Abstract
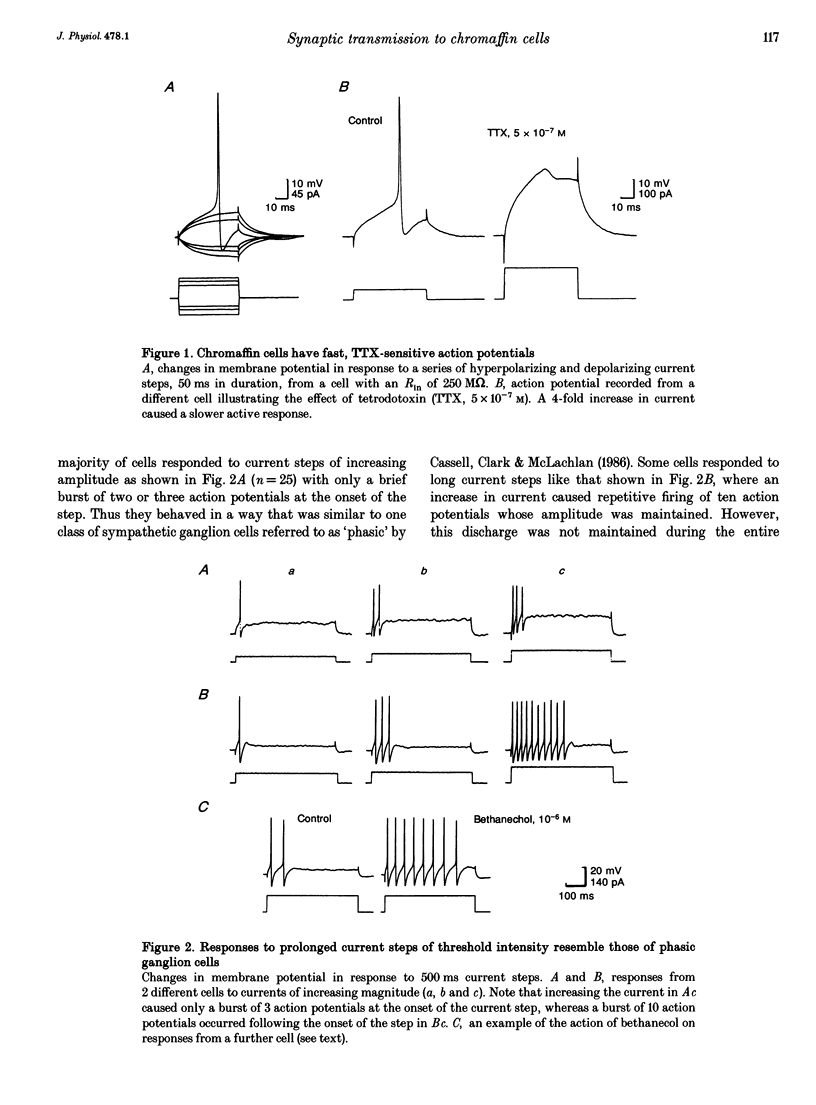
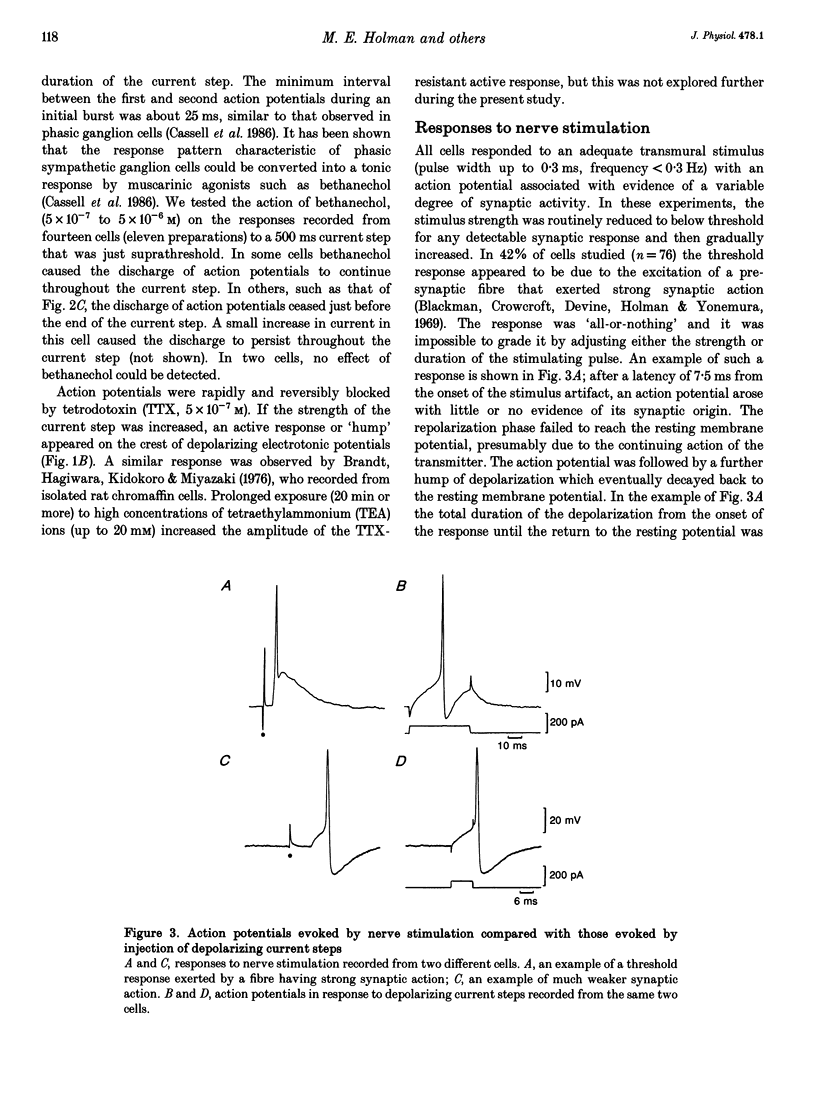
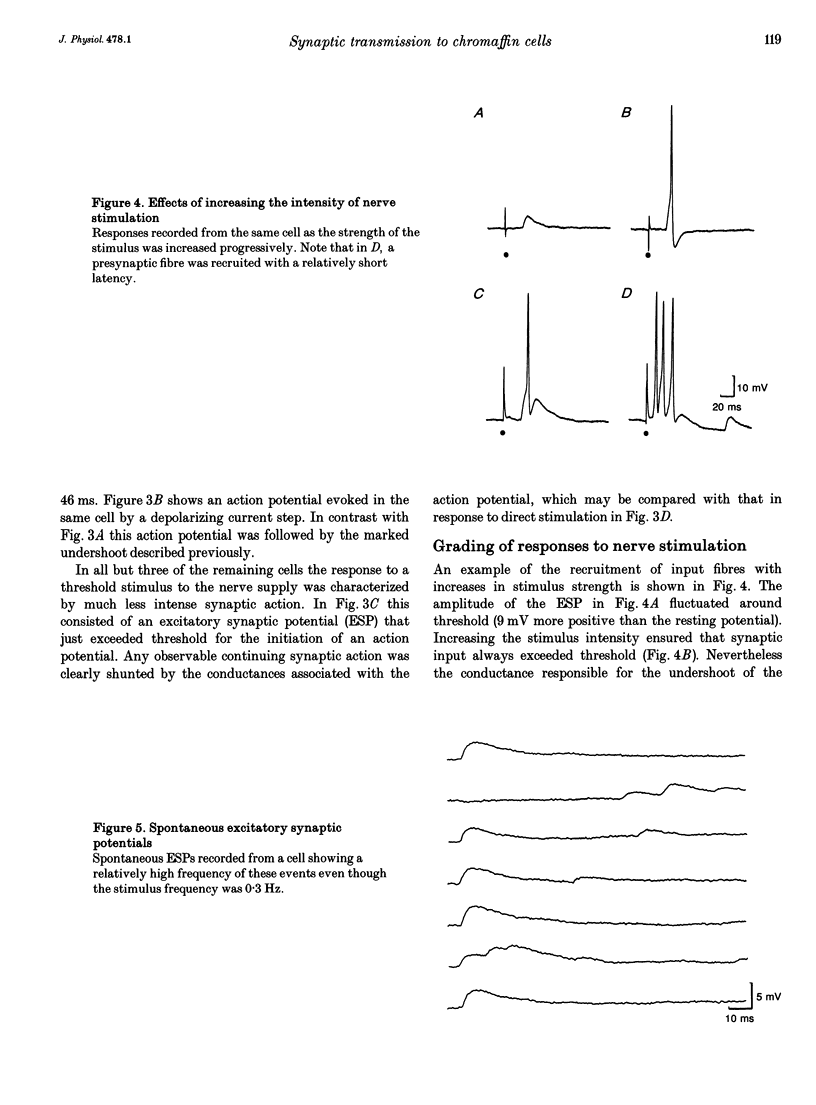
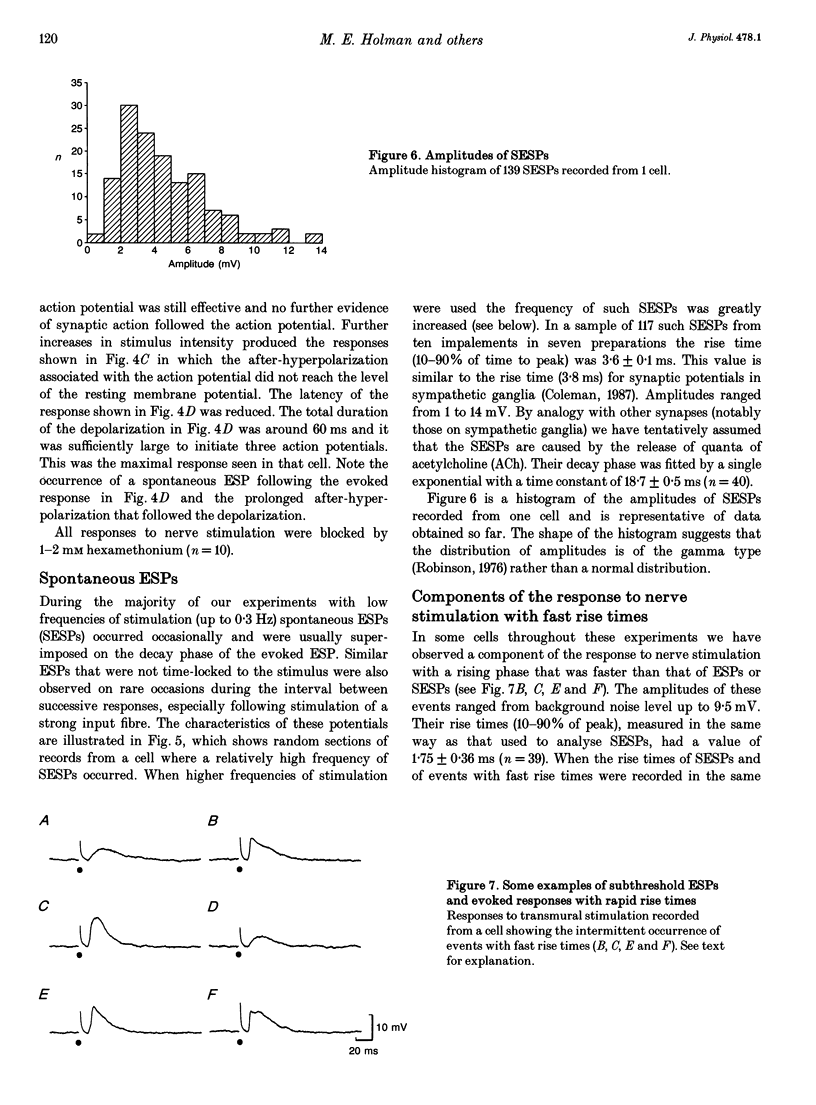
1. Membrane potentials were recorded with conventional intracellular microelectrodes from chromaffin cells in isolated, bisected adrenal glands from guinea-pigs. 2. All cells were electrically excitable and responded to depolarizing current with all-or-nothing action potentials that were blocked by tetrodotoxin. 3. Input resistance was 180 +/- 14 M omega and this was lower than that reported for isolated chromaffin cells using patch electrodes. 4. All cells responded to transmural stimulation with action potentials that arose from excitatory synaptic potentials in response to the excitation of one or more preganglionic fibres, many having strong synaptic action. Other fibres had weaker synaptic action but in all cases, maximal transmural stimulation caused depolarization well above threshold for action potential initiation. 5. Spontaneous excitatory synaptic potentials were observed whose frequency was greatly increased by repetitive stimulation at 10 or 30 Hz. 6. No evidence was found for the desensitization of nicotinic receptors in response to acetylcholine released from presynaptic nerve terminals. 7. These experiments show that there are many similarities between the responses to splanchnic nerve stimulation of guinea-pig chromaffin cells in situ and sympathetic ganglion cells from the same species.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., McLachlan E. M. An electrophysiological analysis of the synthesis of acetylcholine in preganglionic nerve terminals. J Physiol. 1972 Mar;221(3):669–682. doi: 10.1113/jphysiol.1972.sp009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman J. G., Crowcroft P. J., Devine C. E., Holman M. E., Yonemura K. Transmission from pregnanglionic fibres in the hypogastric nerve to peripheral ganglia of male guinea-pigs. J Physiol. 1969 May;201(3):723–743. doi: 10.1113/jphysiol.1969.sp008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell J. F., Clark A. L., McLachlan E. M. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol. 1986 Mar;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman H. A. Multiple sites for the initiation of action potentials in neurons of the inferior mesenteric ganglion of the guinea-pig. Neuroscience. 1987 Feb;20(2):357–363. doi: 10.1016/0306-4522(87)90097-2. [DOI] [PubMed] [Google Scholar]
- Coupland R. E. Electron microscopic observations on the structure of the rat adrenal medulla: II. Normal innervation. J Anat. 1965 Apr;99(Pt 2):255–272. [PMC free article] [PubMed] [Google Scholar]
- Engel E., Barcilon V., Eisenberg R. S. The interpretation of current-voltage relations recorded from a spherical cell with a single microelectrode. Biophys J. 1972 Apr;12(4):384–403. doi: 10.1016/S0006-3495(72)86091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynszpan-Wynograd O., Nicolas G. Intercellular junctions in the adrenal medulla: a comparative freeze-fracture study. Tissue Cell. 1980;12(4):661–672. doi: 10.1016/0040-8166(80)90020-8. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Matsumoto G., Kidokoro Y. Synaptic activation of rat adrenal medulla examined with a large photodiode array in combination with a voltage-sensitive dye. Neuroscience. 1992 Nov;51(1):211–219. doi: 10.1016/0306-4522(92)90486-l. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Muscarine induces two distinct current responses in adrenal chromaffin cells of the guinea-pig. Jpn J Physiol. 1990;40(5):679–691. doi: 10.2170/jjphysiol.40.679. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kuriyama H. Properties of the nicotinic-receptor-activated current in adrenal chromaffin cells of the guinea-pig. Pflugers Arch. 1991 Aug;419(1):13–20. doi: 10.1007/BF00373741. [DOI] [PubMed] [Google Scholar]
- Kayaalp S. O., McIsaac R. J. Muscarinic component of splanchnic-adrenal transmission in the dog. Br J Pharmacol. 1969 Jun;36(2):286–293. doi: 10.1111/j.1476-5381.1969.tb09506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Miyazaki S., Ozawa S. Acetylcholine-induced membrane depolarization and potential fluctuations in the rat adrenal chromaffin cell. J Physiol. 1982 Mar;324:203–220. doi: 10.1113/jphysiol.1982.sp014107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley P., Livett B. G. Neuropeptides in the autonomic nervous system. CRC Crit Rev Clin Neurobiol. 1985;1(3):201–283. [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Matthews E. K. Membrane potential measurement in cells of the adrenal gland. J Physiol. 1967 Mar;189(1):139–148. doi: 10.1113/jphysiol.1967.sp008159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Pollard H. B., Rojas E. Electrical activity in chromaffin cells of intact mouse adrenal gland. Am J Physiol. 1988 May;254(5 Pt 1):C675–C683. doi: 10.1152/ajpcell.1988.254.5.C675. [DOI] [PubMed] [Google Scholar]
- Robinson J. Estimation of parameters for a model of transmitter release at synapses. Biometrics. 1976 Mar;32(1):61–68. [PubMed] [Google Scholar]
- Role L. W., Perlman R. L. Both nicotinic and muscarinic receptors mediate catecholamine secretion by isolated guinea-pig chromaffin cells. Neuroscience. 1983 Nov;10(3):979–985. doi: 10.1016/0306-4522(83)90236-1. [DOI] [PubMed] [Google Scholar]
- Sacchi O., Perri V. Quantal release of acetylcholine from the nerve endings of the guinea-pig superior cervical ganglion. Pflugers Arch. 1971;329(3):207–219. doi: 10.1007/BF00586615. [DOI] [PubMed] [Google Scholar]
- Wakade A. R. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]


