Abstract
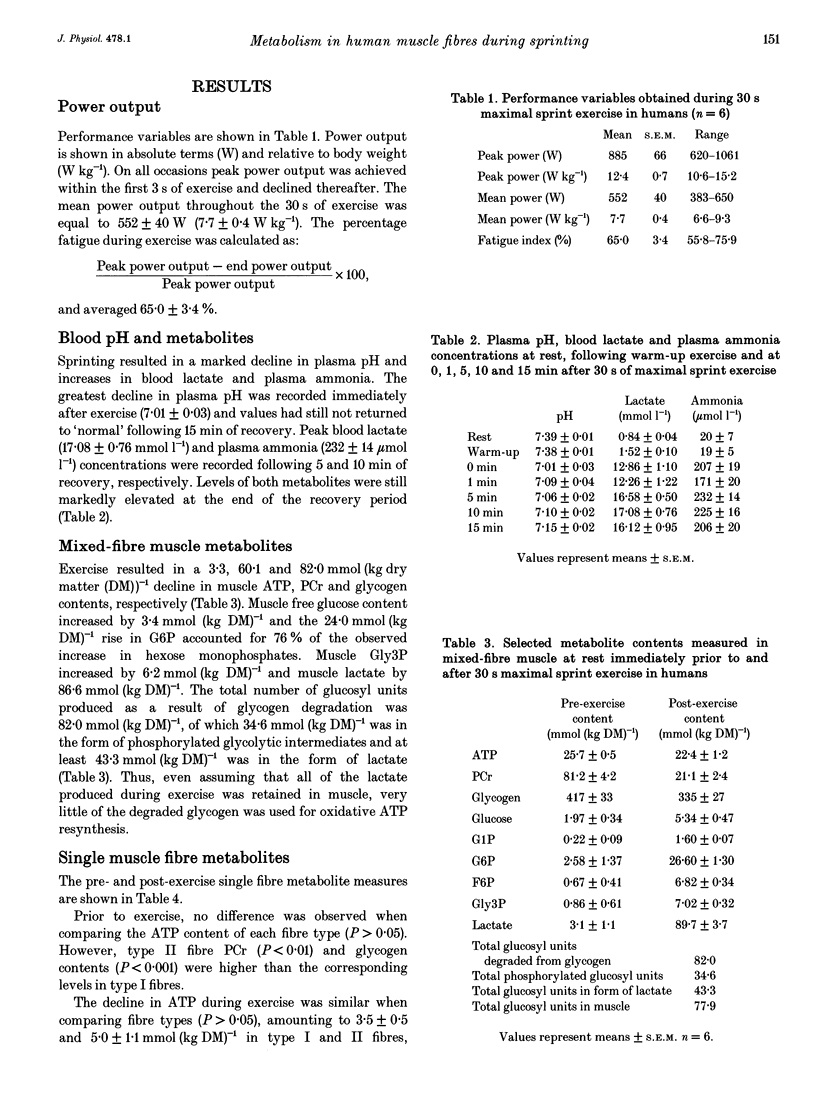
1. Muscle biopsy samples were obtained from the vastus lateralis of six healthy volunteers before and after 30 s of treadmill sprinting. A portion of each biopsy sample was used for mixed-fibre metabolite analysis. Single fibres were dissected from the remaining portion of each biopsy and were used for ATP, phosphocreatine (PCr) and glycogen determination. 2. Before exercise, PCr and glycogen contents were higher in type II fibres (79.3 +/- 2.7 and 472 +/- 35 mmol (kg dry matter (DM)-1, respectively) compared with type I fibres (71.3 +/- 3.0 mmol (kg DM)-1, P < 0.01 and 375 +/- 25 mmol (kg DM)-1, P < 0.001, respectively). 3. Peak power output was 885 +/- 66 W and declined by 65 +/- 3% during exercise. Phosphocreatine and glycogen degradation in type II fibres during exercise (74.3 +/- 2.5 and 126.3 +/- 15.8 mmol (kg DM)-1, respectively) was greater than the corresponding degradation in type I fibres (59.1 +/- 2.9 mmol (kg DM)-1, P < 0.001 and 77.0 +/- 14.3 mmol (kg DM)-1, P < 0.01, respectively). The decline in ATP during exercise was similar when comparing fibre types (P > 0.05). 4. Compared with previous studies involving similar durations of maximal cycling exercise, isokinetic knee extension and intermittent isometric contraction, the rates of substrate utilization recorded in type I fibres were extremely high, being close to the rapid rates observed in this fibre type during intense contraction with limb blood flow occluded.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Cheetham M. E., Boobis L. H., Brooks S., Williams C. Human muscle metabolism during sprint running. J Appl Physiol (1985) 1986 Jul;61(1):54–60. doi: 10.1152/jappl.1986.61.1.54. [DOI] [PubMed] [Google Scholar]
- Cheetham M. E., Williams C., Lakomy H. K. A laboratory running test: metabolic responses of sprint and endurance trained athletes. Br J Sports Med. 1985 Jun;19(2):81–84. doi: 10.1136/bjsm.19.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essén B., Jansson E., Henriksson J., Taylor A. W., Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975 Oct;95(2):153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- Fridén J., Seger J., Ekblom B. Topographical localization of muscle glycogen: an ultrahistochemical study in the human vastus lateralis. Acta Physiol Scand. 1989 Mar;135(3):381–391. doi: 10.1111/j.1748-1716.1989.tb08591.x. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Ren J. M., Söderlund K., Hultman E. Energy metabolism in single human muscle fibers during contraction without and with epinephrine infusion. Am J Physiol. 1991 May;260(5 Pt 1):E713–E718. doi: 10.1152/ajpendo.1991.260.5.E713. [DOI] [PubMed] [Google Scholar]
- Greenhaff P. L., Söderlund K., Ren J. M., Hultman E. Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J Physiol. 1993 Jan;460:443–453. doi: 10.1113/jphysiol.1993.sp019480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., Essén B., Hultman E. Glycogen phosphorylase activity in biopsy samples and single muscle fibres of musculus quadriceps femoris of man at rest. Scand J Clin Lab Invest. 1976 Oct;36(6):521–526. doi: 10.1080/00365517609054473. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hultman E., Nordesjö L. O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974 Apr;33(2):109–120. [PubMed] [Google Scholar]
- Hultman E., Greenhaff P. L., Ren J. M., Söderlund K. Energy metabolism and fatigue during intense muscle contraction. Biochem Soc Trans. 1991 Apr;19(2):347–353. doi: 10.1042/bst0190347. [DOI] [PubMed] [Google Scholar]
- Hultman E., Spriet L. L. Skeletal muscle metabolism, contraction force and glycogen utilization during prolonged electrical stimulation in humans. J Physiol. 1986 May;374:493–501. doi: 10.1113/jphysiol.1986.sp016093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I., Bar-Or O., Karlsson J., Dotan R., Tesch P., Kaiser P., Inbar O. Changes in muscle metabolites in females with 30-s exhaustive exercise. Med Sci Sports Exerc. 1982;14(6):457–460. doi: 10.1249/00005768-198206000-00009. [DOI] [PubMed] [Google Scholar]
- Jacobs I., Tesch P. A., Bar-Or O., Karlsson J., Dotan R. Lactate in human skeletal muscle after 10 and 30 s of supramaximal exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):365–367. doi: 10.1152/jappl.1983.55.2.365. [DOI] [PubMed] [Google Scholar]
- Jansson E., Dudley G. A., Norman B., Tesch P. A. ATP and IMP in single human muscle fibres after high intensity exercise. Clin Physiol. 1987 Aug;7(4):337–345. doi: 10.1111/j.1475-097x.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Jones N. L., McCartney N., Graham T., Spriet L. L., Kowalchuk J. M., Heigenhauser G. J., Sutton J. R. Muscle performance and metabolism in maximal isokinetic cycling at slow and fast speeds. J Appl Physiol (1985) 1985 Jul;59(1):132–136. doi: 10.1152/jappl.1985.59.1.132. [DOI] [PubMed] [Google Scholar]
- Katz A., Sahlin K., Henriksson J. Muscle ATP turnover rate during isometric contraction in humans. J Appl Physiol (1985) 1986 Jun;60(6):1839–1842. doi: 10.1152/jappl.1986.60.6.1839. [DOI] [PubMed] [Google Scholar]
- Maughan R. J. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-mul blood sample. Clin Chim Acta. 1982 Jul 1;122(2):231–240. doi: 10.1016/0009-8981(82)90282-0. [DOI] [PubMed] [Google Scholar]
- McCartney N., Spriet L. L., Heigenhauser G. J., Kowalchuk J. M., Sutton J. R., Jones N. L. Muscle power and metabolism in maximal intermittent exercise. J Appl Physiol (1985) 1986 Apr;60(4):1164–1169. doi: 10.1152/jappl.1986.60.4.1164. [DOI] [PubMed] [Google Scholar]
- Nevill M. E., Boobis L. H., Brooks S., Williams C. Effect of training on muscle metabolism during treadmill sprinting. J Appl Physiol (1985) 1989 Dec;67(6):2376–2382. doi: 10.1152/jappl.1989.67.6.2376. [DOI] [PubMed] [Google Scholar]
- Ren J. M., Hultman E. Regulation of phosphorylase a activity in human skeletal muscle. J Appl Physiol (1985) 1990 Sep;69(3):919–923. doi: 10.1152/jappl.1990.69.3.919. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Gorski J., Edström L. Influence of ATP turnover and metabolite changes on IMP formation and glycolysis in rat skeletal muscle. Am J Physiol. 1990 Sep;259(3 Pt 1):C409–C412. doi: 10.1152/ajpcell.1990.259.3.C409. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Lindinger M. I., McKelvie R. S., Heigenhauser G. J., Jones N. L. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol (1985) 1989 Jan;66(1):8–13. doi: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Söderlund K., Bergström M., Hultman E. Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol (1985) 1987 Feb;62(2):611–615. doi: 10.1152/jappl.1987.62.2.611. [DOI] [PubMed] [Google Scholar]
- Söderlund K., Greenhaff P. L., Hultman E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol Scand. 1992 Jan;144(1):15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Tesch P. A., Thorsson A., Fujitsuka N. Creatine phosphate in fiber types of skeletal muscle before and after exhaustive exercise. J Appl Physiol (1985) 1989 Apr;66(4):1756–1759. doi: 10.1152/jappl.1989.66.4.1756. [DOI] [PubMed] [Google Scholar]
- Thorstensson A., Sjödin B., Tesch P., Karlsson J. Actomyosin ATPase, myokinase, CPK and LDH in human fast and slow twitch muscle fibres. Acta Physiol Scand. 1977 Feb;99(2):225–229. doi: 10.1111/j.1748-1716.1977.tb10373.x. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Tabata I., Medbø J. I. Glycogen breakdown in different human muscle fibre types during exhaustive exercise of short duration. Acta Physiol Scand. 1992 Feb;144(2):135–141. doi: 10.1111/j.1748-1716.1992.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Wibom R., Söderlund K., Lundin A., Hultman E. A luminometric method for the determination of ATP and phosphocreatine in single human skeletal muscle fibres. J Biolumin Chemilumin. 1991 Apr-Jun;6(2):123–129. doi: 10.1002/bio.1170060210. [DOI] [PubMed] [Google Scholar]


