Abstract
Background:
Acinetobacter is a Gram-negative bacterium that causes nosocomial infections, increasing healthcare costs, patient morbidity, and mortality. The rate of carbapenem resistance among Acinetobacter species is rising in several countries, including Saudi Arabia.
Objective:
To determine the risk factors and compare the predictors of mortality in patients infected with carbapenem-susceptible and carbapenem-resistant Acinetobacter strains.
Materials and Methods:
This retrospective study included patients with Acinetobacter infection who were admitted to a community hospital in Madinah, Saudi Arabia, between January 2017 and June 2021. A logistic regression analysis was conducted to assess the risks of acquiring carbapenem-resistant Acinetobacter infections and the mortality risk associated with these infections.
Results:
This study included 138 Acinetobacter-infected cases, of which 114 (82%) were carbapenem-resistant infections. Between 2017 and 2020, resistance rates increased from 75% to 87%. Patients with carbapenem-resistant Acinetobacter infections had higher 90-day mortality than those with carbapenem-susceptible infection (62% vs. 29%, P = 0.006). The risk factors for carbapenem-resistant Acinetobacter infections were prior antimicrobial therapy (aOR: 8.36 [1.69–41.29]; P = 0.009) and mechanical ventilation (aOR: 6.07 [1.82–20.20]; P = 0.003). Among all patients with Acinetobacter infections, significant predictors of 90-day mortality were carbapenem resistance (aOR: 3.26 [1.19–8.90]; P = 0.021) and Charlson comorbidity score (aOR: 1.19 [1.06–1.34]; P = 0.004).
Conclusion:
The increase in carbapenem-resistant Acinetobacter cases in this study was consistent with the findings of other studies from Saudi Arabia. This, together with the high associated mortality rates, indicates the urgent need for effective antimicrobials and infection prevention strategies to combat carbapenem-resistant Acinetobacter infections in hospitals.
Keywords: Acinetobacter, carbapenem-resistant, mortality, outcome, predictors, risk factor, Saudi Arabia
INTRODUCTION
Acinetobacter is a multidrug-resistant Gram-negative bacterium that causes skin, blood, respiratory, and urinary tract infections. It is endemic in many hospitals, as it colonizes patients, survives in harsh environments, and is resistant to many antibiotics. Acinetobacter infection prolongs hospital stay, increases healthcare costs, and increases in-hospital mortality.[1] In addition, some Acinetobacter species carry mobile antimicrobial-resistant genes that can transfer antimicrobial resistance to other bacteria.
Carbapenem-resistant Acinetobacter (CRA) represents a substantial threat because carbapenems are last-resort antibiotics. The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have classified CRA infection as a severe threat that needs urgent attention, as it currently has no effective therapy.[2,3] In many countries, including Saudi Arabia, the rate of CRA infections has increased significantly in the past 10 years. For instance, in some Saudi Arabian regions, CRA infections rose from 5.4% to 80% between 2009 and 2019.[4,5] Furthermore, CRA infections causes more than 25% of the nosocomial pneumonia cases in several hospitals in the country.[6]
Many studies have described Acinetobacter as a nosocomial infection and reported multiple risk factors, including extended hospitalization, ICU admission, mechanical ventilation, antimicrobial exposure, recent surgery, and indwelling catheters.[7] Conversely, other studies have described Acinetobacter as a community-acquired infection that causes about 10% of community-acquired pneumonia.[8] Therefore, the exact origin and risk factors of CRA infections remain unclear.
During the COVID-19 pandemic, several hospitals had CRA outbreaks, leading to increased mortality among COVID-19 patients.[9] These increased CRA infection rates were mainly because of breaches in infection prevention measures, increased antimicrobial use, staff shortages, and a shortage of personal protective equipment.[10,11] Recently, in our hospital, there has been an increase in multidrug-resistant (MDR) Acinetobacter infection rates, including CRA; however, the risk factors and outcome of the CRA infections were unclear. Therefore, this study was conducted to describe the risk factors of CRA and carbapenem-susceptible Acinetobacter (CSA) infections and to compare the mortality between these infections.
MATERIALS AND METHODS
Study setting, design, and population
This retrospective study included all adult patients (aged ≥18 years) who were admitted in a community hospital in Madinah, Saudi Arabia, between January 1, 2017, and June 30, 2021, and had a clinical diagnosis of Acinetobacter infection. The included patients had a positive culture for Acinetobacter species that required specific antimicrobial therapy. Cases that were colonized with Acinetobacter species were excluded. For patients with more than one Acinetobacter infection, only the first episode of infection was recorded. All data were collected from patients’ medical records. Ethical approval was obtained from the King Abdullah International Research Center, Jeddah, Saudi Arabia.
Microbiology method
Our laboratory employs an automated blood culture system (BACT/ALERT3D) to process blood cultures and a VITEK 2 instrument to identify bacterial species and perform antimicrobial susceptibility testing (ver. 8, bioMérieux, USA). The latest Clinical and Laboratory Standards Institute breakpoints was used to define susceptibility patterns. Acinetobacter isolates were considered as carbapenem-resistant if they had a minimal inhibitory concentration of ≥8 μg/mL to imipenem, meropenem, or doripenem.[12]
Definitions and outcomes
The following risk factors for Acinetobacter-infected cases were explored if they occurred within 3 months before the infection: patient demographics, comorbidities, antimicrobial therapy, interhospital transfer, central venous line, indwelling urinary catheter, mechanical ventilation, recent trauma, receipt of wound care, and hemodialysis. For the outcome variables, the length of the hospital and ICU stays and the duration of mechanical ventilation were calculated. In addition, the 30-day, 90-day, and in-hospital mortalities were calculated.
Using the National Healthcare Safety Network definition, hospital-acquired infection was defined as an infection that occurred 2 days after hospital admission. The sites were classified as lung, skin, soft tissue, central venous catheter, urinary tract, and intra-abdominal infections.[13] The severity of infection was assessed using a quick sequential organ failure assessment (SOFA) score and the occurrence of septic shock. The Charlson comorbidity index was used to categorize comorbidities.[14] Confounders were accounted for in the adjusted analysis, including comorbidities, infection severity, and appropriate antimicrobial therapy for Acinetobacter infection.
Statistical analysis
The mean, median, standard deviation (SD), and interquartile range (IQR) were calculated for each continuous variable and the percentage for categorical variables in the descriptive statistics. Fisher’s exact test was used to compare categorical variables and Student’s t-test to compare normally distributed continuous variables. For non-normally distributed continuous variables, the Wilcoxon rank-sum test was used. A multivariate logistic regression analysis was performed to estimate the risk factor of and mortality due to CRA infection. Using the backward stepwise selection method, variables that were significant in the univariate analysis were included in the final model. All data were analyzed using the STATA software, version 13 (StataCorp, Texas, USA). A P value <0.05 was considered statistically significant.
RESULTS
A total of 196 hospitalized patients had a positive culture for Acinetobacter. Of these, 58 cases (29%) were considered colonized with Acinetobacter and excluded from the analysis. Of the remaining 138 Acinetobacter-infected patients, 66% were male, with a mean and median age of 69 and 72 years, respectively (IQR: 61–81 years) [Table 1].
Table 1.
Clinical characteristics of the Acinetobacter-infected cases with resistant and susceptible isolates
| Characteristics | All cases (N=138) N (%) | CRA (n=114) n (%) | CSA (n=24) n (%) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Mean | 69.24 (17) | 70.93 (16) | 61.2 (19) | 0.033* |
| Median | 72 (61–81) | 73 (63–81) | 63 (50–79) | |
| Male | 91 (66) | 78 (68) | 13 (54) | 0.236 |
| Female | 47 (34) | 36 (32) | 11 (46) | 0.236 |
| Mechanical ventilation | 98 (76) | 91 (81) | 7 (47) | 0.007* |
| Healthcare-associated infection | 104 (75) | 94 (82) | 10 (42) | 0.001* |
| Wound care | 26 (19) | 25 (22) | 1 (4) | 0.046* |
| Hemodialysis | 17 (12) | 17 (15) | 0 | 0.043* |
| Urinary catheter | 32 (23) | 29 (25) | 3 (13) | 0.286 |
| Tracheostomy | 8 (6) | 8 (7) | 0 | 0.350 |
| Venous catheter | 30 (22) | 30 (26) | 0 | 0.002* |
| Transfer from another hospital | 46 (33) | 44 (37) | 2 (8) | 0.004* |
| Recent antimicrobial treatment | 64 (46) | 58 (51) | 6 (25) | 0.025* |
| Intensive care unit admission | 111 (87) | 101 (89) | 10 (67) | 0.030* |
| Diabetes mellitus | 91 (66) | 81 (71) | 10 (42) | 0.009* |
| Chronic lung diseases | 22 (16) | 19 (17) | 3 (13) | 0.765 |
| Malignancy | 12 (9) | 10 (9) | 2 (8) | 0.999 |
| Heart failure | 30 (22) | 26 (23) | 4 (17) | 0.597 |
| Charlson comorbidity score (median) | 5 (3–8) | 6 (4–8) | 3.5 (1–5.5) | 0.006* |
| Bacteremia | 28 (20) | 27 (23) | 1 (4) | 0.001* |
| Site of the infections | ||||
| Skin and soft tissues | 29 (21) | 27 (24) | 2 (8) | 0.106 |
| Intra-abdominal | 4 (3) | 2 (2) | 2 (8) | 0.140 |
| Pneumonia | 92 (67) | 85 (75) | 7 (29) | 0.001* |
| Bone and joint | 6 (4) | 6 (5) | 0 | 0.590 |
| Venous catheters | 13 (9) | 12 (11) | 1 (4) | 0.466 |
| Urinary tract | 29 (21) | 16 (14) | 13 (54) | 0.001* |
*Significant at P<0.05. Median are presented with interquartile range. CRA – Carbapenem-resistant Acinetobacter; CSA – Carbapenem-susceptible Acinetobacter
Most patients had been admitted to the ICU during hospitalization (87%) and required mechanical ventilation (76%). Bacteremia occurred in 20% of the cases, and most infections were nosocomial (75%). The most common sites of the infection were lungs, urinary tract, and skin and soft tissues. Within the 3 months of the current Acinetobacter infection, 33% of the cases had been hospitalized, 19% received outpatient wound care, and 12% were on regular hemodialysis. The median quick SOFA score for the Acinetobacter-infected cases was 2 (IQR = 1–2), and half of the subjects developed septic shock. The mortality rate was 32% in 2019 and 41% in 2020. The overall 30- and 90-day mortality rates were 39% and 57%, respectively. The median length of the hospital and ICU stays and mechanical ventilation were 33, 21, and 18 days, respectively [Table 2].
Table 2.
Outcome and laboratory parameters of carbapenem-resistant and carbapenem-susceptible Acinetobacter infected cases
| Characteristics | All (n=138) n (%) | CRA (n=114) n (%) | CSA (n=24) n (%) | P |
|---|---|---|---|---|
| Days of ICU stay | ||||
| Mean | 31 (49) | 32 (50) | 26 (30) | - |
| Median | 21 (12–37) | 21 (12–37) | 23 (4–30) | 0.473 |
| Days of hospital stay | ||||
| Mean | 54 (68) | 56 (70) | 44 (56) | - |
| Median | 33 (18–60) | 36 (18–61) | 26 (16–32) | 0.227 |
| Days of mechanical ventilation | ||||
| Mean | 21 (18) | 21 (19) | 22 (11) | - |
| Median | 18 (9–29) | 17 (9–28) | 22 (14–34) | 0.422 |
| Quick SOFA score | ||||
| Mean | 1.47 (0.93) | 1.6 (0.9) | 0.8 (0.85) | - |
| Median | 2 (1–2) | 2 (1–2) | 1 (0–1) | 0.001* |
| Septic shock | 73 (52) | 69 (61) | 4 (17) | 0.001* |
| 30-day mortality | 54 (39) | 49 (43) | 2 (20) | 0.050 |
| 90-day mortality | 78 (57) | 71 (62) | 7 (29) | 0.006* |
| In-hospital mortality | 93 (67) | 86 (75) | 7 (29) | 0.001* |
| Serum creatinine (µmol/L) | ||||
| Mean | 133 (118) | 141 (124) | 90 (61) | - |
| Median | 83 (61–150) | 94 (61–164) | 73 (58–94) | 0.093 |
| White blood cells per µL | ||||
| Mean | 13 (9) | 13.4 (10) | 11 (6) | - |
| Median | 11 (7.4–16) | 11 (7.4–16) | 10 (7.2–15) | 0.001* |
| C-reactive protein (mg/dL) | ||||
| Mean | 121 (94) | 126 (96) | 73 (51) | - |
| Median | 96 (50–184) | 113 (54–201) | 62 (33–88) | 0.091 |
| Platelet per µL | ||||
| Mean | 253 (189) | 246 (197) | 298 (120) | - |
| Median | 219 (113–355) | 209 (101–340) | 321 (228–397) | 0.034* |
*Significant at P<0.05. Median are presented with interquartile range. ICU – Intensive care unit; CRA – Carbapenem-resistant Acinetobacter; CSA – Carbapenem-susceptible Acinetobacter; Quick SOFA score – Quick sequential organ failure assessment score
Acinetobacter susceptibility pattern
Overall, 83% of the 138 Acinetobacter isolates were resistant to carbapenems; however, the resistance rates increased between 2017 and 2020 from 75% to 87%, respectively [Figure 1]. In addition, high resistance rates were observed among other antimicrobials, including ceftazidime (87%), piperacillin/tazobactam (85%), and ciprofloxacin (85%). Meanwhile, 89%, 55%, and 48% of the isolates were susceptible to colistin, tigecycline, and sulfamethoxazole/trimethoprim, respectively [Figure 2].
Figure 1.
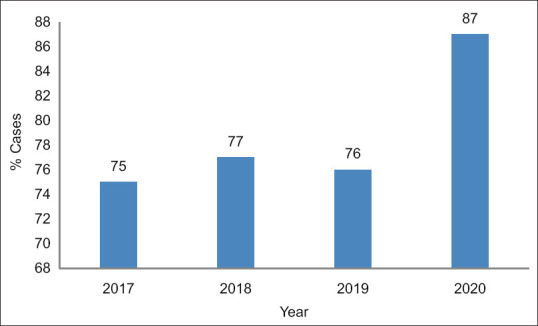
Trends in carbapenem-resistant Acinetobacter cases: 2017–2020
Figure 2.
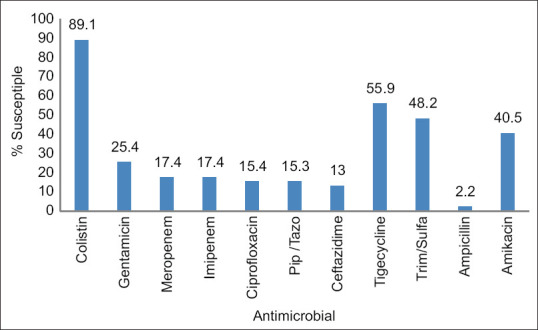
Acinetobacter susceptibility to antimicrobials: 2017–2020
Risk factors for carbapenem-resistant Acinetobacter infection
Table 1 compares the characteristics of CRA and CSA infections. In the univariate analysis, several risk factors were found to be associated with CRA, including age, prior antimicrobial therapy, mechanical ventilation, Charlson comorbidity score, quick SOFA score, diabetes mellitus, culture site, and ICU admission. However, in the adjusted multivariate analysis, the risk factors that remained significantly associated with CRA were prior antimicrobial therapy (odds ratio [OR], 95% confidence interval [CI]: 8.36 [1.69–41.29]; P = 0.009) and mechanical ventilation (OR: 6.07 [1.82–20.20]; P = 0.003).
Table 2 compares the outcomes of CRA and CSA infections. Patients with CRA had higher 30- and 90-day mortality than those with CSA (43% vs. 20%, P = 0.05; and 62% vs. 29%, P = 0.006, respectively). Overall, the in-hospital mortality was also significantly higher in CRA cases than in CSA cases (75% vs. 29%; P = 0.001). No significant difference was found in the length of hospitalization, ICU stays, or duration of mechanical ventilation between cases with CRA and CSA.
Patients who died within 90 days of hospitalization were older (P = 0.049), had a higher median Charlson comorbidity score (P = 0.001), higher rates of CRA (P = 0.006), septic shock (P = 0.026), and more likely to have diabetes mellitus (P = 0.007) than those who survived [Table 3]. Patients who survived were more likely to have Acinetobacter urinary tract infections than those who died (P = 0.018). There was no difference in antimicrobial treatment among those who survived and those who did not. After adjusting for confounding factors, the only significant predictors of 90-day mortality among cases infected with Acinetobacter were carbapenem resistance (OR: 3.26, 95% CI: 1.19–8.90; P = 0.021) and Charlson comorbidity score (OR: 1.19, 95% CI: 1.06–1.34; P = 0.004) [Table 4].
Table 3.
Clinical characteristics of patients with Acinetobacter infection at 90 days after hospitalization
| Characteristics | All (N=138) N (%) | Nonsurvivors (n=78) n (%) | Survivors (n=60) n (%) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Mean | 69 (17) | 72 (13) | 65 (20) | - |
| Median | 72 (61–81) | 73 (63–81) | 69 (48–79) | 0.049* |
| Age groups (years) | ||||
| 15–39 | 10 (7.25) | 1 (1.28) | 9 (15) | - |
| 40–59 | 22 (15.94) | 12 (15.38) | 10 (16.67) | - |
| >60 | 106 (76.81) | 65 (83.33) | 41 (68.33) | 0.007* |
| Gender | ||||
| Male | 91 (65.94) | 54 (69.23) | 37 (61.67) | 0.371 |
| Female | 47 (34.06) | 24 (30.77) | 23 (38.33) | - |
| Diabetes mellitus | 91 (65.94) | 59 (75.64) | 32 (53.33) | 0.007* |
| Lung diseases | 22 (15.94) | 13 (16.67) | 9 (15) | 0.819 |
| Malignancy | 12 (8.70) | 10 (12.82) | 2 (3.33) | 0.068 |
| Heart failure | 30 (21.74) | 22 (28.210) | 8 (13.33) | 0.039* |
| Mechanical ventilation | 98 (76.56) | 61 (78.21) | 37 (74) | 0.670 |
| Charlson comorbidity score (mean) | 5.72 (3.39) | 6.57 (3.17) | 4.61 (3.36) | - |
| Charlson comorbidity score (median) | 5 (3–8) | 7 (4–9) | 5 (2.5–7) | 0.001* |
| Quick SOFA score (mean) | 1.47 (0.93) | 1.58 (0.88) | 1.32 (0.99) | 0.091 |
| Quick SOFA score (median) | 2 (1–2) | 2 (1–2) | 1 (1–2) | - |
| Septic shock | 73 (52.9) | 48 (61.54) | 25 (41.67) | 0.026* |
| Bacteremia | 28 (20) | 9 (11.54) | 19 (31.67) | 0.005* |
| Appropriate antimicrobials | 120 (86) | 68 (87) | 52 (86) | 0.929 |
| Carbapenem-resistance | 114 (82.61) | 71 (91.03) | 43 (71.67) | 0.006* |
| Site of the infection | ||||
| Skin and soft tissue | 29 (21.01) | 20 (25.64) | 9 (15) | 0.145 |
| Intra-abdominal infection | 4 (2.90) | 3 (3.85) | 1 (1.67) | 0.632 |
| Pneumonia | 92 (66.67) | 57 (73.08) | 35 (58.33) | 0.101 |
| Bone and joint infection | 6 (4.38) | 3 (3.90) | 3 (5) | 0.999 |
| Venous-catheter infection | 13 (9.42) | 4 (5.13) | 9 (15) | 0.076 |
| Urinary tract | 29 (21) | 11 (14) | 18 (30) | 0.018* |
*Significant at P<0.05. Median are presented with interquartile range. N – Values represent all patients; n/N (%) – % a proportion of subset; CRS – Carbapenem-resistant Acinetobacter; CSA – Carbapenem-susceptible Acinetobacter; Quick SOFA score – Quick Sequential organ failure assessment score; IQR – Interquartile range
Table 4.
Risk factors for 90-day mortality of all cases with Acinetobacter infection using unadjusted and adjusted multivariable logistic analysis
| Characteristics | 90-day mortality | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Carbapenem-resistance | 4.00 | 1.54–10.45 | 0.005 | 3.26 | 1.19–8.90 | 0.021 |
| Charlson comorbidity score | 1.20 | 1.08–1.35 | 0.001 | 1.19 | 1.06–1.34 | 0.004 |
| Septic shock | 2.24 | 1.13–4.45 | 0.021 | - | - | - |
| Age (years) | 1.03 | 1.00–1.05 | 0.017 | - | - | - |
| Urinary tract | 0.38 | 0.16–2.34 | 0.026 | - | - | - |
| Diabetes mellitus | 2.71 | 1.31–5.60 | 0.007 | - | - | - |
| Heart failure | 2.55 | 1.04–6.23 | 0.040 | - | - | - |
| Appropriate antimicrobials administration | 1.04 | 0.39–2.83 | 0.929 | 1.72 | 0.58–5.08 | 0.324 |
OR – Odds ratio; CI – Confidence interval
DISCUSSION
This study found that among adult patients who were found to have an Acinetobacter infection after admission to a community hospital in Madinah, Saudi Arabia, most CRA isolates were hospital-acquired and resistant to multiple antimicrobial classes, including carbapenems. In addition, the main risk factors for CRA infections were mechanical ventilation and prior antimicrobial therapy. Compared with CSA, CRA infections were associated with increased mortality, with patients’ comorbidities being the main predictor of mortality.
In this study, most Acinetobacter species were multidrug-resistant and were mainly susceptible to polymyxins. This represents a significant increase in the past 10 years: in 2009, 5.4% of Acinetobacter infections were resistant to imipenem,[4] while the current study and another recent study found 83% and 94% CRA infection rates, respectively.[5] Another study from Saudi Arabia has reported that the increased rate of CRA is due to extreme use of wide-spectrum antimicrobial drugs and use of invasive medical devices.[15]
The current study found that patients with CRA infections had a twofold higher 90-day mortality rate than those with CSA infections, which is in coherence with the findings of previous studies, including a meta-analysis.[16,17] While a few studies found this association in the unadjusted analysis, we could not confirm the same due to the small sample size.[18,19,20] Therefore, there is a need for larger, more controlled studies to better understand the impact of CRA infections on mortality outcomes. The increased mortality in patients with CRA infections may be attributed to several factors, such as underlying comorbidities, the severity of the infection, and inadequate antimicrobial therapy. Nonetheless, in our study, the association between CRA infection and higher mortality rates remained significant even after adjusting for confounding factors.
While the majority of cases infected with Acinetobacter were treated with active antimicrobials based on laboratory susceptibility testing, it is important to mention that cases of CRA were primarily treated with colistin and tigecycline, unlike cases of CSA. Nevertheless, multiple studies have demonstrated that these antimicrobials are less effective against carbapenem-resistant Gram-negative bacteria than alternative agents.[21,22,23,24] Therefore, the absence of efficacious antimicrobials that specifically target CRA may be a factor contributing to the difference in mortality rates between patients with CRA and CSA.
Comorbidities were found to be a significant factor associated with 90-day mortality, which is in accordance with the findings of several other studies.[17,25] Since CRA infections were more frequent in patients with chronic comorbidities, increased mortality in the CRA group may be due to underlying chronic illnesses. The higher 90-day mortality than 30-day mortality in patients with CRA may also support the association between comorbidities and increased mortality, as the progression of the underlying comorbidities could contribute to the increased mortality observed at 90 days. However, Acinetobacter infection is likely to contribute to mortality rates, as some studies have shown that patients with Acinetobacter infections, regardless of antimicrobial susceptibility, had higher mortality than uninfected patients.[26]
In line with previous studies, we found that mechanical ventilation is a risk factor for CRA infection.[27,28,29] Two-thirds of the cases in this study had Acinetobacter ventilator-associated pneumonia, of which 75% were CRA infections. It was also the most common site of infection (73%) among non-survivors. Acinetobacter can survive in harsh hospital environments for long periods and is difficult to eradicate with current infection control practices. Therefore, new prevention intervention strategies are needed to eliminate it from hospital environments, especially mechanical ventilators.
We found that prior antimicrobial therapy was associated with CRA infection. This result has also been demonstrated in many previous studies.[27,30] However, those previous studies examined different antimicrobial agents and various time intervals before CRA infection. For instance, Huang et al. showed that the risk of CRA infection was increased with prior use of piperacillin/tazobactam and cefepime,[31] while Zheng et al. found that prior carbapenem therapy was associated with CRA infection.[27] Our study limited the interval before CRA infection to 3 months and examined all antimicrobials. In the same context, previous studies showed that prior antimicrobial therapy was associated with carbapenem resistance in Enterobacterales, supporting our findings.[32] Although prior antimicrobial treatment increases the risk of resistance, Acinetobacter survival in the environment is a prerequisite to the emergence of CRA.[33]
Limitations
This is a retrospective study and has the inherent limitations of this study design. Another limitation of this study is that the relatively small sample size may have resulted in missing some risk factors for CRA infections that were significant in our unadjusted analysis but not in the adjusted analysis. Future studies with a larger sample are needed to clarify these risk factors. In addition, the mortality rate may have been underestimated, as recording death after discharge was beyond the scope of the study. Lastly, we could not verify the timing of antimicrobial administration, which may have influenced the mortality in both CRA and CSA groups.
CONCLUSION
This study found that in patients admitted to our community hospital, most Acinetobacter isolates were carbapenem-resistant, and that there was a gradual increase in the resistance over the study period. CRA infections were mostly hospital-acquired and associated with higher mortality than CSA. The main risk factors for CRA infections were mechanical ventilation and prior antimicrobial therapy. With the current Acinetobacter eradication strategies within hospitals proving to be less effective, there is a need for newer eradication strategies and to ensure limiting its spread to chronically hospitalized patients, particularly ventilated patients.
Ethical considerations
The study was approved by the Research Ethics Committee (Ref. no. roj-data/om/2021/rc/083; date: March 16, 2021), King Abdullah International Research Center, Jeddah. Saudi Arabia. Requirement for patient consent was waived owing to the study design. The study adhered to the principles of the Declaration of Helsinki, 2013.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Data availability statement
The data underlying this study are limited and restricted. Access to the data is controlled by Prince Mohammed Bin Abdulaziz Hospital, Ministry of National Guard Health Affairs. Due to privacy and confidentiality considerations, the data cannot be shared publicly. For researchers interested in accessing the data, inquiries should be directed to Prince Mohammed Bin Abdulaziz Hospital. Requests for access will be evaluated on a case-by-case basis in accordance with institutional policies and regulations.
Author contributions
Conceptualization: Z.G., R.F., and A.A.; Methodology: Z.G. and R.F.; Data analysis: Z.G.; Writing–original draft preparation: Z.G., R.F., A.A., R.A., L.H., H.O.; Writing – review and editing: Z.G., R.F., A.A., R.A., L.H., M.M., H.O.; Supervision: Z.G. and R.F.
All authors have read and agreed to the published version of the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: Risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65:204–11. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Biggest Threats and Data. Centers for Disease Control and Prevention. 2019. [[Last accessed: January 2021]]. Available from: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html .
- 3.World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. Geneva: World Health Organization; 2017. [[Last accessed: January 2021]]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493062 . [PubMed] [Google Scholar]
- 4.Memish ZA, Shibl AM, Kambal AM, Ohaly YA, Ishaq A, Livermore DM. Antimicrobial resistance among non-fermenting gram-negative bacteria in Saudi Arabia. J Antimicrob Chemother. 2012;67:1701–5. doi: 10.1093/jac/dks091. [DOI] [PubMed] [Google Scholar]
- 5.Kharaba A, Algethamy H, Hussein M, Al-Hameed FM, Alghamdi A, Hamdan A, et al. Incidence, outcomes, and predictors of Acinetobacter infection in Saudi Arabian critical care units. J Crit Care. 2021;66:109–16. doi: 10.1016/j.jcrc.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Balkhy HH, El-Saed A, Maghraby R, Al-Dorzi HM, Khan R, Rishu AH, et al. Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann Thorac Med. 2014;9:104–11. doi: 10.4103/1817-1737.128858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maragakis LL, Perl TM. Antimicrobial resistance: Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–63. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 8.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin Microbiol Rev. 2017;30:409–47. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rangel K, Chagas TP, De-Simone SG. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens. 2021;10:1006. doi: 10.3390/pathogens10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verroken A, Scohy A, Gérard L, Wittebole X, Collienne C, Laterre PF. Co-infections in COVID-19 critically ill and antibiotic management: A prospective cohort analysis. Crit Care. 2020;24:410. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intra J, Sarto C, Beck E, Tiberti N, Leoni V, Brambilla P. Bacterial and fungal colonization of the respiratory tract in COVID-19 patients should not be neglected. Am J Infect Control. 2020;48:1130–1. doi: 10.1016/j.ajic.2020.06.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries R, Bobenchik AM, Hindler JA, Schuetz AN. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J Clin Microbiol. 2021;59:e0021321. doi: 10.1128/JCM.00213-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Healthcare Safety Network (NHSN) Patient Safety Component Manual. CDC. 2021. [[Last accessed: March 2021]]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf .
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim ME. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann Clin Microbiol Antimicrob. 2019;18:1. doi: 10.1186/s12941-018-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, et al. Amulticenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14:e764–9. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 17.Lemos EV, de la Hoz FP, Einarson TR, McGhan WF, Quevedo E, Castañeda C, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: Systematic review and meta-analysis. Clin Microbiol Infect. 2014;20:416–23. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 18.Esterly JS, Griffith M, Qi C, Malczynski M, Postelnick MJ, Scheetz MH. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother. 2011;55:4844–9. doi: 10.1128/AAC.01728-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Nanas S, Markaki V, et al. Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: Risk factors, clinical features and outcomes. Infection. 2010;38:173–80. doi: 10.1007/s15010-010-0008-1. [DOI] [PubMed] [Google Scholar]
- 20.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: A systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73:22–32. doi: 10.1093/jac/dkx368. [DOI] [PubMed] [Google Scholar]
- 22.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 23.Aydemir H, Akduman D, Piskin N, Comert F, Horuz E, Terzi A, et al. Colistin versus the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect. 2013;141:1214–22. doi: 10.1017/S095026881200194X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of AmpC ?-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74:2089–114. doi: 10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 25.Brotfain E, Borer A, Koyfman L, Saidel-Odes L, Frenkel A, Gruenbaum SE, et al. Multidrug resistance Acinetobacter bacteremia secondary to ventilator-associated pneumonia: Risk factors and outcome. J Intensive Care Med. 2017;32:528–34. doi: 10.1177/0885066616632193. [DOI] [PubMed] [Google Scholar]
- 26.Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: A systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, et al. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am J Infect Control. 2013;41:e59–63. doi: 10.1016/j.ajic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Mah MW, Memish ZA, Cunningham G, Bannatyne RM. Outbreak of Acinetobacter baumannii in an intensive care unit associated with tracheostomy. Am J Infect Control. 2001;29:284–8. doi: 10.1067/mic.2001.114232. [DOI] [PubMed] [Google Scholar]
- 29.El-Ageery SM, Abo-Shadi MA, Alghaithy AA, Ahmad MA, Alsharif NH, Alharbi SA. Epidemiological investigation of nosocomial infection with multidrug-resistant Acinetobacter baumannii. Eur Rev Med Pharmacol Sci. 2012;16:1834–9. [PubMed] [Google Scholar]
- 30.Chopra T, Marchaim D, Awali RA, Krishna A, Johnson P, Tansek R, et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob Agents Chemother. 2013;57:6270–5. doi: 10.1128/AAC.01520-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang ST, Chiang MC, Kuo SC, Lee YT, Chiang TH, Yang SP, et al. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2012;45:356–62. doi: 10.1016/j.jmii.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Zhou M, Yan Q, Jian Z, Liu W, Li H. Risk factors for carbapenem-resistant enterobacterales infection among hospitalized patients with previous colonization. J Clin Lab Anal. 2022;36:e24715. doi: 10.1002/jcla.24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlosser B, Weikert B, Fucini GB, Kohlmorgen B, Kola A, Weber A, et al. Risk factors for transmission of carbapenem-resistant Acinetobacter baumannii in outbreak situations: Results of a case-control study. BMC Infect Dis. 2024;24:120. doi: 10.1186/s12879-024-09015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study are limited and restricted. Access to the data is controlled by Prince Mohammed Bin Abdulaziz Hospital, Ministry of National Guard Health Affairs. Due to privacy and confidentiality considerations, the data cannot be shared publicly. For researchers interested in accessing the data, inquiries should be directed to Prince Mohammed Bin Abdulaziz Hospital. Requests for access will be evaluated on a case-by-case basis in accordance with institutional policies and regulations.


