Abstract
1. The fluorescent dye fluo-3, in its permeant acetoxymethyl form, was used to monitor calcium transients during twitch and tetanus of single fibres isolated from the anterior tibialis muscle of Rana temporaria (2-5 degrees C). 2. Fluo-3 was loaded into the muscle fibre by diffusion. Under the experimental conditions used, approximately 45% of maximal fluorescence was reached during a 1 s fused isometric tetanus. Fluo-3 had no detectable effect on the mechanical response of the fibre. 3. The free calcium concentration in the myoplasm, [Ca2+]i, and its variation with time, was calculated from the fluorescence signal by accounting for the on- and off-rate constants for the binding of calcium to the dye. The time course of the calcium transient during twitch and tetanus determined in this way agreed well with previous measurements based on fast-reacting calcium-sensitive dyes. 4. [Ca2+]i declined steeply during the initial phase of force relaxation in both twitch and tetanus, but exhibited a secondary rise that closely coincided with the pseudoexponential fall of tension after the shoulder in the tetanus myogram. The rate of decay of [Ca2+]i during relaxation and the rate of decline of force both became progressively reduced by repetitive stimulation. 5. Stretch and shortening ramps performed during the plateau of an isometric tetanus had no detectable effect upon the calcium transient during the movement. By contrast, shortening and stretch imposed during the linear phase of relaxation both led to an increase of [Ca2+]i and to a steepening of the relaxation phase. 6. The results strongly suggest that the non-uniform length changes that are known to occur along a muscle fibre during relaxation enhance the release of calcium from the contractile system. The calcium mobilized in this way probably accounts for the transitory increase of [Ca2+]i that is observed during the latter part of force relaxation.
Full text
PDF


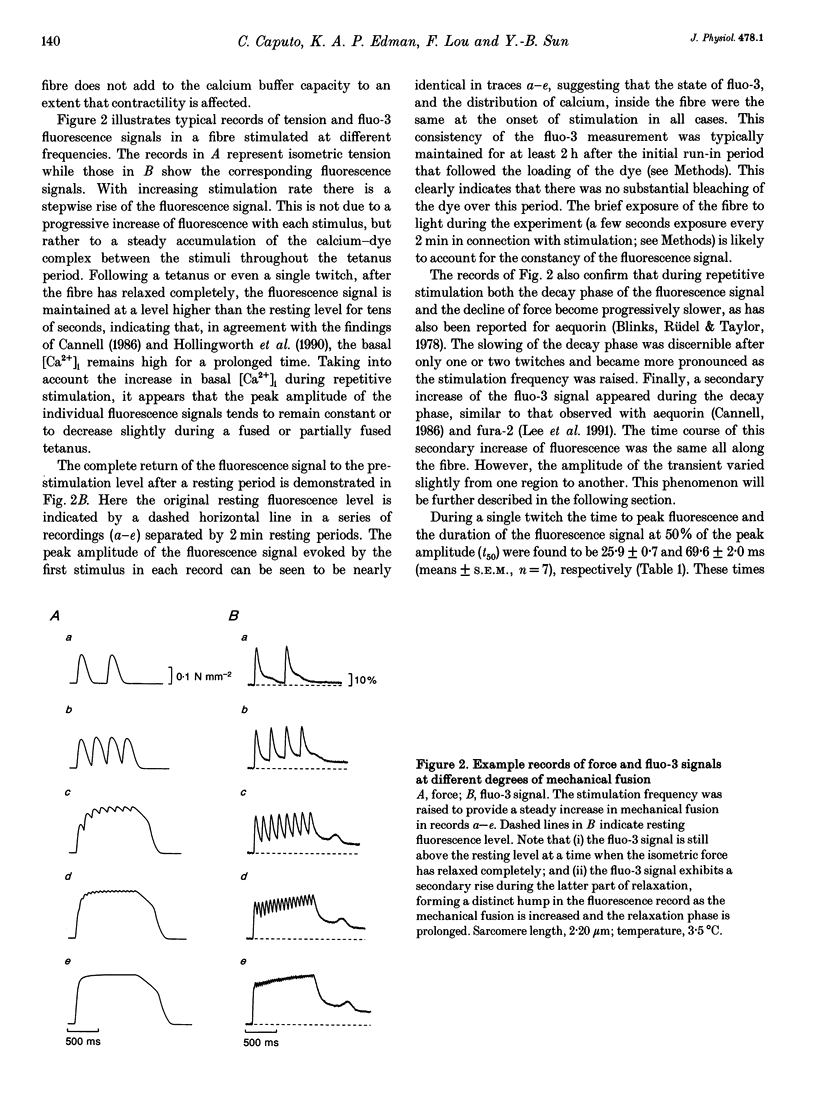
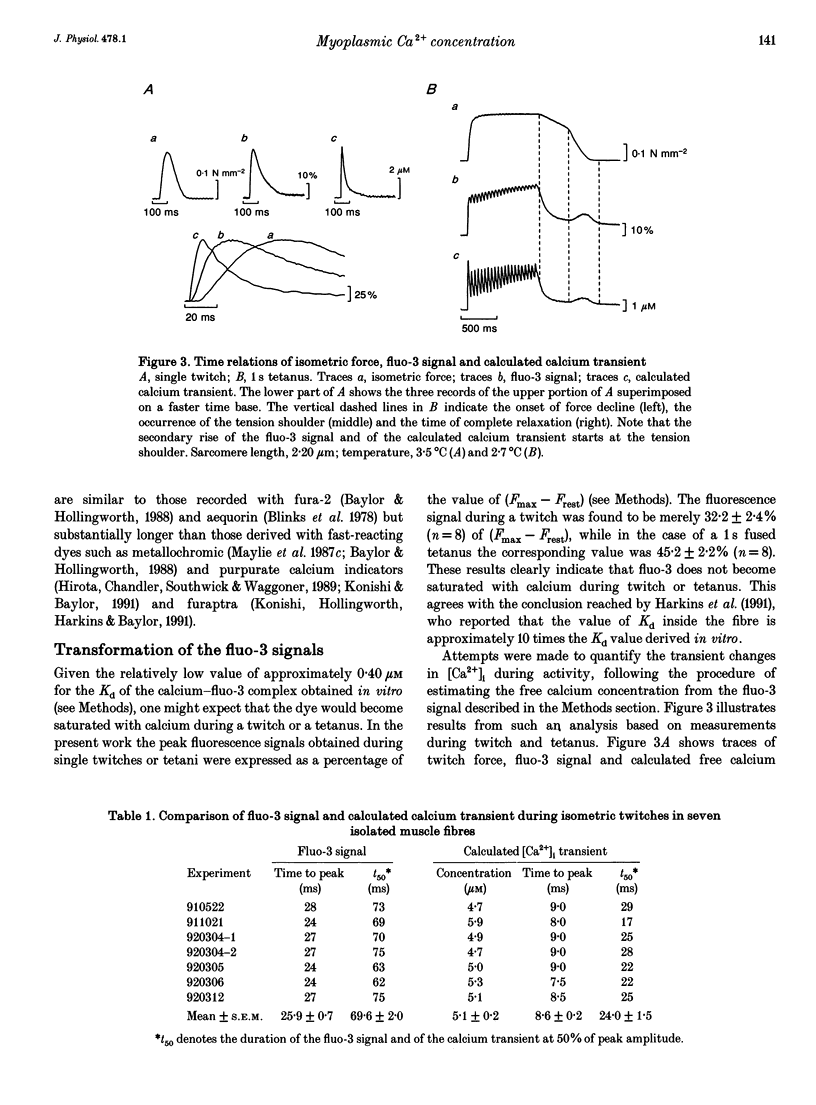
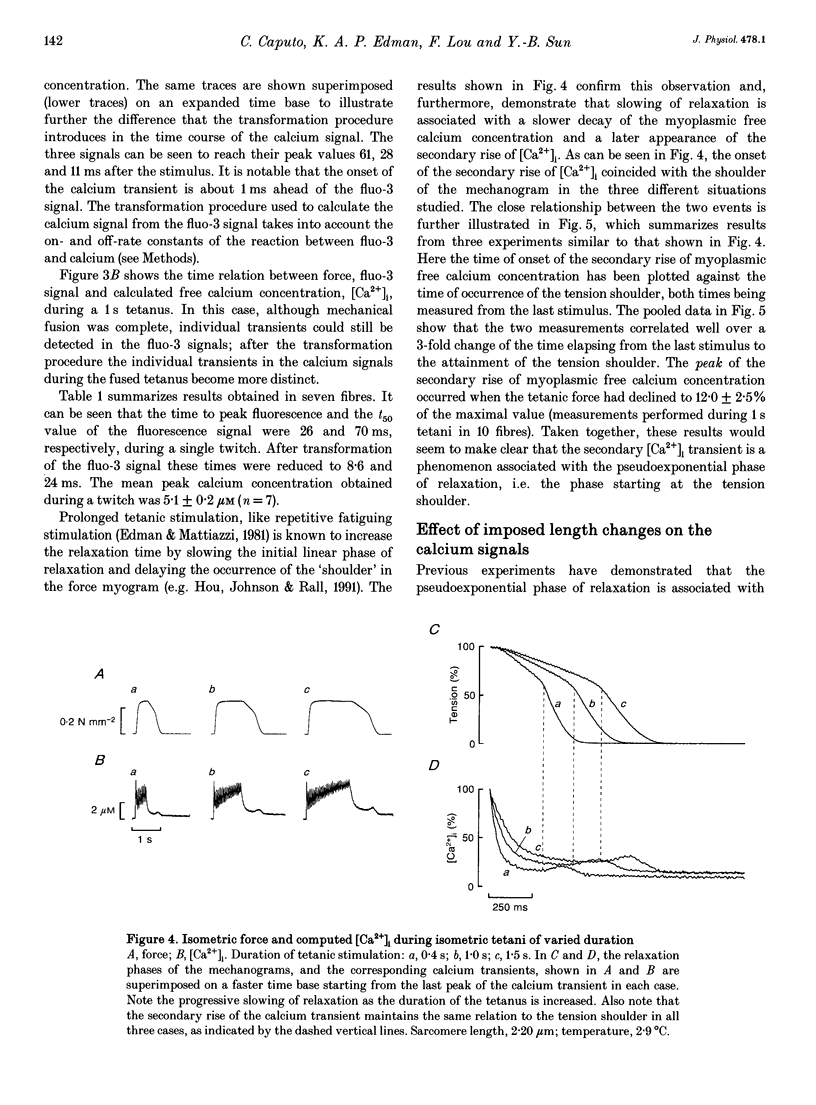
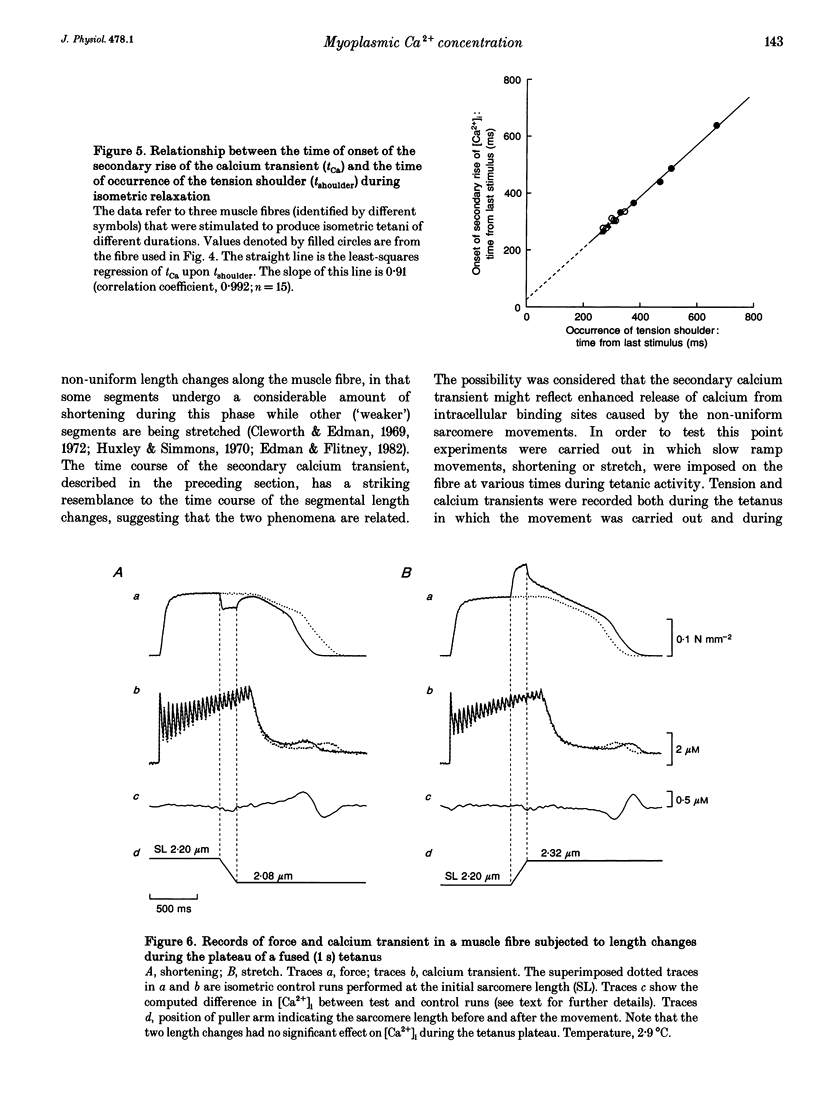




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Ashley C. C., Ridgway E. B. Simultaneous recording of membrane potential, calcium transient and tension in single muscle fibers. Nature. 1968 Sep 14;219(5159):1168–1169. doi: 10.1038/2191168a0. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Rüdel R., Taylor S. R. Calcium transients in isolated amphibian skeletal muscle fibres: detection with aequorin. J Physiol. 1978 Apr;277:291–323. doi: 10.1113/jphysiol.1978.sp012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D., Edman K. A. Laser diffraction studies on single skeletal muscle fibers. Science. 1969 Jan 17;163(3864):296–298. doi: 10.1126/science.163.3864.296. [DOI] [PubMed] [Google Scholar]
- Curtin N. A., Edman K. A. Effects of fatigue and reduced intracellular pH on segment dynamics in 'isometric' relaxation of frog muscle fibres. J Physiol. 1989 Jun;413:159–174. doi: 10.1113/jphysiol.1989.sp017647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard M., Erne P. Kinetics of calcium binding to fluo-3 determined by stopped-flow fluorescence. Biochem Biophys Res Commun. 1989 Aug 30;163(1):309–314. doi: 10.1016/0006-291x(89)92136-0. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Caputo C., Lou F. Depression of tetanic force induced by loaded shortening of frog muscle fibres. J Physiol. 1993 Jul;466:535–552. [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Flitney F. W. Laser diffraction studies of sarcomere dynamics during 'isometric' relaxation in isolated muscle fibres of the frog. J Physiol. 1982 Aug;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Mattiazzi A. R. Effects of fatigue and altered pH on isometric force and velocity of shortening at zero load in frog muscle fibres. J Muscle Res Cell Motil. 1981 Sep;2(3):321–334. doi: 10.1007/BF00713270. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol. 1984 Jun;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The role of non-uniform sarcomere behaviour during relaxation of striated muscle. Eur Heart J. 1980;Suppl A:49–57. doi: 10.1093/eurheartj/1.suppl_1.49. [DOI] [PubMed] [Google Scholar]
- Gillis J. M. Relaxation of vertebrate skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochim Biophys Acta. 1985 Jun 3;811(2):97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirota A., Chandler W. K., Southwick P. L., Waggoner A. S. Calcium signals recorded from two new purpurate indicators inside frog cut twitch fibers. J Gen Physiol. 1989 Oct;94(4):597–631. doi: 10.1085/jgp.94.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyde M. J., Potter J. D., Solaro R. J. The calcium binding properties of phosphorylated and unphosphorylated cardiac and skeletal myosins. J Biol Chem. 1979 Jul 25;254(14):6478–6482. [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Rapid 'give' and the tension 'shoulder' in the relaxation of frog muscle fibres. J Physiol. 1970 Sep;210(1):32P–33P. [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Konishi M., Baylor S. M. Myoplasmic calcium transients monitored with purpurate indicator dyes injected into intact frog skeletal muscle fibers. J Gen Physiol. 1991 Feb;97(2):245–270. doi: 10.1085/jgp.97.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Ríos E., Schneider M. F. Calcium transients and intramembrane charge movement in skeletal muscle fibres. Nature. 1979 May 31;279(5712):391–396. doi: 10.1038/279391a0. [DOI] [PubMed] [Google Scholar]
- Kubota T., Hagiwara N., Fujimoto M. Intracellular calcium measurements with PVC-resin Ca-selective microelectrodes in frog proximal tubules and sartorius muscle fibers. Jpn J Physiol. 1990;40(1):79–95. doi: 10.2170/jjphysiol.40.79. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Boyarsky G., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing tetramethylmurexide. J Gen Physiol. 1987 Jan;89(1):145–176. doi: 10.1085/jgp.89.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Comparison of arsenazo III optical signals in intact and cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):41–81. doi: 10.1085/jgp.89.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescence measurement and photochemical manipulation of cytosolic free calcium. Trends Neurosci. 1988 Oct;11(10):419–424. doi: 10.1016/0166-2236(88)90192-0. [DOI] [PubMed] [Google Scholar]
- Vergara J., DiFranco M., Compagnon D., Suarez-Isla B. A. Imaging of calcium transients in skeletal muscle fibers. Biophys J. 1991 Jan;59(1):12–24. doi: 10.1016/S0006-3495(91)82193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



