Abstract
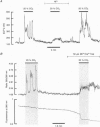
1. An acid-induced rise in the intracellular calcium concentration ([Ca2+]i) of type I cells is thought to play a vital role in pH/PCO2 chemoreception by the carotid body. In this present study we have investigated the cause of this rise in [Ca2+]i in enzymatically isolated, neonatal rat type I cells. 2. The rise in [Ca2+]i induced by a hypercapnic acidosis was inhibited in Ca(2+)-free media, and by 2 mM Ni2+. Acidosis also increased Mn2+ permeability. The rise in [Ca2+]i is dependent, therefore, upon a Ca2+ influx from the external medium. 3. The acid-induced rise in [Ca2+]i was attenuated by both nicardipine and methoxyverapamil (D600), suggesting a role for L-type Ca2+ channels. 4. Acidosis depolarized type I cells and often (approximately 50% of cells) induced action potentials. These effects coincided with a rise in [Ca2+]i. When membrane depolarization was prevented by a voltage clamp, acidosis failed to evoke a rise in [Ca2+]i. The acid-induced rise in [Ca2+]i is a consequence, therefore, of membrane depolarization. 5. Acidosis decreased the resting membrane conductance of type I cells. The reversal potential of the acid-sensitive current was about -75 mV. 6. A depolarization (30 mM [K+]o)-induced rise in [Ca2+]i was blocked by either the removal of extracellular Ca2+ or the presence of 2 mM Ni2+, and was also substantially inhibited by nicardipine. Under voltage-clamp conditions, [Ca2+]i displayed a bell-shaped dependence on membrane potential. Depolarization raises [Ca2+]i, therefore, through voltage-operated Ca2+ channels. 7. Caffeine (10 mM) induced only a small rise in [Ca2+]i (< 10% of that induced by 30 mM extracellular K+). Ca(2+)-induced Ca2+ release is unlikely, therefore, to contribute greatly to the rise in [Ca2+]i induced by depolarization. 8. Although the replacement of extracellular Na+ with N-methyl-D-glucamine (NMG), but not Li+, inhibited the acid-induced rise in [Ca2+]i, this was due to membrane hyperpolarization and not to the inhibition of Na(+)-Ca2+ exchange or Na(+)-dependent action potentials. 9. The removal of extracellular Na+ (NMG substituted) did not have a significant effect upon the resting [Ca2+]i, and only slowed [Ca2+]i recovery slightly following repolarization from 0 to -60 mV. Therefore, if present, Na(+)-Ca2+ exchange plays only a minor role in [Ca2+]i homeostasis. 10. In summary, in the neonatal rat type I cell, hypercapnic acidosis raises [Ca2+]i through membrane depolarization and voltage-gated Ca2+ entry.
Full text
PDF














Images in this article
Selected References
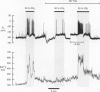
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Krishtal O. A., Maruyama T. Proton-induced sodium current in frog isolated dorsal root ganglion cells. J Neurophysiol. 1990 Apr;63(4):805–813. doi: 10.1152/jn.1990.63.4.805. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Lab M. J., Orchard C. H. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. J Physiol. 1983 Dec;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of acidic stimuli on intracellular calcium in isolated type I cells of the neonatal rat carotid body. Pflugers Arch. 1993 Oct;425(1-2):22–27. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D., Peers C., Lagadic-Gossmann D., Nye P. C. Effects of extracellular pH, PCO2 and HCO3- on intracellular pH in isolated type-I cells of the neonatal rat carotid body. J Physiol. 1991 Dec;444:703–721. doi: 10.1113/jphysiol.1991.sp018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D., Peers C., Nye P. C. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991 May;436:107–129. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Ikeuchi M., Fujimoto W. Y. Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):269–271. doi: 10.1038/311269a0. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by Cai and ATP. Cai-dependent Nai/Cao and Nai/Nao exchange modes. J Gen Physiol. 1987 Oct;90(4):505–525. doi: 10.1085/jgp.90.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Almaraz L., Obeso A., Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992 Apr;15(4):146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S. F., Wei J. Y., Eyzaguirre C. Influence of intracellular pH on the membrane potential of cultured carotid body glomus cells. Brain Res. 1993 Jan 22;601(1-2):353–357. doi: 10.1016/0006-8993(93)91736-c. [DOI] [PubMed] [Google Scholar]
- He S. F., Wei J. Y., Eyzaguirre C. Intracellular pH and some membrane characteristics of cultured carotid body glomus cells. Brain Res. 1991 May 3;547(2):258–266. doi: 10.1016/0006-8993(91)90969-3. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J., Miyamae S., Noma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987 Mar;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T. Interactions of divalent cations with single calcium channels from rat brain synaptosomes. J Gen Physiol. 1986 Feb;87(2):201–222. doi: 10.1085/jgp.87.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rigual R., Gonzalez E., Gonzalez C., Fidone S. Synthesis and release of catecholamines by the cat carotid body in vitro: effects of hypoxic stimulation. Brain Res. 1986 May 21;374(1):101–109. doi: 10.1016/0006-8993(86)90398-7. [DOI] [PubMed] [Google Scholar]
- Rigual R., López-López J. R., Gonzalez C. Release of dopamine and chemoreceptor discharge induced by low pH and high PCO2 stimulation of the cat carotid body. J Physiol. 1991 Feb;433:519–531. doi: 10.1113/jphysiol.1991.sp018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher A., Obeso A., Gonzalez C., Herreros B. Ionic mechanisms for the transduction of acidic stimuli in rabbit carotid body glomus cells. J Physiol. 1991 Feb;433:533–548. doi: 10.1113/jphysiol.1991.sp018442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Alexander S. A., Nurse C. A. Effects of pHi and pHe on membrane currents recorded with the perforated-patch method from cultured chemoreceptors of the rat carotid body. Brain Res. 1991 Dec 13;567(1):83–90. doi: 10.1016/0006-8993(91)91439-8. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Chloride channels in cultured glomus cells of the rat carotid body. Am J Physiol. 1989 Aug;257(2 Pt 1):C174–C181. doi: 10.1152/ajpcell.1989.257.2.C174. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Trieschmann U., Isenberg G. Ca2+-activated K+ channels contribute to the resting potential of vascular myocytes. Ca2+-sensitivity is increased by intracellular Mg2+-ions. Pflugers Arch. 1989;414 (Suppl 1):S183–S184. doi: 10.1007/BF00582296. [DOI] [PubMed] [Google Scholar]
- Ueno S., Nakaye T., Akaike N. Proton-induced sodium current in freshly dissociated hypothalamic neurones of the rat. J Physiol. 1992 Feb;447:309–327. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding T. J., Cheng B., Roos A. pH regulation in adult rat carotid body glomus cells. Importance of extracellular pH, sodium, and potassium. J Gen Physiol. 1992 Oct;100(4):593–608. doi: 10.1085/jgp.100.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]