Abstract
Objectives:
The HealthPartners’ Vaccine Safety Datalink (VSD) team maintains standardized files of vaccines from medical and pharmacy claims and electronic health records (established data sources) for safety surveillance. Since 2021, for selected vaccines, data from the Minnesota Immunization Information Connection (MIIC), Minnesota’s immunization information system, have been added to the HealthPartners’ VSD files. We examined how MIIC data have enhanced the identification of novel and routine vaccines.
Methods:
We describe the approach to incorporating MIIC data. We determined and compared the number and proportion of vaccines identified from established data sources with the additional capture of vaccine data identified from MIIC, in which age group and period of observation varied by vaccine.
Results:
As of December 31, 2023, of 1 099 411 people in the HealthPartners’ VSD cohort, 1 001 400 people (91%) were linked with an MIIC record. Across all data sources, for the full cohort, >2.7 million COVID-19 vaccine doses were recorded since 2020, >4000 mpox vaccine doses since 2022, >7.3 million influenza vaccine doses since 2004, >600 000 human papillomavirus (HPV) vaccine doses since 2006, and >1.1 million diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine doses since 2004. For COVID-19 vaccines, about 30% of vaccine doses were exclusively captured from MIIC, with the remaining 70% from established data sources. For the mpox vaccine, about 42% were exclusively from MIIC. For influenza, HPV, and DTaP vaccines, about 20%, 14%, and 17%, respectively, were exclusively identified from MIIC.
Conclusions:
Incorporation of data from state immunization information systems into existing vaccine data files can enhance monitoring on the safety of novel vaccines administered outside traditional health care settings and can enhance data quality for routine childhood and adult vaccines.
Keywords: vaccination, immunization information system, safety, surveillance, vaccine uptake
The Vaccine Safety Datalink (VSD) is a collaborative project between the Centers for Disease Control and Prevention’s (CDC’s) Immunization Safety Office and 13 integrated health systems; the VSD includes data on more than 3% of the US population. 1 The primary aim of the VSD is to monitor the safety of vaccines in use in the United States, informing vaccine recommendations and guidelines.2 -4 Full capture of vaccine data is key to monitoring the safety and effectiveness of vaccines after vaccine approval. HealthPartners, an integrated health system in Minnesota and western Wisconsin, has been a member of the VSD since 2000. The VSD team relies on electronic health records (EHRs) and medical and pharmacy claims (established data sources) to identify vaccines administered in the VSD population. 5 The accuracy of vaccine data in the VSD, compared with self-reported data or data obtained from medical records, is generally high, although accuracy may be lower for vaccines that are administered outside traditional sites for vaccination, such as clinics and pharmacies. 6
The Minnesota Immunization Information Connection (MIIC) has been in use as a statewide immunization information system (IIS) since 2002; as of 2023, MIIC contained data on more than 125 million immunizations received by more than 8.6 million people of all ages. 7 Participation in MIIC is widespread throughout Minnesota. All 10 major health systems in the state report vaccines administered to MIIC. Pharmacies, including the large commercial chains, report to MIIC as mandated by state law. 8 In addition, 94% of health care providers enrolled in the Minnesota Vaccines for Children program, regardless of practice size or volume, participate in MIIC. MIIC has supported bidirectional information exchange since 2009, before efforts were made to increase the meaningful use of EHR systems (hereinafter, meaningful use program). 9 The national standard bidirectional option (Simple Object Access Protocol Web Service with the CDC Web Services Definition Language) was introduced in 2011 to ensure that MIIC could accommodate health care providers interested in participating in the meaningful use program. 9 Data from state IISs, including MIIC, continue to be used to evaluate vaccine uptake and vaccine coverage.10 -12 However, the importance of data collected by MIIC in supporting surveillance of health system–based vaccine safety has not been previously described.
In Minnesota, COVID-19 vaccines were distributed by the state department of health. Especially early in the vaccination campaign, most doses were administered outside traditional health care settings without a medical or pharmacy claim being generated and with mandated reporting to MIIC. As such, EHRs and medical and pharmacy claims could have substantial lags in identifying the number of COVID-19 vaccines administered. Thus, starting in May 2021, HealthPartners established a manual process for receiving bulk vaccine data from MIIC for use in clinical care and near–real-time surveillance of vaccine safety.13 -15 The availability of MIIC data was expected to improve the capture of information on vaccines known to be administered in pharmacies or community vaccination settings, such as for the COVID-19 vaccine and later for the mpox vaccine. However, whether the incorporation of MIIC data would also benefit safety surveillance of routine childhood, adolescent, and adult vaccines was not clear. 16 We aimed to describe how incorporation of MIIC data into VSD vaccine files has enhanced the capture of data on the administration of both novel and routine vaccines.
Methods
Study Design
We conducted an observational, descriptive, single-site study. HealthPartners’ institutional review board (IRB) reviewed the study and waived informed consent (IRB no. A01-023), consistent with applicable federal law and CDC policy (eg, 45 CFR part 46; 21 CFR part 56; 42 USC §241[d]; 5 USC §552a; 44 USC §3501 et seq).
Setting and Study Population
HealthPartners serves more than 1.8 million members in its medical and dental health insurance plan and more than 1.2 million patients in its clinics and hospitals. HealthPartners is an open network health system in which both members and patients are not limited to a single care system. HealthPartners’ project investigators and the data manager for the VSD study defined the HealthPartners VSD population as members in the HealthPartners’ health insurance plan with at least 1 ambulatory or virtual visit in the HealthPartners’ health system since January 1, 2004. For this project, we identified a subset of the HealthPartners’ VSD population with health insurance enrollment since December 15, 2020, aligning with the COVID-19 vaccine rollout, and at least 1 outpatient or virtual encounter at a HealthPartners facility since January 1, 2018. We included the 2 additional years before rollout of the COVID-19 vaccine (ie, 2018 and 2019) to improve capture of vaccine data for members with low levels of health care use.
Vaccine Data Sources
In the past, our HealthPartners’ VSD team identified vaccines from 3 established data sources (EHRs, medical claims, and pharmacy claims). EHR data include vaccines administered to patients in the HealthPartners health system and historical vaccines reported by the patient or manually reconciled from MIIC during a health care encounter. Claims data include information from both medical and pharmacy claims for vaccine-related services billed to HealthPartners health insurance. To consolidate vaccine data from various data sources, we maintain a crosswalk for vaccines that can be mapped to the standard code set of vaccines administered, commonly known as the Vaccine Administered Code Set (CVX). 9 For EHR data, we use the immunization identifier from the immunization table that corresponds to the type of vaccine administered. We update EHR data in the VSD files on a weekly basis. For claims data, we identify vaccine-related Current Procedural Terminology (CPT) codes from medical claims and generic product identifiers from pharmacy claims and update these data monthly. In May 2021, we began incorporating immunization data on 8 vaccine groups (COVID-19, influenza, mpox, human papillomavirus [HPV], rotavirus, diphtheria and tetanus toxoids and acellular pertussis [DTaP], pneumococcal conjugate vaccine [PCV13], and measles-mumps-rubella [MMR]) from MIIC; we use both the immunization record identifier and vaccine-related CPT codes to map to the standard CVX code set. We update and add the MIIC data to the VSD vaccine data files every 2 weeks. We also have access to information on vaccine manufacturer, anatomic site of vaccine administration, and vaccine lot number from EHR data and MIIC data but not from claims data.
Approach to Data Exchange With MIIC
Every 2 weeks, we prepare a new batch of data consisting of eligible members of the HealthPartners’ VSD cohort and upload the data batch to the Minnesota Department of Health (MDH) Cloud Drive for matching through probabilistic linkage by MDH. We receive a data file of individuals from the VSD cohort matched to MIIC records and data on vaccines administered for each individual, which also includes vaccine-related CVX codes and CPT codes, vaccine manufacturer, anatomic site of vaccine administration, vaccine lot number, and date of administration. For the matched cohort, we maintain a vaccine crosswalk for the 8 selected vaccine groups (COVID-19, influenza, mpox, HPV, rotavirus, DTaP, PCV13, and MMR). We identified DTaP vaccines if administered as individual vaccines or as part of combination vaccines, such as DTaP, hepatitis B, inactivated poliovirus or DTaP, inactivated poliovirus, or Haemophilus influenzae type b. For all vaccines, we implement deduplication and data quality check procedures locally. Similarly, we deduplicate records within each established data source. We deduplicate vaccine records found from multiple data sources by using a hierarchy with EHR data assigned as the highest rank, followed by MIIC, then medical claims, and finally pharmacy claims; we retain the record with the highest rank along with the data source. In addition, when deduplicating records between the EHR and MIIC, we retrieve information about vaccine manufacturer, anatomic site of vaccine administration, and vaccine lot number, depending on the availability from either data source. After the processing of MIIC data is completed, we combine the MIIC vaccine data with data available through EHRs and medical and pharmacy claims to generate the final vaccine file.
Statistical Analysis
We reported count data from the eligible HealthPartners VSD cohort uploaded for MIIC data exchange and the count and proportion with linked MIIC data. We also compared demographic characteristics between people with linked MIIC data and people without linked MIIC data.
Age groups (at the time of vaccination) and the period of evaluation of the additional capture of vaccine data from MIIC varied by vaccine, consistent with the recommended age of administration and vaccine availability. We described the capture of COVID-19 vaccines among people aged 6 months and older starting in December 2020 and influenza vaccines among people aged 6 months and older starting in January 2004. We described the capture of mpox vaccines among adults aged ≥18 years starting in June 2022. We described capture of HPV vaccines among people aged 9 through 26 years starting in January 2006. We described the capture of PCV13 vaccines in infants and children aged ≤2 years starting in January 2010. We described the capture of rotavirus vaccines among infants aged 0 through 8 months, DTaP vaccines among infants and children aged ≤6 years, and MMR vaccines among children aged 1 through 6 years, all starting in January 2004. For all of these vaccines, we reported counts and proportions of doses from each respective data source (ie, MIIC, EHR, medical and pharmacy claims) after hierarchical deduplication. For analyses, we included vaccines administered as of December 31, 2023. We performed statistical analyses with SAS version 9.4 (SAS Institute, Inc).
Results
Of 1 099 411 HealthPartners VSD cohort members eligible for MIIC data exchange on December 31, 2023, 1 001 400 (91.1%) had linked MIIC data and 98 011 (8.9%) did not have linked MIIC data. Characteristics of people with and without linked MIIC data by sex and age were similar (Table 1). Proportions of people who identified as Asian (6.0% vs 3.7%), Black (10.3% vs 7.7%), and Hispanic (5.2% vs 3.2%) were higher among people with linked MIIC data than among people without linked MIIC data.
Table 1.
Demographic characteristics of people in the HealthPartners Vaccine Safety Datalink cohort (N = 1 099 411) as of December 31, 2023, with and without linked MIIC vaccine data
| Characteristic | No. (%) a | |
|---|---|---|
| People with linked MIIC data (n = 1 001 400) | People without linked MIIC data (n = 98 011) | |
| Sex | ||
| Female | 544 048 (54.3) | 52 542 (53.6) |
| Male | 457 264 (45.7) | 45 463 (46.4) |
| Unknown | 88 (0) | 6 (<0.1) |
| Age, y | ||
| <18 | 174 924 (17.5) | 8074 (8.2) |
| ≥18 | 826 476 (82.5) | 89 937 (91.8) |
| Race and ethnicity | ||
| American Indian/Alaska Native | 2257 (0.2) | 220 (0.2) |
| Asian | 60 390 (6.0) | 3598 (3.7) |
| Hispanic | 52 283 (5.2) | 3172 (3.2) |
| Non-Hispanic Black/African American | 102 614 (10.2) | 7507 (7.7) |
| Non-Hispanic White | 694 279 (69.3) | 75 095 (76.6) |
| Multiple race b | 58 577 (5.8) | 3740 (3.8) |
| Other race and ethnicity c | 31 000 (3.1) | 4679 (4.8) |
Abbreviation: MIIC, Minnesota Immunization Information Connection.
Not all percentages total to 100 because of rounding.
People who identified as ≥2 races.
People who reported being Hawaiian/Pacific Islander, other, or unknown.
As of December 31, 2023, in the VSD cohort, all data sources (EHR, MIIC, medical and pharmacy claims) identified 2 715 519 COVID-19 vaccine doses, 4335 mpox vaccine doses, 7 318 748 influenza vaccine doses, 606 533 HPV vaccine doses, 371 883 rotavirus vaccine doses, 1 135 733 DTaP vaccine doses, 441 346 PCV13 vaccine doses, and 425 391 MMR vaccine doses (Table 2).
Table 2.
Number of vaccine doses identified from all data sources and established data sources by vaccine group for the eligible HealthPartners Vaccine Safety Datalink population (N = 1 001 400), as of December 31, 2023 a
| Vaccine | Age group at time of vaccination | No. of doses identified from all data sources | No. of doses identified from established data sources b | Percentage of doses identified solely from MIIC |
|---|---|---|---|---|
| COVID-19 | ≥6 mo | 2 715 519 | 1 915 144 | 29.5 |
| mpox | ≥18 y | 4335 | 2536 | 41.5 |
| Influenza | ≥6 mo | 7 318 748 | 5 887 824 | 19.6 |
| HPV | 9-26 y | 606 533 | 520 207 | 14.2 |
| Rotavirus | 0-8 mo | 371 883 | 327 289 | 12.0 |
| DTaP | 0-6 y | 1 135 733 | 946 185 | 16.7 |
| PCV13 | 0-2 y | 441 346 | 393 345 | 10.9 |
| MMR | 1-6 y | 425 391 | 367 105 | 13.7 |
Abbreviations: DTaP, diphtheria and tetanus toxoids and acellular pertussis; HPV, human papillomavirus; MIIC, Minnesota Immunization Information Connection; MMR, measles-mumps-rubella; PCV13, pneumococcal conjugate vaccine.
For COVID-19 vaccines, doses were recorded since December 15, 2020; for mpox vaccines, doses were recorded since June 1, 2022; for influenza vaccines, doses were recorded since July 1, 2004; for HPV vaccines, doses were recorded since January 1, 2006; for PCV13 vaccines, doses were recorded since January 1, 2010; for rotavirus, DTaP, and MMR vaccines, doses were recorded since January 1, 2004.
Established data sources include electronic health records and medical and pharmacy claims.
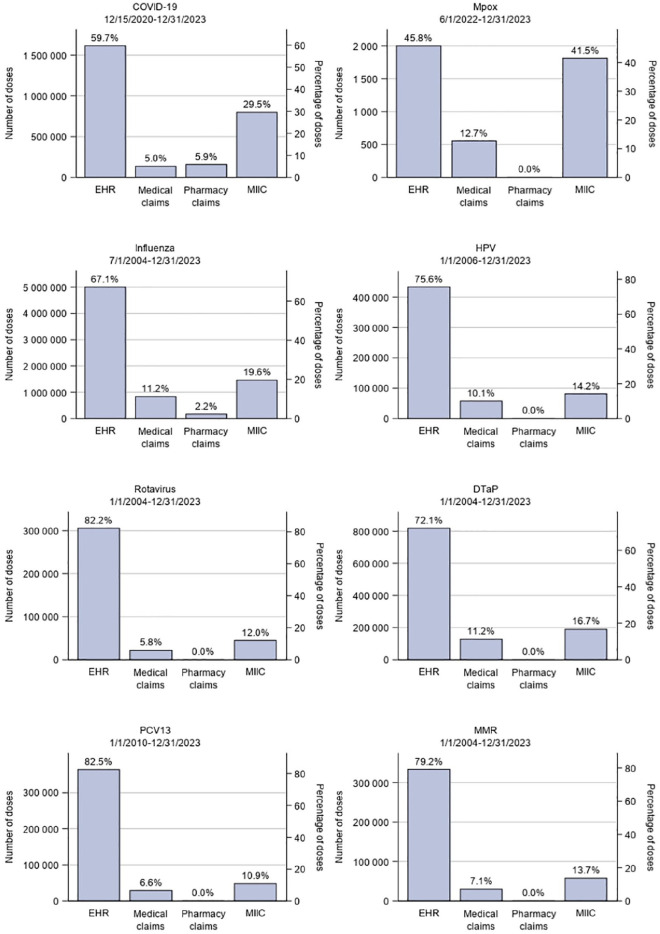
For COVID-19 vaccines, 800 375 doses administered from December 15, 2020, through December 31, 2023, were exclusively captured from MIIC, accounting for 29.5% of the total doses (Figure). For mpox vaccines, 1799 doses administered from June 1, 2022, through December 31, 2023, were exclusively captured from MIIC, representing 41.5% of the total doses. For influenza vaccines, 1 430 924 doses administered from July 1, 2004, through December 31, 2023, were exclusively captured from MIIC, representing 19.6% of the total doses. For HPV vaccines, 86 326 doses administered from January 1, 2006, through December 31, 2023, were exclusively captured from MIIC, representing 14.2% of the total doses. For PCV13 vaccines, 48 001 doses administered from January 1, 2010, through December 31, 2023, were exclusively captured from MIIC, representing 10.9% of the total doses. For rotavirus, DTaP, and MMR vaccines administered from January 1, 2004, through December 31, 2023, MIIC data contributed 44 594 additional rotavirus vaccine doses, 189 548 additional DTaP vaccine doses, and 58 286 additional MMR vaccine doses, accounting for 12.0%, 16.7%, and 13.7% of the total doses, respectively. We also observed that, among the 8 vaccine groups that we evaluated, the proportion of doses from medical claims (ie, CPT codes) was below 10% for 4 vaccine groups (5.0% for COVID-19 vaccines, 5.8% for rotavirus vaccines, 6.6% for PCV13 vaccines, and 7.1% for MMR vaccines) and ranged from 10.1% to 12.7% for the other vaccine groups.
Figure.
Number and percentage of doses of COVID-19, mpox, influenza, human papillomavirus (HPV), rotavirus, diphtheria and tetanus toxoids and acellular pertussis (DTaP), pneumococcal conjugate vaccine (PCV13), and measles-mumps-rubella (MMR) vaccines administered to individuals in the HealthPartners’ Vaccine Safety Datalink cohort, by data source. The HealthPartners Vaccine Safety Datalink cohort was defined as patients having enrollment in HealthPartners insurance since December 15, 2020, or at least 1 outpatient or virtual encounter at a HealthPartners facility since January 18, 2018. Data sources were electronic health records (EHRs), medical claims, pharmacy claims, and the Minnesota Immunization Information Connection (MIIC).
Discussion
Many VSD sites have established data exchange with state or regional IISs, including the Colorado Immunization Information System, Oregon’s ALERT Immunization Information System, the Washington Immunization Information System, and the Wisconsin Immunization Registry. 13 Compared with other states’ IISs, MIIC stands out for its comprehensive data capture, high participation rates, and effective integration with various health care systems. 17 The real-time data exchange we implemented has ensured timely updates to immunization records, thus facilitating real-time vaccine safety surveillance.
In our evaluation of 13 019 488 vaccine doses for 8 vaccine types among 1 001 400 individuals in the HealthPartners VSD cohort, a substantial number of vaccines were identified exclusively from MIIC data, with the proportion exclusively identified from MIIC ranging from about 11% of total doses for PCV13 vaccines to about 42% of total doses for mpox vaccines. Incorporation of MIIC data has substantially improved the identification of vaccines administered in the HealthPartners VSD population. The incorporation of IIS data into existing vaccine data files is critical for monitoring vaccine coverage, safety, and effectiveness of vaccines administered outside traditional health care settings. 18 With the availability of more comprehensive, timely, and accurate vaccine data in VSD populations, misclassification bias is substantially reduced. In addition, the availability of MIIC data can broaden the scope of surveillance and helps in the identification of rare adverse events after vaccination. In contrast, compared with vaccine data from MIIC, we found that only a small proportion of our vaccine data were from CPT codes.
The finding on similar proportions of people with and without MIIC linked data by sex and age group highlighted that people in the linked MIIC data were well represented. Differences in race and ethnicity among people with and without linked MIIC data likely represent differences in the location where care was received; large health systems and those participating in the Vaccines for Children program will have increased reporting to MIIC compared with small independent practices.
We previously described, in an adult population without health insurance, how access to both EHR and claims data for the identification of tetanus-containing vaccines increased data capture compared with the use of either data source alone. 19 In this current study, we highlighted the importance of MIIC as a comprehensive IIS in Minnesota. Muscoplat and Rajamani 17 described how MIIC could be used to capture and manage vaccine data and the potential of MIIC to support bidirectional data exchange with health care systems. Since that publication, MIIC has grown both in the amount of data in the system and in the volume of data exchanged annually. From July 2022 through June 2023, MIIC processed more than 77 million messages; 78% of those messages were queries from bidirectional interfaces (email communication with M.H.M. and A.B., September 18, 2023), meaning that MIIC data were heavily used by partners across the state. Similar to the report by Muscoplat et al, this study demonstrated the importance of capturing vaccine data from multiple sources, including EHRs, medical and pharmacy claims, and MIIC, to ensure the comprehensive capture of vaccine administration.
Limitations
Our study had several limitations. First, our data were from a single, large, integrated health system and a single state IIS; thus, our findings may not be applicable to other sites conducting vaccine safety surveillance for vaccines approved for use in the United States or in other countries. Similarly, selection bias may be an issue with a single-site study, and results could have been different had we used a different definition for our VSD population. Second, the accuracy of the data used in our study may have influenced the reliability of the findings. Our study relied on automated data from HealthPartners’ EHR and claims database and MIIC, and issues such as data entry errors or inconsistencies could have affected the analysis and interpretation of the results. In particular, loss of granularity may occur when CPT codes are converted to CVX codes. The mapping between CPT codes and CVX codes is not always one-to-one, and both code sets are updated periodically, requiring updates to the CPT to CVX crosswalk.
Conclusions
This study evaluated the incorporation of data from the Minnesota IIS into the VSD vaccine data files at HealthPartners to enhance the capture of data on the administration of novel and routine vaccines. We found that the inclusion of MIIC data substantially increased the capture of data on COVID-19, influenza, mpox, HPV, rotavirus, DTaP, PCV13, and MMR vaccines across various age groups. Including data from MIIC is essential for monitoring the safety of novel vaccines, such as COVID-19 vaccines, which were mostly administered outside traditional health care settings and can enhance data quality for safety surveillance of additional routine and seasonal childhood, adolescent, and adult vaccines.
Acknowledgments
The authors acknowledge staff from the Minnesota Department of Health, who set up the data exchange process for the Minnesota Immunization Information Connection.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.V.B. reports research funding for unrelated work from Sanofi Pasteur and AbbVie, and H.C.G. reports research funding for unrelated work from Moderna.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centers for Disease Control and Prevention (contract no. 75D30122D15421).
ORCID iD: Jingyi Zhu, PhD  https://orcid.org/0009-0008-1725-5305
https://orcid.org/0009-0008-1725-5305
References
- 1. McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32(42):5390-5398. doi: 10.1016/j.vaccine.2014.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goddard K, Donahue JG, Lewis N, et al. Safety of COVID-19 mRNA vaccination among young children in the Vaccine Safety Datalink. Pediatrics. 2023;152(1):e2023061894. doi: 10.1542/peds.2023-061894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenigsberg TA, Hanson KE, Klein NP, et al. Safety of simultaneous vaccination with COVID-19 vaccines in the Vaccine Safety Datalink. Vaccine. 2023;41(32):4658-4665. doi: 10.1016/j.vaccine.2023.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panagiotakopoulos L, McCarthy NL, Tepper NK, et al. Evaluating the association of stillbirths after maternal vaccination in the Vaccine Safety Datalink. Obstet Gynecol. 2020;136(6):1086-1094. doi: 10.1097/AOG.0000000000004166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(suppl 1):S45-S53. doi: 10.1542/peds.2010-1722H [DOI] [PubMed] [Google Scholar]
- 6. Daley MF, Reifler LM, Shoup JA, et al. Influenza vaccination among pregnant women: self-report compared with vaccination data from electronic health records, 2018-2020 influenza seasons. Public Health Rep. 2023;138(3):456-466. doi: 10.1177/00333549221099932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minnesota Immunization Information Connection. MIIC status and overall characteristics. Data reported October 2023. Accessed October 10, 2023. https://www.health.state.mn.us/people/immunize/miic/stats/clients.html
- 8. Minnesota Board of Pharmacy. Pharmacy immunization practice in Minnesota. February 2016. Accessed July 26, 2024. https://mn.gov/boards/assets/MNPharmacyImmPractice_2016_February_tcm21-29195.pdf
- 9. Centers for Disease Control and Prevention. Meaningful use and immunization information systems. October 3, 2022. Accessed October 16, 2023. https://www.cdc.gov/vaccines/programs/iis/meaningful-use/index.html
- 10. Groene EA, Horvath KJ, Yared N, et al. Missed opportunities for human papillomavirus vaccination by parental nativity, Minnesota, 2015-2018. Public Health Rep. 2022;137(5):867-877. doi: 10.1177/00333549211027244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Immunization information systems (IIS): current HL7 standard code set CVX—vaccines administered. September 22, 2022. Accessed October 16, 2023. https://www2.cdc.gov/vaccines/iis/iisstandards/vaccines.asp?rpt=cvx
- 12. LoMurray K, Sander M. Using the North Dakota Immunization Information System to determine adolescent vaccination rates and uptake. Public Health Rep. 2011;126(suppl 2):S78-S86. doi: 10.1177/00333549111260S210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groom HC, Crane B, Naleway AL, et al. Monitoring vaccine safety using the Vaccine Safety Datalink: assessing capacity to integrate data from immunization information systems. Vaccine. 2022;40(5):752-756. doi: 10.1016/j.vaccine.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326(16):1629-1631. doi: 10.1001/jama.2021.15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390-1399. doi: 10.1001/jama.2021.15072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bumatay S, Dickinson C, Larsen R, et al. A comparison of electronic health records and the Oregon state immunization registry for human papilloma virus vaccine delivery (2005-2022). Vaccine. 2023;41(39):5758-5762. doi: 10.1016/j.vaccine.2023.08.017 [DOI] [PubMed] [Google Scholar]
- 17. Muscoplat MH, Rajamani S. Immunization information system and informatics to promote immunizations: perspective from Minnesota Immunization Information Connection. Biomed Inform Insights. 2017;9:1178222616688893. doi: 10.1177/1178222616688893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stockwell MS, Natarajan K, Ramakrishnan R, et al. Immunization data exchange with electronic health records. Pediatrics. 2016;137(6):e20154335. doi: 10.1542/peds.2015-4335 [DOI] [PubMed] [Google Scholar]
- 19. Kharbanda EO, Parker ED, Nordin JD, Hedblom BD, Rolnick SJ. Influenza and pertussis vaccination coverage among privately insured women of reproductive age. Matern Child Health J. 2013;17(9):1631-1637. doi: 10.1007/s10995-012-1176-7 [DOI] [PubMed] [Google Scholar]