. R. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neurosci. 1988 Jun;8(6):2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji K., Matsuki M., Shimizu H., Iwane T., Takahashi R., Maruyama M., Masuko K. Low-frequency, weak extradural stimulation in the management of intractable pain. Br J Anaesth. 1977 Nov;49(11):1081–1086. doi: 10.1093/bja/49.11.1081. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Terui N., Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. J Neurophysiol. 1989 Oct;62(4):834–840. doi: 10.1152/jn.1989.62.4.834. [DOI] [PubMed] [Google Scholar]
- Todd A. J., Lochhead V. GABA-like immunoreactivity in type I glomeruli of rat substantia gelatinosa. Brain Res. 1990 Apr 23;514(1):171–174. doi: 10.1016/0006-8993(90)90454-j. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The gate control theory of pain mechanisms. A re-examination and re-statement. Brain. 1978 Mar;101(1):1–18. doi: 10.1093/brain/101.1.1. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983 Dec 10;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Membrane properties of rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):109–118. doi: 10.1152/jn.1989.62.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. M. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989 Jul;62(1):96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990 Nov;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]


