Abstract
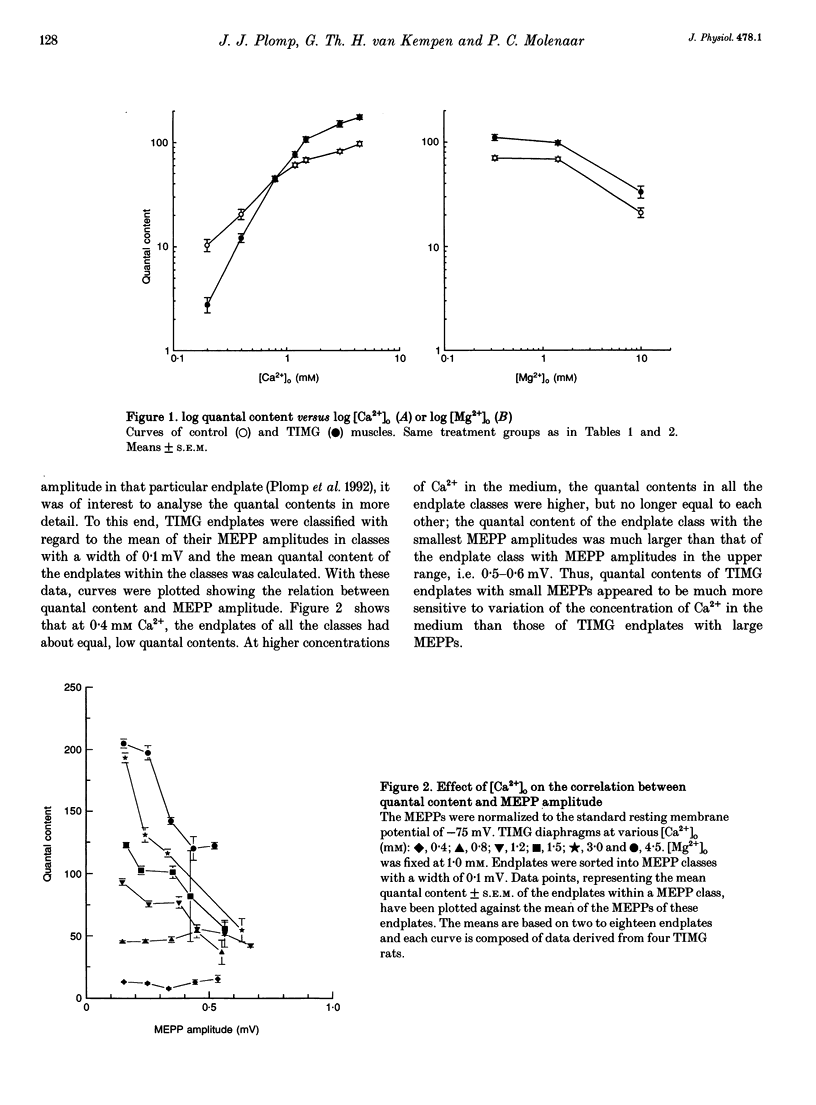
1. The presynaptic component of an adaptive feedback mechanism leading to increased acetylcholine (ACh) release was studied in endplates of diaphragms from rats treated chronically with alpha-bungarotoxin (alpha BTX). 2. Quantal contents were calculated 'directly' from the amplitude of miniature endplate potentials (MEPPs) and endplate potentials (EPPs) which were recorded after mu-conotoxin treatment to prevent muscle action potentials. 3. In vitro application of the Ca2+ channel blockers nifedipine (10 microM) or omega-conotoxin (40 nM) had no significant effect on the increased quantal content of endplates from alpha BTX-treated rats. 4. At control endplates, in vitro block of presynaptic K+ channels by 5 microM 3,4-diaminopyridine did increase the quantal content to a level which was similar to that found in endplates of alpha BTX-treated rats but also induced a broadening of EPPs, which was not found at endplates after alpha BTX treatment. 5. The difference between quantal contents of alpha BTX-treated and control rats was highly dependent on the [Ca2+]o/[Mg2+]o ratio when [Mg2+]o was fixed at 1 mM. At low [Ca2+]o, the quantal content of endplates from alpha BTX-treated rats was lower than that of controls while at [Ca2+]o in the normal and high range this was reversed. However, changing the [Ca2+]o/[Mg2+]o ratio by means of [Mg2+]o, at a fixed [Ca2+]o of 2 mM, did not influence the relative increase of quantal contents at endplates from alpha BTX-treated rats. Double logarithmic plots of the 'toxin-induced' myasthenia gravis (TIMG) and control quantal content versus [Ca2+]o had an approximately linear part between 0.2 and 1.5 mM [Ca2+]o. The slopes of the TIMG and control lines were 1.81 and 0.96, indicating that the ACh release in TIMG muscles was more sensitive to changes of [Ca2+]o than controls. 6. At normal [Ca2+]o and [Mg2+]o, the depression of EPP amplitude during stimulation of the phrenic nerve at 30-50 Hz was somewhat larger at endplates from alpha BTX-treated rats than at control endplates. At low [Ca2+]o, the potentiation of EPP amplitudes during a stimulus train was much larger at endplates from alpha BTX-treated rats than from controls. 7. The results do not support the idea that the increased release of ACh is caused via regulatory effects on the presynaptic Ca2+ or K+ channels. Instead, the anomalous dependency of ACh release on Ca2+ in muscles of alpha BTX-treated rats suggests that a cytoplasmic, Ca(2+)-dependent, component is involved in the adaptive change of transmitter release.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. L., Rossier M. F., Shcherbatko A. D., White R. E. Enzymatic gating of voltage-activated calcium channels. Ann N Y Acad Sci. 1991;635:26–34. doi: 10.1111/j.1749-6632.1991.tb36478.x. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon R. J., Breedlove S. M., Bernstein S., Lichtman J. W. Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci. 1990 Aug;10(8):2660–2671. doi: 10.1523/JNEUROSCI.10-08-02660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- Caratsch C. G., Schumacher S., Grassi F., Eusebi F. Influence of protein kinase C-stimulation by a phorbol ester on neurotransmitter release at frog end-plates. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jan;337(1):9–12. doi: 10.1007/BF00169469. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Hong S. J. Dissociation of the end-plate potential run-down and the tetanic fade from the postsynaptic inhibition of acetylcholine receptor by alpha-neurotoxins. Exp Neurol. 1987 Dec;98(3):509–517. doi: 10.1016/0014-4886(87)90260-3. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Gray W. R., Olivera B. M., Zeikus R. D., Kerr L., Yoshikami D., Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J Biol Chem. 1985 Aug 5;260(16):9280–9288. [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Trautmann A., Uchitel O. D. On the release of transmitter at normal, myasthenia gravis and myasthenic syndrome affected human end-plates. J Physiol. 1980 Feb;299:621–638. doi: 10.1113/jphysiol.1980.sp013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo Egidio, Rossi Paola, Tanzi Franco, Taglietti Vanni. Protein Kinase C Facilitation of Acetylcholine Release at the Rat Neuromuscular Junction. Eur J Neurosci. 1992;4(9):823–831. doi: 10.1111/j.1460-9568.1992.tb00192.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y., Poo M. M. Hebbian depression of isolated neuromuscular synapses in vitro. Science. 1992 Jun 12;256(5063):1570–1573. doi: 10.1126/science.1317971. [DOI] [PubMed] [Google Scholar]
- De Luca A., Li C. G., Rand M. J., Reid J. J., Thaina P., Wong-Dusting H. K. Effects of omega-conotoxin GVIA on autonomic neuroeffector transmission in various tissues. Br J Pharmacol. 1990 Oct;101(2):437–447. doi: 10.1111/j.1476-5381.1990.tb12727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E. R., Dani J. A. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990 Oct;10(10):3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gregorio F., Fesce R., Cereser S., Favaro G., Fiori M. G. Spontaneous and nerve-evoked quantal transmission in regenerated motor terminals. Cell Biol Int Rep. 1989 Dec;13(12):1119–1126. doi: 10.1016/0309-1651(89)90025-8. [DOI] [PubMed] [Google Scholar]
- Gallant P. E. The relationship between anti-acetylcholine receptor antibody levels and neuromuscular function in chronically myasthenic rats. J Neurol Sci. 1982 Apr;54(1):129–141. doi: 10.1016/0022-510x(82)90225-8. [DOI] [PubMed] [Google Scholar]
- Glavinović M. I. Presynaptic action of curare. J Physiol. 1979 May;290(2):499–506. doi: 10.1113/jphysiol.1979.sp012786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C., Meldolesi J., Ceccarelli B. The phorbol ester, 12-O-tetradecanoyl-phorbol-13-acetate, enhances the evoked quanta release of acetylcholine at the frog neuromuscular junction. Pflugers Arch. 1987 Jan;408(1):27–31. doi: 10.1007/BF00581836. [DOI] [PubMed] [Google Scholar]
- Hamilton B. R., Smith D. O. Calcium currents in rat motor nerve terminals. Brain Res. 1992 Jul 3;584(1-2):123–131. doi: 10.1016/0006-8993(92)90885-d. [DOI] [PubMed] [Google Scholar]
- Harish O. E., Poo M. M. Retrograde modulation at developing neuromuscular synapses: involvement of G protein and arachidonic acid cascade. Neuron. 1992 Dec;9(6):1201–1209. doi: 10.1016/0896-6273(92)90077-q. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden A. L. The field potential profile during activation of the avian optic tectum. J Physiol. 1968 Jan;194(1):75–90. doi: 10.1113/jphysiol.1968.sp008395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Facilitation by 3,4-diaminopyridine of regenerative acetylcholine release from mouse motor nerve. Br J Pharmacol. 1990 Dec;101(4):793–798. doi: 10.1111/j.1476-5381.1990.tb14159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol. 1989 Jul;97(3):934–940. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Salpeter M. M. Absence of [125I] alpha-bungarotoxin binding to motor nerve terminals of frog, lizard and mouse muscle. J Neurosci. 1983 Feb;3(2):326–331. doi: 10.1523/JNEUROSCI.03-02-00326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Neher E. Dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels in a mammalian neuroblastoma-glioma cell line. J Physiol. 1992 Mar;448:161–188. doi: 10.1113/jphysiol.1992.sp019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Malinow R., Schulman H., Tsien R. W. Persistent signalling and changes in presynaptic function in long-term potentiation. Ciba Found Symp. 1992;164:176–196. doi: 10.1002/9780470514207.ch12. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Oen B. S., Plomp J. J., Van Kempen G. T., Jennekens F. G., Hesselmans L. F. A non-immunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol. 1991 Apr 10;196(1):93–101. doi: 10.1016/0014-2999(91)90413-k. [DOI] [PubMed] [Google Scholar]
- Murphy R. L., Smith M. E. Effects of diacylglycerol and phorbol ester on acetylcholine release and action at the neuromuscular junction in mice. Br J Pharmacol. 1987 Feb;90(2):327–334. doi: 10.1111/j.1476-5381.1987.tb08962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., Cruz L. J. Diversity of Conus neuropeptides. Science. 1990 Jul 20;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Plomp J. J., van Kempen G. T., Molenaar P. C. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992 Dec;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N. Compensatory plasticity of aging at the neuromuscular junction. Exp Gerontol. 1992;27(1):75–81. doi: 10.1016/0531-5565(92)90030-4. [DOI] [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Strong J. A., Fox A. P., Tsien R. W., Kaczmarek L. K. Stimulation of protein kinase C recruits covert calcium channels in Aplysia bag cell neurons. Nature. 1987 Feb 19;325(6106):714–717. doi: 10.1038/325714a0. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Wilkinson R. S., Lunin S. D., Stevermer J. J. Regulation of single quantal efficacy at the snake neuromuscular junction. J Physiol. 1992 Mar;448:413–436. doi: 10.1113/jphysiol.1992.sp019049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y., Abe T., Satake M. Mu-conotoxins share a common binding site with tetrodotoxin/saxitoxin on eel electroplax Na channels. J Neurosci. 1987 May;7(5):1498–1502. doi: 10.1523/JNEUROSCI.07-05-01498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


