Abstract
Alzheimer’s disease (AD) disproportionately impacts Black Americans, who are three times more likely to develop AD. While heart-healthy diets have shown potential in reducing AD risk, research on adapted dietary interventions for Black American communities remains limited. This pilot study assessed the feasibility and acceptability of an adapted brain healthy diet intervention (MIND + SOUL) and explored changes in cardiometabolic risk and cognition. Twenty-nine participants completed the 12-week intervention, which included culturally tailored health education, cooking classes, health coaching, and groceries. Feasibility was assessed by attendance and retention rates, while acceptability was measured by two questionnaires. Participants had a mean age of 70.3, with 10.3% male. The intervention demonstrated high feasibility (89.3% retention) and acceptability (mean = 71.9, SD = 8.59), with meaningful improvements in body mass index (estimate = −0.54, P = 0.009), dietary intake (estimate = 28.39, P = 0.042), and executive function (estimate = 3.32, P < 0.001). However, no significant changes in blood-based biomarkers were observed. The MIND + SOUL intervention demonstrated high feasibility and acceptability, improvements in body composition, cognitive function, and dietary behaviors, despite no significant changes in blood-based biomarkers. Findings suggest potential benefits for reducing AD risk factors and promoting healthy aging. Clinical Trials Registry: ClinicalTrials.Gov; NCT05414682.
Keywords: diet intervention, MIND diet, older adults, black adults, Alzheimer’s disease, cultural adaptation, brain health
“Dietary intake among participants improved, specifically improved veggie meter scores and self-reported fruit intake were observed.”
Introduction
Racial Disparities in AD/Impact on Black Community
It is estimated that 6.9 million Americans aged 65 and older are affected by Alzheimer’s disease (AD), a figure projected to triple by 2060 due to the aging population and rising life expectancy rates in the United States (U.S.).1,2 However, this prevalence varies significantly among racial groups within the U.S. Specifically, Black Americans are two to three times more likely to develop Alzheimer’s disease (AD) compared to non-Hispanic Whites. 3 Health disparities, particularly prevalent within Black communities are believed to contribute significantly to the elevated dementia risk observed in this population. Structural racism can both directly and indirectly impact the risk for AD by shaping social determinants of health, including access to quality education, housing, health care, employment opportunities, and exposure to environmental toxins.4-8 Additionally, structural racism affects healthy dietary consumption by limiting access to affordable, nutritious foods through the creation of food deserts and food swamps in predominantly Black neighborhoods. This lack of access to healthy foods contributes to poor dietary patterns, increasing the risk of conditions like hypertension, diabetes, and cardiovascular disease, all of which are associated with a higher risk of AD.9,10 While important to note that race is a socially constructed concept lacking significant genetic or biological foundation, these systemic issues contribute to racial and ethnic disparities in health outcomes, including an increased risk of AD among Black adults.9,10
Diet and AD
Although age and genetic makeup are nonmodifiable risk factors for dementia, 40% of cases are due to modifiable factors such as lifestyle and diet. 11 Heart-healthy diets, such as the Mediterranean Diet (MeDi), Dietary Approaches to Stop Hypertension (DASH), and the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, emphasize consumption of fruits, vegetables, whole grains, healthy fats, poultry, beans, and nuts, while limiting sugar, red meats, and saturated fats. 12 The MIND diet, combining elements of MeDi and DASH, focuses on neuroprotective foods like berries and leafy greens and has been linked to reduced AD risk and slower cognitive decline.13-16 Adhering to the MIND diet has been associated in observational studies with a cognitive decline rate equivalent to being 7.5 years younger, with improvements in cognitive domains by 30%-78%. 17 However, a recent trial found no significant cognitive change over a three year period, possibly due to selection bias as participants tended to be more educated, had healthier medical histories, and had higher baseline MIND diet scores compared to those in other studies where the MIND diet showed benefits.17,18 Despite the findings, a more recent systematic review supports the MIND diet’s protective effects on global cognition. 19
The MIND diet is rich in nutrients critical for supporting brain health. Berries and vegetables provide polyphenols and antioxidants, nuts and olive oil offer vitamin E with anti-inflammatory and vascular health benefits, and fish reduce amyloid-β formation and inflammation due to omega-3 fatty acids.20-22 Leafy greens contain flavonoids and carotenoids that may influence cognitive function indirectly by modulating neuronal receptors. 23 Additionally, whole grains, poultry, and leafy greens supply B vitamins essential for regulating homocysteine levels. 24 Preventive dietary interventions are most effective when implemented before initial dementia symptoms, typically around age 50, when nutritional status and cognitive function can compensate for neuropathological changes. 25 However, much research on AD prevention focused on diet has not adequately represented populations disproportionately affected by the disease, limiting the generalizability of findings to underrepresented racial and ethnic groups in aging research. 26
Barriers to Healthy Dietary Patterns Among Black Americans
Previous research indicates that studies aimed at promoting behavioral change in diverse cohorts have, in certain studies resulted in lower levels of adherence among Black Americans. 27 Diet-related disparities are intricate and influenced by various demographic (e.g., age, race/ethnicity, gender), psychosocial factors (e.g., self-efficacy and social support), and systematic social determinants of health (structural racism, education quality, economic stability).28,29 Additionally, it is important to recognize that Black Americans are not a homogeneous group. Variations in food choices, religious practices, health beliefs, and socioeconomic conditions exist across this population, which can further influence dietary behaviors. Studies suggest that the perceived absence of social support, social determinants of health (e.g., perceived affordability of healthy foods and limited access to quality food options), significant cultural influences on food preferences and preparation, together contribute to reduced adherence and acceptability seen among Black Americans.27,29-32 Cultural tailoring of interventions is a vital approach to bridging the health disparities gap. Specifically, previous studies have suggested that modifying traditional soul food to align better with nutritional guidelines would be more effective than advocating for the elimination of such foods from a healthy diet altogether. 33 While several behavioral interventions tailored to Black American populations have shown improvements in reduction in cardiovascular risk factors related to AD (e.g., weight loss) and dietary behaviors, there has been limited research focused on cultural adaptation with respect to dietary AD prevention interventions within Black American communities.34,35
Community Engaged Research Interventions for Prevention, Reducing Health Disparities, and as a Means to Address Structural Disparities and Inequalities
Community engaged research (CEnR) is increasingly recognized as a critical approach to address health disparities. This asset-based approach values the experiences and knowledge of communities facing inequalities, marking a significant paradigm shift in scientific practice. 36 CEnR actively involves underrepresented communities in various forms, such as collaborating to formulate research questions and design and disseminate studies.36-38 Community leaders and minority serving organizations play pivotal roles by leveraging their credibility and longstanding partnerships to engage communities meaningfully in research. 37 CEnR combined with cultural tailoring has shown positive outcomes in dietary interventions focused on Black communities. For example, a 12-week culturally tailored plant-based intervention for rural Black adults resulted in significant reductions in weight (9.6%), total cholesterol (10.9%), low-density lipoprotein (13.9%), and C-reactive protein (25.8%). 39 Similarly, a 9-month community faith-based diet intervention among Black Americans saw increased vegetable consumption in 47% of participants, improved overall diet quality, and reduced blood pressure. 40 Despite these successes, culturally tailored dietary interventions targeting brain health in Black Americans remain limited.
A critical step in developing such interventions is to assess feasibility and acceptability prior to proceeding to a large-scale replication. 41 The primary aim of this study was to assess feasibility and acceptability of a community informed, culturally tailored brain healthy dietary intervention for older Black Americans aged 55 years and older. The secondary, which was exploratory in nature, was aimed to assess changes in cardiometabolic risk, body composition, nutritional health status, and cognitive function.
Methods
This study was approved by the University of Kansas Medical Center Institutional Review Board (IRB#00148307) and was registered with ClinicalTrials.gov (NCT# 05414682). The study was conducted from August 2022 to November 2024.
Study Design
This was a single arm study conducted to assess feasibility and acceptability of the adapted brain healthy diet intervention. The secondary aim explored changes in cognitive functions associated with the MIND + SOUL diet. Given that the primary aim of this study was feasibility and acceptability, no sample size calculations were conducted. However, it is important to note that pilot studies that have sample sizes of at least 12 yield a reliable estimate of standard deviation, with a probability of less than 20% from the power to fall below 60%. 42
Formative Work
The design and development of the MIND + SOUL intervention was informed by the capability, opportunity, motivation, and behavioral (COM-B) framework, 43 MIND dietary model,18,44 extensive preliminary research focused on culturally sensitive nutrition interventions among Black adults,45-49 and a non-interventional mixed methods study that explored knowledge and beliefs that influence dietary practices among older Midwestern Black Americans to inform a culturally tailored brain healthy diet intervention. 50 In our former CEnR work using focus group discussions, the MIND diet emerged as the most favored and feasible dietary model due to its food variety and alignment with practices common among members of the Black community, compared to the MeDi and DASH diets. 50 Participants expressed the need for modifications in the MIND dietary model to increase acceptability (e.g., increasing recommended butter and cheese amounts and offering plant-based alternatives). Additionally, there was a consensus among participants for a culturally adapted intervention that incorporates Christian faith components (e.g., scripture) and ongoing education with resources such as cooking classes, simple recipes, and preparation videos to better reflect the cultural practices common among members of the Black community. 50 To develop the MIND + SOUL intervention ongoing discussions between the principal investigator, three academic research experts (neurology, community-based research, dietetics), a registered dietician, and three community leaders took place over a six-month period. All aspects of the intervention were thoroughly examined and discussed to ensure its scientific and cultural suitability, resulting in only minimal adjustments to the intervention protocol.
MIND + SOUL Intervention
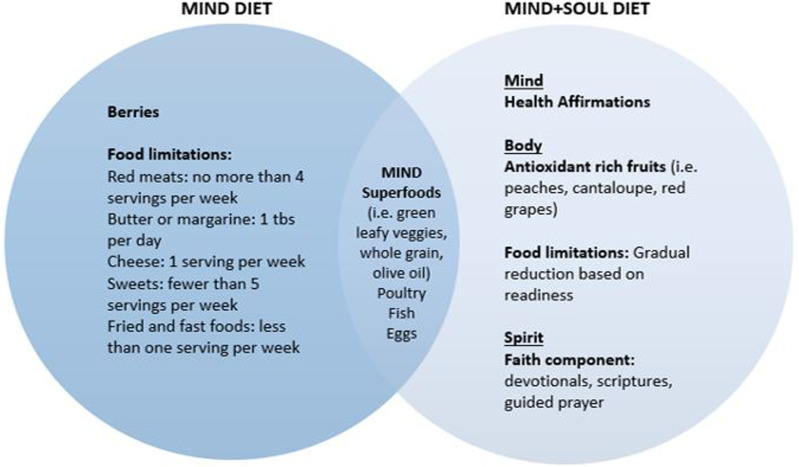
The MIND + SOUL diet builds upon the foundations of the MIND diet by incorporating a holistic approach to healthy eating encompassing aspects of the mind, body, and spirit (Figure 1). This dietary approach is designed to align with the cultural dietary practices in the Black community and is made feasible by food variability. The MIND + SOUL intervention incorporates education, skill-building cooking classes, health coaching, and financial support as part of its holistic approach.
Figure 1.
MIND Diet vs. MIND+SOUL Diet.
Education and skill-building cooking classes were conducted in group sessions over six consecutive weeks as part of the MIND + SOUL diet intervention and held at the University of Kansas Medical Center. Each of the six sessions (Table 1) lasted 60 minutes and was facilitated by a registered dietitian, a white female with prior experience working with this population. The behavioral strategies shared during these sessions, aimed at enhancing dietary consumption and adherence to the MIND + SOUL diet, were based on the principles of the COM-B framework. Specifically, participants were provided with education and skills to enhance both psychological and physical skills to improve dietary consumption and adherence to the MIND + SOUL diet. Furthermore, participants received various educational resources during the first six weeks, including sample weekly menu plans, recipe cards, culturally tailored recipe books and handouts, grocery shopping guides, Christian faith-related materials such as culturally adapted devotional books and scripture readings focusing on healthy eating, affirmations for healthy eating, and a manual outlining the MIND + SOUL diet goals.
Table 1.
Demographics of Participants in the MIND + SOUL Pilot Study.
| Baseline Characteristics | N = 29 |
|---|---|
| Age | 70.3 (6.69) |
| Years of education | 21.3 (3.11) |
| Gender | |
| Male | 3 (10.3%) |
| Female | 26 (89.7%) |
| Marital status | |
| Single (never married) | 5 (17.2%) |
| Married | 11 (37.9%) |
| Widowed | 6 (20.7%) |
| Divorced | 7 (24.1%) |
| Separated | 0 (0.0%) |
| Primary race | |
| Black or African American | 29 (100.0%) |
| Additional race | |
| American Indian or Alaska Native | 2 (6.9%) |
| Not reported | 27 (93.1%) |
| Ethnicity | |
| Hispanic | 0 (0.0%) |
| Not Hispanic or Latino | 28 (96.6%) |
| Not reported | 1 (3.4%) |
Health coaching sessions were conducted by two Black American community health coaches, one female and one male, both of whom had prior experience serving in the Black community. Coaches offered ongoing support and continuing education tailored to the MIND + SOUL diet which was guided by the Telephonic Health Coaching Intervention Toolkit; an established process that promotes healthy dietary behaviors. 51 During these sessions, participants received personalized education and recommendations based on their individual needs, preferences, and local circumstances. The health coaches assisted participants in setting dietary goals aligned with the MIND + SOUL diet, aided in developing action plans for various scenarios (e.g., holiday parties and vacations), and provided encouragement to foster the adoption of healthy eating habits. All health coaching sessions were conducted via Zoom online platform, and occurred bi-weekly for 20 minutes over 12 weeks (totaling six sessions per participant).
To enhance adherence to the MIND + SOUL intervention, each participant received weekly groceries valued at up to $50 per week (with an average total of $600) for a total of 12 weeks. These groceries were exclusively comprised of items from the specific food groups in the MIND + SOUL diet. Groceries were selected each week by the research team from a community supermarket.
Recruitment and Participants
We employed both purposive and convenience sampling techniques to recruit cognitively normal older Black adults, aged 55 years and older, with 1 or more cardiovascular risk factors from a Midwestern metropolitan area between June 2022 and August 2023.52,53 Our recruitment strategies included utilization of existing outreach and recruitment registries, distribution of flyers by community partners, and leveraging an email list server established through previous outreach efforts by the PI. Those interested in participating contacted the research staff via phone and underwent a brief telephone screening to determine eligibility.
The study team employed a brief phone pre-screening interview guide (32-item) to assess eligibility prior to scheduling in-person baseline assessments. Study eligibility requirements included (1) self-identifying as African American or Black (2) 55 years and older in age (3) English speaking (4) 1 or more of the following cardiovascular risk factors (high blood pressure, high cholesterol, controlled type 2 diabetes overweight/obesity with a BMI of 25 or more as calculated from weight and height at baseline visit) (4) cognitively normal with an Eight-item Informant Interview to Differentiate Aging and Dementia (AD8) < 2 (normal cognition), 54 (5) no active depression as determined by a score of below 4 on the Patient Health Questionnaire (PHQ-9). AD8 was selected due to its ability to identify early cognitive changes associated with dementia-related conditions like AD with high sensitivity (> 84%), where a score below 2 on the AD8 indicates normal cognitive function. 55 PHQ-9 was selected due to it acceptability and ease among patients in previous studies and its modest performance in dementia diagnosis. 56
If deemed potentially eligible based upon completion of a pre-screening interview, individuals were invited to a baseline visit in which study staff followed a guided script that explained the study’s objectives, potential risks, benefits, and addressed any outstanding questions individuals may have had. Written informed consent was obtained from each of the participants prior to the start of baseline assessments. At the baseline visit, assessments were conducted to confirm the one or more cardiovascular conditions for eligibility, defined as high blood pressure, high cholesterol, controlled type 2 diabetes overweight/obesity with a BMI of 25 as calculated from weight and height. If an individual was considered ineligible based on measured CV outcomes, they were promptly notified and withdrawn from the study. Eligible participants were subsequently invited to complete study assessments at the University of Kansas Medical Center. Individuals were excluded from participation in the study for the following reasons (1) no CVD risk factor (2) score above a 2 on AD8 assessment and (3) dietary restriction.
Data Collection
All study visits were conducted by trained study staff, including individuals who identified as Black American and White, all of whom had prior experience working with this population. The visits took place in a dedicated private room at the University of Kansas Medical Center. Specifically, all study staff were trained in clinical protocols, data collection, data entry, confidentiality, and safety procedures. Each study visit lasted ∼2.5 hours.
Blood Draw
Participants were instructed to abstain from consuming any food or beverages except water for 12 hours prior to their scheduled appointment. Confirmation of fasting status was obtained from the participant prior to the blood draw. Blood draws were administered to gather cardiometabolic measurements including lipid profile (cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides), glucose metabolism (fasting glucose, fasting insulin, HbA1c), and inflammatory biomarkers of AD (IL-6, IL-8, CRP). Approximately ∼10cc of blood was drawn into an EDTA vacutainer tube both at baseline and at 12 weeks (with a variation of ± 2 weeks). Lipid profile was evaluated through a lipid panel, 57 while glucose metabolism was assessed via fasting glucose, fasting insulin, and hemoglobin A1c (HbA1c) using the Siemens DCA Vantage HbA1c Analyzer. Inflammation and CRP were evaluated using ELISA. 58
Blood Pressure
Blood pressure was assessed at baseline and at 12 weeks (with a variation of ± 2 weeks) using an automated sphygmomanometer (DinaMap ProCare 100). We followed the National Health and Nutrition Examination Survey (NHANES) protocol 59 and the recommendations of the American Heart Association 60 for conducting all blood pressure assessments.
Body Composition
Body composition was measured using dual energy x-ray absorptiometry (DXA),61,62 waist circumference, and body mass index (BMI) which was calculated by height and weight. DXA (GE Lunar iDXA) was used to assess fat-free mass, fat mass, and percent body fat uses very low X-ray doses. DXA used low-Xray doses (0.02mREM) to detect changes in body composition on the order of 1.6-3.8%. Weight was measured using a digital scale (± 0.1 kg; Befour Inc model #PS6600, Saukville, WI) and height was measured using a stadiometer (Model PE-WM-60-84, Perspective Enterprises, Portage, MI). To calculate BMI the study staff entered participants height (feet and inches) and weight (pounds) into REDCap database that automatically calculated BMI.63,64 Waist circumference was measured to the nearest 0.1 cm using measuring tape, following standard protocols in which the waist was measured at the inferior margin of the last rib and the crest of the ilium. 65 All body composition assessments were measured at baseline and at 12 weeks (with a variation of ± 2 weeks)
Dietary Assessments
Nutritional health status was assessed during the clinic visit using the NHANES Dietary Screener Questionnaire (31-item) which assessed intake frequency of food groups consumed (e.g., fruits, vegetables) in the past 30 days. Also, nutritional health status was assessed using the skin carotenoid assessment (Veggie Meter) 66 which assessed skin carotenoids as a proxy for dietary intake of carotenoid rich foods such as fruits and vegetables. All nutritional assessments were assessed at baseline and at 12 weeks (with a variation of ± 2 weeks).
Cognitive Assessments
Psychometricians administered cognitive assessments using the NIH Toolbox to evaluate memory, executive function, language, reading, vocabulary, and processing speed. 67 Specifically, the following tests were conducted during the study visit: NIH-TB List Sorting Working Memory Test, NIH-TB Picture Vocabulary Test, NIH-TB Oral Reading Recognition Test, and the NIH-TB Pattern Comparison Processing Speed Test, and NIH-TB Flanker Inhibitory Control and Attention at baseline and at 12 weeks (with a variation of ± 2 weeks).
Satisfaction and Acceptability Surveys
Participants completed 2 surveys at the 12 weeks (± 2 weeks) study visit. The acceptability survey (23-item) was guided by the theoretical framework of acceptability 68 and focused on the domains of affective attitude (how a participant feels about the MIND + SOUL intervention), burden (perceived amount of effort required to participate in the MIND + SOUL intervention), ethicality (extent to which the intervention has good fit with the participants’ value system), perceived effectiveness (extent to which the intervention is perceived as likely to achieve its purpose), and self-efficacy (participants’ confidence that they can perform the behaviors required to participate in the intervention).
The second survey was a 10-item satisfaction survey, where participants rated various aspects of the MIND + SOUL diet using a visual analog scale (VAS). For each item, participants rated their satisfaction on a scale of 0 to 10 where 0 indicated “not satisfied at all” and 10 indicated “completely satisfied.” The frequency of each rating was recorded, providing a detailed distribution of participant responses.
Data Analysis
We conducted descriptive statistics for all variables which included frequencies and percentages for categorical variables and the mean (standard deviation) for continuous variables. For the acceptability survey, we analyzed responses by reporting the frequency and percentage of responses for each domain, including affective attitude, burden, ethicality, perceived effectiveness, and self-efficacy. For the satisfaction survey, which used VAS, we recorded the frequency of each satisfaction rating (from 0 to 10, where 0 indicated “not satisfied at all” and 10 indicated “completely satisfied”). These frequencies provided a detailed distribution of participant responses without calculating mean values, as satisfaction is a subjective measure. Additionally, the median satisfaction score was calculated for each item to represent central tendencies in participants’ ratings. Changes in cardiometabolic risk factors, body composition, nutritional health status, and cognitive function from baseline to 12 weeks were calculated using a linear mixed-effect model. The models were adjusted for age and years of education. Missing data were sparse and considered randomly distributed. Statistical significance was defined as two-sided alpha <0.05. Statistical significance was defined as two-sided alpha <0.05. All statistical analyses were performed using SPSS (Version 27.0. IBM Corp: Armonk, NY, USA).
Results
Demographics for the MIND + SOUL trial are displayed in Table 1. The mean age of the participants was 70.3 years (SD 6.69) ranging from 58 to 84 years. The mean years of formal education among participants was 21.3 years (SD 3.11), which includes K-12, college, and postgraduate education, with a range from 14 to 28 years. The marital status of participants was heterogeneous, ranging from married (37.9%), divorced (24.1%), and widowed (20.7%).
The MIND + SOUL yielded mixed outcomes across various health measures among participants. While systolic and diastolic blood pressure showed non-significant increases post-intervention, significant improvements were observed in body composition, including reductions in total fat mass, android fat mass, and BMI. The unexpected non-significant increases in blood pressure and lipids may be explained by unreported changes in medication use, which were not tracked during the study, or by the 12-week duration, which may not have been sufficient to observe changes in these biomarkers. Dietary intake revealed a significant increase in self-reported fruit consumption and improvements in the veggie meter score, indicating enhanced fruit and vegetable consumption. Cognitive function saw significant improvements in executive function, while working memory, processing speed, and total cognitive composite scores remained stable. A full description can be found in Table 2.
Table 2.
Mean Values and Standard Deviations (SD) of Blood Pressure, Body Composition, Lipid Profile, Inflammation, Glucose Metabolism, Dietary Intake, and Cognitive Function at Baseline and Post-Intervention (12-Week).
| Variable | Baseline Mean (SD) | Post-Intervention Mean (SD) | P-Value |
|---|---|---|---|
| Blood pressure | |||
| Systolic blood pressure (mmHg) | 127.31 (12.421) | 132.40 (14.602) | 0.152 |
| Diastolic blood pressure (mmHg) | 77.07 (5.467) | 78.92 (6.048) | 0.059 |
| Body composition | |||
| Total fat mass (g) | 39324.7 (12204.6) | 37395.1 (13218.4) | 0.046 |
| Android fat mass (g) | 3590.5 (1684.1) | 3301.2 (1749.3) | 0.001 |
| Gynoid fat mass (g) | 6278.5 (1998.2) | 6046.6 (2013.0) | 0.328 |
| BMI | 33.2 (6.5) | 32.4 (6.1) | 0.009 |
| Waist circumference (in) | 99.0(15.3) | 98.5 (14.0) | 0.719 |
| Lipid profile | |||
| Total cholesterol (mg/dL) | 177.5 (36.46) | 181.5 (53.88) | 0.719 |
| Triglycerides (mg/dL) | 81.3 (26.88) | 78.2 (23.32) | 0.833 |
| LDL (mg/dL) | 99.7 (32.48) | 102.2 (47.99) | 0.747 |
| HDL (mg/dL) | 60.5 (15.08) | 62.2 (16.77) | 0.860 |
| Inflammatory biomarkers | |||
| IL-6 | 1.816 (0.9418) | 1.872 (1.3169) | 0.348 |
| CRP (mg/dL) | 8265.3 (7044.25) | 7122.8 (5705.84) | 0.818 |
| Glucose metabolism | |||
| Glucose (mg/dL) | 96.38 (16.648) | 94.48 (14.350) | 0.357 |
| HbA1C | 6.13 (0.358) | 6.04 (0.276) | 0.237 |
| Dietary intake | |||
| Vegetable consumption (daily) | 0.141 | ||
| Fruit consumption (daily) | 0.626 (0.5634) | 0.891 (0.6052) | 0.004 |
| Veggie meter score | 245.2 (96.45) | 270.9 (91.24) | 0.042 |
| Cognitive function | |||
| Executive function | 43.7 (5.31) | 47.6 (7.45) | <0.001 |
| Working memory | 49.7 (6.06) | 50.9 (7.20) | 0.243 |
| Processing speed | 46.8 (19.10) | 52.4 (18.95) | 0.100 |
| Total cognitive composite score | 53.6 (9.04) | 51.7 (10.44) | 0.161 |
Most participants had a retention rate of 80% or higher (N = 25, 89.3%), meaning most participants stayed engaged with the study and completed the intervention. This suggests high overall adherence to the study protocol. Half of the participants (N = 14, 50.0%) attended all six health coach sessions and 8 participants (28.6%) attended 5 out of the 6 health coaching sessions, indicating strong feasibility. Most of the participants (N = 21, 75%) attended all 6 cooking sessions and 7 participants (25%) attended 5 of the 6 classes indicating strong participation and feasibility of the trial. A full description of participant retention and participation in the trial can be found in Table 3.
Table 3.
Participant Retention and Attendance in the MIND + SOUL Pilot Study.
| Characteristic | N = 28 |
|---|---|
| Retention ≥80% | 25 (89.3%) |
| Health coaching attendance | |
| 0 sessions | 1 (3.6%) |
| 2 sessions | 1 (3.6%) |
| 4 sessions | 4 (14.3%) |
| 5 sessions | 8 (28.6%) |
| 6 sessions | 14 (50.0%) |
| Health education/cooking demonstration attendance | |
| 5 sessions | 7 (25.0%) |
| 6 sessions | 21 (75.0%) |
Acceptability results of the MIND + SOUL pilot study are displayed in Table 4. Overall, the MIND + SOUL diet intervention was well-accepted by participants (71.9). Most participants (92.4%) found the diet easy to follow, indicating high acceptability. Also, most participants (77%) found the MIND + SOUL diet flavorful and tasty, though there was some variation in responses, with a small percentage of participant (7.7%) disagreeing. A significant number of participants (84.6%) perceived that the food preparation practices aligned with their values and was effective in improving their overall health (80.8%). Furthermore, a majority of participants (92%) felt confident that they could eat healthy on a budget after completing the MIND + SOUL intervention, which is a strong indication of acceptability.
Table 4.
Acceptability of the MIND + SOUL Pilot Study.
| Characteristic | N = 26 |
|---|---|
| Acceptability questionnaire score | 71.9 (8.59) |
| Overall, I felt that the MIND + SOUL diet was easy to follow | Strongly Agree (12, 46.2%) |
| Agree (12, 46.2%) | |
| Neutral (2, 7.7%) | |
| Foods on the MIND + SOUL diet were flavorful and tasty | Strongly Agree (12, 46.2%) |
| Agree (8, 30.8%) | |
| Neutral (4, 15.4%) | |
| Disagree (2, 7.7%) | |
| The food preparation practices of the MIND + SOUL align with my values | Strongly Agree (9, 34.6%) |
| Agree (13, 50.0%) | |
| Neutral (2, 7.7%) | |
| Disagree (2, 7.7%) | |
| Following the MIND + SOUL diet was effective in improving my overall health | Strongly Agree (11, 42.3%) |
| Agree (10, 38.5%) | |
| Neutral (3, 11.5%) | |
| Disagree (2, 7.7%) | |
| I feel confident that I can eat healthy on budget after participating in the MIND + SOUL diet intervention | Strongly Agree (11, 44.0%) |
| Agree (12, 48.0%) | |
| Neutral (2, 8.0%) |
As detailed in Table 5, most participants reported high levels of satisfaction across all components of the intervention. The highest satisfaction was with the health education classes, where 73.1% of participants rated their satisfaction as a 10, indicating that these classes were particularly well-received. Similarly, the spiritual health resources and grocery delivery services were rated highly, with 69.2% and 57.7% of participants giving a rating of 10, respectively. The grocery delivery services, while generally rated positively, showed the most variability in satisfaction, with responses ranging from 0 to 10, reflecting differing participant experiences.
Table 5.
Satisfaction of the MIND + SOUL Pilot Study.
| Characteristic | Median | Satisfaction Response [n (%)] |
|---|---|---|
| Satisfaction visual analog scale (0-70) | 65.0 | - |
| I am satisfied overall with the MIND + SOUL diet intervention | 10.0 | 1 (3.8%) answered 4, 1 (3.8%) answered 5, 1 (3.8%) answered 6, 1 (3.8%) answered 7, 3 (11.5%) answered 8, 5 (19.2%) answered 9, 14 (53.8%) answered 10 |
| I am satisfied with the foods on the MIND + SOUL diet | 9.5 | 1 (3.8%) answered 4, 1 (3.8%) answered 5, 1 (3.8%) answered 6, 2 (7.7%) answered 7, 3 (11.5%) answered 8, 5 (19.2%) answered 9, 13 (50.0%) answered 10 |
| I am satisfied with the health education classes | 10.0 | 1 (3.8%) answered 5, 2 (7.7%) answered 7, 2 (7.7%) answered 8, 2 (7.7%) answered 9, 19 (73.1%) answered 10 |
| I am satisfied with the health cooking demonstrations | 9.5 | 1 (3.8%) answered 3, 1 (3.8%) answered 5, 3 (11.5%) answered 7, 4 (15.4%) answered 8, 4 (15.4%) answered 9, 13 (50.0%) answered 10 |
| I am satisfied with the spiritual health resources | 10.0 | 3 (11.5%) answered 6, 1 (3.8%) answered 7, 1 (3.8%) answered 8, 2 (7.7%) answered 9, 1 (3.8%) answered 10, 18 (69.2%) answered 10 |
| I am satisfied with the health coaching sessions | 9.5 | 1 (3.8%) answered 4, 1 (3.8%) answered 5, 2 (7.7%) answered 6, 3 (11.5%) answered 7, 2 (7.7%) answered 8, 4 (15.4%) answered 9, 13 (50.0%) answered 10 |
| I am satisfied with the grocery delivery services | 10.0 | 1 (3.8%) answered 0, 2 (7.7%) answered 3, 1 (3.8%) answered 4, 2 (7.7%) answered 6, 5 (19.2%) answered 7, 15 (57.7%) answered 10 |
Footnote: Each satisfaction question was rated on a scale of 0 to 10, with 0 being “Not satisfied at all” and 10 being “Completely satisfied.” The frequencies show how many participants rated each level of satisfaction for each item.
There were no statistically significant changes in blood pressure or blood-based biomarkers (NF-light, Abeta 42, Abeta 40, GFAP, ALZpath Simoa pTau-217 v2), indicating limited impact on these measures. However, participants showed significant reductions in total fat mass, android fat mass, and BMI, reflecting positive effects on body composition. Statistically significant improvements in executive function (estimate = 3.32, SE = 0.83, P < 0.001), were observed, alongside a significant increase in self-reported fruit consumption and veggie meter score (estimate = 28.39, SE = 13.28, P = 0.042), suggesting potential benefits for dietary behaviors. In Table 6, SE reflects the variability of the estimates, with the relatively small SE for BMI reduction (SE = 0.19) indicating a high level of precision in estimating the effect of the intervention on BMI changes. Full details of changes in outcomes from baseline to 12-week can be found in Table 6.
Table 6.
Change of Continuous Outcomes at Baseline and Week 12 Using Linear Mixed Models in the MIND + SOUL Pilot Study.
| Outcome | Estimate | SE | 95% CI | P-Value |
|---|---|---|---|---|
| Blood pressure | ||||
| Systolic blood pressure | 5.11 | 3.47 | −2.00, 12.22 | 0.152 |
| Diastolic blood pressure | 2.17 | 1.10 | −0.088, 4.42 | 0.059 |
| Blood-based biomarkers | ||||
| NF-light (pg/mL) | 0.52 | 1.20 | −2.01, 3.06 | 0.670 |
| Abeta 42 (pg/mL) | −0.16 | 0.23 | −0.64, 0.32 | 0.499 |
| Abeta 40 (pg/mL) | −7.10 | 3.50 | −14.54, 0.34 | 0.060 |
| GFAP (pg/mL) | 2.16 | 7.68 | −14.10, 18.43 | 0.782 |
| ALZpath Simoa pTau-217 v2 | −0.01 | 0.02 | −0.046, 0.024 | 0.509 |
| Body composition | ||||
| Total fat mass (g) | −725.44 | 335.11 | −1435.70, −15.19 | 0.046 |
| Android fat mass (g) | −162.37 | 40.90 | −249.05, −75.69 | 0.001 |
| Gynoid fat mass (g) | −65.95 | 65.37 | −204.50, 72.60 | 0.328 |
| BMI | −0.54 | 0.19 | −0.93, −0.15 | 0.009 |
| Waist circumference (cm) | 0.49 | 1.36 | −2.30, 3.29 | 0.719 |
| CRP | ||||
| CRP | −185.19 | 789.77 | −1862.10, 1491.73 | 0.818 |
| Cognitive function | ||||
| Executive function | 3.32 | 0.83 | 1.61, 5.04 | <0.001 |
| Working memory | 1.27 | 1.07 | −0.92, 3.47 | 0.243 |
| Processing speed | 4.33 | 2.53 | −0.88, 9.54 | 0.100 |
| Total cognition composite score | −1.50 | 1.03 | −3.64, 0.64 | 0.161 |
| Dietary measures | ||||
| Vegetable consumption (daily frequency) | 0.13 | 0.08 | −0.045, 0.30 | 0.141 |
| Fruit consumption (daily frequency) | 0.28 | 0.09 | 0.097, 0.47 | 0.004 |
| Veggie meter | 28.39 | 13.28 | 1.07, 55.71 | 0.042 |
| Glucose metabolism | ||||
| Glucose | −2.05 | 2.18 | −6.53, 2.44 | 0.357 |
| HbA1c % | −0.10 | 0.08 | −0.26, 0.069 | 0.237 |
| Inflammation | ||||
| IL-6 | 0.16 | 0.16 | −0.19, 0.51 | 0.348 |
| Lipid profile | ||||
| Total cholesterol | 1.97 | 5.38 | −9.46, 13.40 | 0.719 |
| Triglycerides | −0.98 | 4.56 | −10.66, 8.70 | 0.833 |
| Low-density lipoprotein | 1.56 | 4.74 | −8.52, 11.63 | 0.747 |
| High-density lipoprotein | 0.43 | 2.40 | −4.66, 5.52 | 0.860 |
Discussion
MIND + SOUL is a novel, culturally tailored pilot dietary intervention aimed to evaluate feasibility, acceptability, and potential impact among older Black adults. Consistent with previous culturally tailored lifestyle interventions,39,69,70 our study demonstrated high retention rates (89.3%) and acceptability (71.9%), underscoring that cultural tailoring of the MIND diet can enhance participant engagement and overall feasibility of a dietary intervention among older Black adults. This is crucial for supporting behavioral changes aimed at reducing the risk of AD as previous literature suggests that lower levels of adherence and acceptability to dietary interventions are seen among Black Americans.30,31,71 The high acceptability and satisfaction results highlight the MIND + SOUL intervention’s potential to be integrated into participants’ lifestyles without compromising cultural values, which is a well-known barrier to dietary lifestyle changes in Black communities.
High acceptability and satisfaction suggest that the MIND + SOUL intervention effectively addressed barriers to healthy eating, such as access, cost, and taste, which were noted in our previous work and are well-documented in the literature.50,72 The MIND + SOUL intervention included culturally relevant modifications of traditional soul food to better align with evidence-based MIND dietary guidelines. For example, participants were encouraged to increase their consumption of leafy greens (e.g., collard greens), antioxidant rich fruits (e.g., cantaloupe, plums), whole grains (e.g., brown rice), poultry (e.g., chicken), and fish (e.g., salmon) into their diet. Participants were also encouraged to use traditional spices commonly found in soul food (e.g., black pepper, cayenne pepper, paprika, and thyme) to enhance flavor and familiarity while potentially supporting cardiovascular and brain health due to their anti-inflammatory and antioxidant properties.73-76 Moreover, research has shown that addressing these barriers is critical to enhancing dietary adherence and acceptability in Black American populations, as demonstrated in the MIND + SOUL pilot. By addressing these barriers and modifying culturally familiar foods, the MIND + SOUL pilot achieved positive outcomes in retention, acceptability, and satisfaction. Addressing multifaceted barriers through culturally tailored interventions, such as the MIND + SOUL pilot, is essential to bridging the health disparities gap seen in AD.
This pilot study resulted in several positive health outcome changes including body composition and cognitive function. Specifically, significant reductions in total fat mass, android fat mass, and BMI were observed, indicating a positive impact on body composition, a well-known risk factor of AD.77,78 These positive anthropometric changes are critical given the association between central obesity and AD among older adults. 79 Furthermore, these finding are in line with other evidence-based dietary patterns that have similar beneficial anthropometric effects.80,81 Significant improvements in cognitive function, particularly in the executive function domain (estimate = 3.32, P < 0.001), suggest that the MIND + SOUL diet may support cognitive health. However, the possibility of practice effects from repeated cognitive assessments should be considered, and future studies with a control group will be necessary to better isolate these effects. This complements prior research indicating a positive association between healthier food consumption and cognitive domains. 82
While results are encouraging, significant changes were not observed in other domains including working memory, processing speed, or total cognition composite score, potentially due to the short duration of the trial as previous research has shown positive changes in cognition for longer follow-up periods.83,84 Additionally, it is important to note that no significant improvements were observed in blood pressure and lipid values, which are critical risk factors for cognitive decline and cardiovascular events. 85 It is possible that medication changes or dietary nonadherence may have contributed to these findings. Moreover, the adapted dietary approach may have been too modest in its prescribed reduction of certain foods including those in saturated fats, such as butter and cheese, which are known to impact blood pressure and lipid profiles. 86 This modest reduction in such foods may have limited the intervention’s efficacy in improving cardiovascular risk markers.
Dietary intake among participants improved, specifically improved veggie meter scores and self-reported fruit intake were observed. These findings suggest that culturally tailored interventions such as the MIND + SOUL can effectively increase the consumption of nutrient-rich fruits and vegetables among older Black adults. Mechanistic evidence supports the relationship between increased intake of these foods and improved brain health. Specifically, fruits and vegetables are rich in antioxidants, which mitigate oxidative stress, a contributor to cognitive decline.21,22 Furthermore, the fiber, vitamins, and minerals present in these foods support overall brain function by reducing inflammation and enhancing vascular health, both of which are crucial for maintaining cognitive health. 20 These results underscore the potential of the MIND + SOUL to promote brain health through improved nutrition.
Despite these positive changes in body composition, dietary intake, and executive function, no significant changes were observed in lipid profiles, inflammatory biomarkers, or glucose metabolism. Results suggest that while the 12-week duration of the MIND + SOUL study may not fully account for the lack of changes in blood pressure and lipid profiles, it could still be a contributing factor. Similar dietary studies, such as those on the MeDi and DASH diets, have shown significant improvements in biomarkers like lipids and glucose metabolism after six months to a year of adherence.87,88 Therefore, longer adherence to the MIND + SOUL diet may be required to observe these effects, alongside other factors like baseline health conditions and participant adherence.
Strenghts, Limitations, and Future Directions
This study had several strengths including a focus on underserved populations disproportionally impacted by AD and often underrepresented in research. Another key strength was the intentional inclusion of Black American research staff and health coaches with prior experience working with older Black adults. This expertise and cultural alignment enhanced trust and rapport, which likely contributed to the high retention and participation rates. Their involvement ensured culturally tailored delivery of the MIND + SOUL intervention, making it more relevant and impactful for the participants. This design choice strengthened the study’s ability to address health disparities and improve engagement within the target population. Additionally, the use of the MIND evidence-based dietary model provided a scientifically supported framework for brain health, increasing the study’s credibility and offering a strong foundation for future replication. The multifaceted approach, including health education, cooking demonstrations, spiritual health resources, and grocery delivery services, enhanced the feasibility of implementing the MIND + SOUL intervention in a community setting. The intervention led to improved dietary intake, particularly with increased fruit and vegetable consumption, further demonstrating the effectiveness of the approach. The structured nature of the intervention provides a replicable framework for future dietary interventions and potential scaling.
While the MIND + SOUL pilot study has many strengths, there were some limitations that should be considered. The study was not randomized, making it difficult to attribute the observed changes solely to the intervention components. Subsequent studies should incorporate a randomized control trial design which will provide more definitive evidence with respect to the effect of the MIND + SOUL diet intervention. The study population predominately consisted of older Black women (89.7%) located solely in the Midwest which limits applicability of findings to Black men and older Black adults located in other geographical areas. Additionally, the small sample size (n = 29) limited generalizability of the findings. Future studies should aim to include a more diverse participant pool in terms of gender, educational status, and geographical area to enhance the generalizability of the results. Moreover, it is important to acknowledge that Black Americans are not a homogeneous group. Differences may exist in what individuals from this community consider culturally sound in terms of food and spirituality elements. Future studies should aim to capture the diverse cultural backgrounds, dietary traditions, and spiritual practices within the Black American community to ensure that interventions are culturally relevant and inclusive.
Due to the pilot intervention being 12 weeks, the duration may not have been sufficient to observe changes in biomarkers and cognition. Extending the duration of the intervention in future studies may provide a better understanding of the long-term effects of the MIND + SOUL diet on health outcomes including metabolism, inflammation, lipid profile, and cognitive performance. Furthermore, investigators should explore the mechanisms through which the MIND + SOUL diet influences health outcomes related to AD with particular focus on body composition and cognitive function to provide further understanding of the effect of the MIND + SOUL intervention.
Conclusion
The MIND + SOUL pilot study demonstrated high feasibility, acceptability, and satisfaction among older Black adults, with positive impacts on body composition, cognitive function, and dietary behaviors. Significant improvements were observed in executive function, total fat mass, android fat mass, and BMI. However, non-significant increases in blood pressure and lipid profiles suggest mixed effects on AD risk factors, indicating a need for further investigation. No substantial changes were detected in blood-based biomarkers, glucose metabolism, or inflammation. These findings suggest that while the MIND + SOUL diet shows promise in reducing AD risk factors and promoting healthy aging, future studies should explore potential modifications to the intervention and consider longer durations to achieve more comprehensive health benefits.
Acknowledgments
We thank the participants in the study. We also appreciate the support from the Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD and our community partners Ms. Estelle Brooks, Mr. Harry Evans, Bright Business Solutions, and the Black Health Care Coalition.
Footnotes
Author Contributions: AS conceptualized the study, designed the methodology, validated findings, performed formal analysis, conducted investigations, provided resources, curated data, wrote the original draft, and participated in review and editing processes. AS also contributed to visualization and secured funding. RH validated findings and contributed to writing review and editing. DM curated data, conducted formal analysis, and contributed to writing review and editing. KY curated data, conducted formal analysis, and contributed to writing review and editing. EV conceptualized the study, developed software, validated findings, curated data, and contributed to writing review and editing. SB validated findings and contributed to writing review and editing. JM curated data, validated findings, and contributed to writing review and editing. MK contributed to writing visualization, wrote the original draft, and participated in writing review and editing. JBP contributed to methodology and writing review and editing. JB supervised the project, administered tasks, and contributed to writing review and editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institute on Aging under grants, K01AG072034, P30AG072973, L60AG069211, R24AG063724 and, the Leo and Anne Albert Charitable Trust.
Ethical Statement
Ethical Approval
This research has been approved by the University of Kanas Medical Center Institutional Review Board (IRB#00148307) on June 02, 2022. All participants provided written informed consent prior to enrollment in the study. This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Informed Consent
Participants enrolled in the study provided written informed consent.
ORCID iD
Ashley R. Shaw https://orcid.org/0000-0003-2018-7274
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.*
References
- 1.Lines LM, Sherif NA, Wiener JM. Racial and ethnic disparities among individuals with Alzheimer’s disease in the United States: A literature review. Research Triangle Park 2014. doi: 10.3768/rtipress.2014.RR.0024.1412 [DOI] [Google Scholar]
- 2.Association As . 2024 Alzheimer's disease facts and figures. 2024; 20. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association As . 2023 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2023;19(4):1598-1695. doi: 10.1002/alz.13016 [DOI] [PubMed] [Google Scholar]
- 4.Bancks MP, Byrd GS, Caban-Holt A, et al. Self-reported experiences of discrimination and incident dementia. Alzheimers Dement. 2023;19(7):3119-3128. doi: 10.1002/alz.12947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson RL, George KM, Barnes LL, et al. Association of timing of school desegregation in the United States with late-life cognition in the study of healthy aging in african Americans (STAR) cohort. JAMA Netw Open. 2021;4(10):e2129052. doi: 10.1001/jamanetworkopen.2021.29052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamar M, Lerner AJ, James BD, et al. Relationship of early-life residence and educational experience to level and change in cognitive functioning: results of the minority aging research study. J Gerontol B Psychol: Ser Bibliogr. 2019;75(7):e81-e92. doi: 10.1093/geronb/gbz031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasefi M, Ghaboolian-Zare E, Abedelwahab H, Osu A. Environmental toxins and Alzheimer's disease progression. Neurochem Int. 2020;141:104852. [DOI] [PubMed] [Google Scholar]
- 8.Qian H, Khadka A, Martinez SM, et al. Food insecurity, memory, and dementia among US adults aged 50 Years and older. JAMA Netw Open. 2023;6(11):e2344186. doi: 10.1001/jamanetworkopen.2023.44186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruthirakuhan M, Swardfager W, Xiong L, et al. Investigating the impact of hypertension with and without diabetes on Alzheimer's disease risk: a clinico-pathological study. Alzheimers Dement. 2024;20(4):2766-2778. doi: 10.1002/alz.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckers K, Schievink SH, Rodriquez MM, et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PLoS One. 2017;12:e0184244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diab A, Dastmalchi LN, Gulati M, Michos ED. A heart-healthy diet for cardiovascular disease prevention: where are we now? Vasc Health Risk Manag. 2023;19:237-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kheirouri S, Alizadeh M. MIND diet and cognitive performance in older adults: a systematic review. Crit Rev Food Sci Nutr. 2022;62(29):8059-8077. doi: 10.1080/10408398.2021.1925220 [DOI] [PubMed] [Google Scholar]
- 14.Barnes LL, Dhana K, Liu X, et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N Engl J Med. 2023;389(7):602-611. doi: 10.1056/NEJMoa2302368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr. 2021;113(1):200-208. doi: 10.1093/ajcn/nqaa303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth SL, Shea MK, Barger K, et al. Association of vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement. 2022;8(1):e12255. doi: 10.1002/trc2.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015-1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11(9):1007-1014. doi: 10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Soest AP, Beers S, van de Rest O, de Groot LC. The mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay (MIND) diet for the aging brain: a systematic review. Adv Nutr. 2024;15(3):100184. doi: 10.1016/j.advnut.2024.100184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006-1015. doi: 10.1016/s1474-4422(18)30338-7 [DOI] [PubMed] [Google Scholar]
- 21.Greiner RS, Moriguchi T, Hutton A, Slotnick BM, Salem N, Jr. Rats with low levels of brain docosahexaenoic acid show impaired performance in olfactory-based and spatial learning tasks. Lipids. 1999;34:S239-S243. doi: 10.1007/bf02562305 [DOI] [PubMed] [Google Scholar]
- 22.Thomas J, Thomas CJ, Radcliffe J, Itsiopoulos C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: a focus on Alzheimer’s disease. BioMed Res Int. 2015;2015:172801. doi: 10.1155/2015/172801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rendeiro C, Rhodes JS, Spencer JPE. The mechanisms of action of flavonoids in the brain: direct versus indirect effects. Neurochem Int. 2015;89:126-139. doi: 10.1016/j.neuint.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 24.Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211-239. doi: 10.1146/annurev-nutr-071715-050947 [DOI] [PubMed] [Google Scholar]
- 25.Barnard ND, Bush AI, Ceccarelli A, et al. Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging. 2014;35:S74-S78. doi: 10.1016/j.neurobiolaging.2014.03.033 [DOI] [PubMed] [Google Scholar]
- 26.Versavel S, Subasinghe A, Johnson K, et al. Diversity, equity, and inclusion in clinical trials: a practical guide from the perspective of a trial sponsor. Contemp Clin Trials. 2023;126:107092. doi: 10.1016/j.cct.2023.107092 [DOI] [PubMed] [Google Scholar]
- 27.Epstein DE, Sherwood A, Smith PJ, et al. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and white adults with high blood pressure: results from the ENCORE trial. J Acad Nutr Diet. 2012;112(11):1763-1773. doi: 10.1016/j.jand.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satia JA. Diet-related disparities: understanding the problem and accelerating solutions. J Am Diet Assoc. 2009;109(4):610-615. doi: 10.1016/j.jada.2008.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odoms-Young A, Bruce MA. Examining the impact of structural racism on food insecurity: implications for addressing racial/ethnic disparities. Fam Community Health. 2018;41:S3-S6. doi: 10.1097/fch.0000000000000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Airhihenbuwa CO, Kumanyika S, Agurs TD, Lowe A, Saunders D, Morssink CB. Cultural aspects of African American eating patterns. Ethn Health. 1996;1(3):245-260. doi: 10.1080/13557858.1996.9961793 [DOI] [PubMed] [Google Scholar]
- 31.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93(3):601-607. doi: 10.3945/ajcn.110.007369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson E, Wei R, Liu B, et al. Improving healthy food choices in low-income settings in the United States using behavioral economic-based adaptations to choice architecture. Front Nutr. 2021;8:734991. doi: 10.3389/fnut.2021.734991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rankins J, Wortham J, Brown LL. Modifying soul food for the Dietary Approaches to Stop Hypertension diet (DASH) plan: implications for metabolic syndrome (DASH of Soul). Ethn Dis. 2007;17:S4-S7. [PubMed] [Google Scholar]
- 34.Joo JY, Liu MF. Culturally tailored interventions for ethnic minorities: a scoping review. Nurs Open. 2021;8(5):2078-2090. doi: 10.1002/nop2.733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadi NM, Asantewa-Ampaduh S, Rivas C, Goff LM. Culturally tailored lifestyle interventions for the prevention and management of type 2 diabetes in adults of Black African ancestry: a systematic review of tailoring methods and their effectiveness. Publ Health Nutr. 2022;25(2):422-436. doi: 10.1017/s1368980021003682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payán D, Zawadzki M, Song A. Advancing community-engaged research to promote health equity: considerations to improve the field. Perspect Public Health. 2022;142(3):139-141. doi: 10.1177/17579139211054118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkins CH, Miller ST, Richmond AN, Carrasquillo O. Community-engaged research — essential to addressing health inequities. N Engl J Med. 2023;389(21):1928-1931. doi: 10.1056/NEJMp2307774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haynes N, Kaur A, Swain J, Joseph JJ, Brewer LC. Community-based participatory research to improve cardiovascular health among US racial and ethnic minority groups. Curr Epidemiol Rep. 2022;9(3):212-221. doi: 10.1007/s40471-022-00298-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sterling SR, Bowen SA. Effect of a plant-based intervention among black individuals in the deep south: a pilot study. J Nutr Educ Behav. 2023;55(1):68-76. doi: 10.1016/j.jneb.2022.08.013 [DOI] [PubMed] [Google Scholar]
- 40.Lynch E, Emery-Tiburcio E, Dugan S, et al. Results of alive: a faith-based pilot intervention to improve diet among african American church members. Prog Community Health Partnersh. 2019;13(1):19-30. doi: 10.1353/cpr.2019.0005 [DOI] [PubMed] [Google Scholar]
- 41.El-Kotob R, Giangregorio LM. Pilot and feasibility studies in exercise, physical activity, or rehabilitation research. Pilot Feasibility Stud. 2018;4:137. doi: 10.1186/s40814-018-0326-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng CH, Sim D. (2021). Sample size planning for pilot studies. arXiv preprint arXiv:2105.05483. doi: 10.48550/arXiv.2105.05483 [DOI]
- 43.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornelis MC, Agarwal P, Holland TM, van Dam RM. MIND dietary pattern and its association with cognition and incident dementia in the UK biobank. Nutrients. 2022;15:32. doi: 10.3390/nu15010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senior J, Cason K, Martinez-Dawson R, Visser R, Dawson P. Culturally tailored nutrition education interventions. Why focus on African American women. J Nutri Health Food Eng. 2015;2(2):1-3. [Google Scholar]
- 46.Di Noia J, Furst G, Park K, Byrd-Bredbenner C. Designing culturally sensitive dietary interventions for African Americans: review and recommendations. Nutr Rev. 2013;71(4):224-238. doi: 10.1111/nure.12009 [DOI] [PubMed] [Google Scholar]
- 47.Williams JH, Auslander WF, de Groot M, Robinson AD, Houston C, Haire-Joshu D. Cultural relevancy of a diabetes prevention nutrition program for african American women. Health Promot Pract. 2006;7(1):56-67. doi: 10.1177/1524839905275393 [DOI] [PubMed] [Google Scholar]
- 48.Kong A, Tussing-Humphreys LM, Odoms-Young AM, Stolley MR, Fitzgibbon ML. Systematic review of behavioural interventions with culturally adapted strategies to improve diet and weight outcomes in African American women. Obes Rev. 2014;15:62-92. doi: 10.1111/obr.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kramer EB, Knight EL, Bryan AD. Cooking interventions for improving diet quality among black Americans: a randomized controlled trial. Ann Behav Med. 2023;57(4):323-333. doi: 10.1093/abm/kaac058 [DOI] [PubMed] [Google Scholar]
- 50.Shaw AR, Key MN, Fikru S, et al. Development of a culturally adapted dietary intervention to reduce Alzheimer's disease risk among older black adults. Int J Environ Res Publ Health. 2023;20:6705. doi: 10.3390/ijerph20176705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaughan M, Brinckman D. Telephonic health coaching (THC) promotes health behavior changes among participants in SNAP-ed. J Nutr Educ Behav. 2017;49(7, Supplement 1):S98. doi: 10.1016/j.jneb.2017.05.176 [DOI] [Google Scholar]
- 52.Andrade C. The inconvenient truth about convenience and purposive samples. Indian J Psychol Med. 2021;43(1):86-88. doi: 10.1177/0253717620977000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris JE, Gleason PM, Sheean PM, Boushey C, Beto JA, Bruemmer B. An introduction to qualitative research for food and nutrition professionals. J Am Diet Assoc. 2009;109(1):80-90. doi: 10.1016/j.jada.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 54.Cordell C, Borson S, Boustani M, et al. The AD8: the Washington University dementia screening test. Fam Med. 2013;25(3):367-382. [Google Scholar]
- 55.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. doi: 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- 56.Hancock P, Larner AJ. Clinical utility of patient health questionnaire-9 (PHQ-9) in memory clinics. Int J Psychiatr Clin Pract. 2009;13(3):188-191. doi: 10.1080/13651500802684500 [DOI] [PubMed] [Google Scholar]
- 57.Diagnostics Q. Lipid panel, standard. https://testdirectory.questdiagnostics.com/test/test-detail/7600/lipid-panel-standard?cc=MASTER
- 58.Abcam. ELISA. Kits. https://www.abcam.com/kits/elisa-kits
- 59.Centers for Disease Control and Prevention . National Health and Nutrition Survey (NHANES): Health Tech/Blood Pressure Procedures Manual. Atlanta, GA: Center for Disease Control and Prevention; 2009. [Google Scholar]
- 60.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of professional and public education of the american heart association council on high blood pressure research. Hypertension. 2005;45(1):142-161. [DOI] [PubMed] [Google Scholar]
- 61.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12(1):45-51. doi: 10.1016/0899-9007(95)00017-8 [DOI] [PubMed] [Google Scholar]
- 62.Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101-105. doi: 10.1016/j.bone.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization . Waist circumference and waist-hip ratio: Report of a WHO expert consultation. World Health Organization. https://www.who.int/publications/i/item/9789241501491 (2008). [Google Scholar]
- 66.Ermakov IV, Whigham LD, Redelfs AH, et al. Skin carotenoids as biomarker for vegetable and fruit intake: validation of the reflection-spectroscopy based “veggie meter”. The FASEB Journal. 2016;30:409.3. doi: 10.1096/fasebj.30.1_supplement.409.3 [DOI] [Google Scholar]
- 67.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54-S64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timm L, Annerstedt KS, Ahlgren J, et al. Application of the Theoretical Framework of Acceptability to assess a telephone-facilitated health coaching intervention for the prevention and management of type 2 diabetes. PLoS One. 2022;17:e0275576. doi: 10.1371/journal.pone.0275576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore SG, Kundra A, Ho P, Bissell E, Apekey T. Feasibility of a community healthy eating and cooking intervention featuring traditional African caribbean foods from participant and staff perspectives. Nutrients. 2023;15:3758. doi: 10.3390/nu15173758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amoah S, Ennin R, Sagoe K, et al. Feasibility of a culturally adapted dietary weight-loss intervention among Ghanaian migrants in Berlin, Germany: the ADAPT study. Int J Environ Res Publ Health. 2021;18:510. doi: 10.3390/ijerph18020510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epstein DE, Sherwood A, Smith PJ, et al. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and white adults with high blood pressure: results from the ENCORE trial. J Acad Nutr Diet. 2012;112(11):1763-1773. doi: 10.1016/j.jand.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richards Adams IK, Figueroa W, Hatsu I, et al. An examination of demographic and psychosocial factors, barriers to healthy eating, and diet quality among African American adults. Nutrients. 2019;11(3):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balakrishnan R, Azam S, Kim IS, Choi DK. Neuroprotective effects of black pepper and its bioactive compounds in age-related neurological disorders. Aging Dis. 2023;14(3):750-777. doi: 10.14336/ad.2022.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasierski M, Szulczyk B. Beneficial effects of capsaicin in disorders of the central nervous system. Molecules. 2022;27:2484. doi: 10.3390/molecules27082484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warman DJ, Jia H, Kato H. Effects of thyme (thymus vulgaris L.) essential oil on aging-induced brain inflammation and blood telomere attrition in chronologically aged C57BL/6J mice. Antioxidants. 2023;12:1178. doi: 10.3390/antiox12061178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda H, Saito S, Nakamura N, Maoka T. Paprika pigments attenuate obesity-induced inflammation in 3T3-L1 adipocytes. ISRN Inflamm. 2013;2013:763758. doi: 10.1155/2013/763758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang SY, Kim YJ, Jang W, Son KY, Park HS, Kim YS. Body mass index trajectories and the risk for Alzheimer's disease among older adults. Sci Rep. 2021;11(1):3087. doi: 10.1038/s41598-021-82593-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolatshahi M, Commean PK, Rahmani F, et al. Alzheimer disease pathology and neurodegeneration in midlife obesity: a pilot study. Aging Dis. 2023;15:1843-1854. doi: 10.14336/ad.2023.0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057-1064. doi: 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 80.Agnoli C, Sieri S, Ricceri F, et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr Diabetes. 2018;8(1):22. doi: 10.1038/s41387-018-0023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soltani S, Shirani F, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes Rev. 2016;17(5):442-454. doi: 10.1111/obr.12391 [DOI] [PubMed] [Google Scholar]
- 82.Gutierrez L, Folch A, Rojas M, et al. Effects of nutrition on cognitive function in adults with or without cognitive impairment: a systematic review of randomized controlled clinical trials. Nutrients. 2021;13(11):3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehtisalo J, Levälahti E, Lindström J, et al. Dietary changes and cognition over 2 years within a multidomain intervention trial—the Finnish geriatric intervention study to prevent cognitive impairment and disability (FINGER). Alzheimers Dement. 2019;15(3):410-417. doi: 10.1016/j.jalz.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 84.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15(4):581-589. doi: 10.1016/j.jalz.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 85.Nakamura H, Tsujiguchi H, Kambayashi Y, et al. Relationship between saturated fatty acid intake and hypertension and oxidative stress. Nutrition. 2019;61:8-15. doi: 10.1016/j.nut.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Manson JE, Forman JP, Gaziano JM, Buring JE, Sesso HD. Dietary fatty acids and the risk of hypertension in middle-aged and older women. Hypertension. 2010;56(4):598-604. doi: 10.1161/hypertensionaha.110.154187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berti V, Walters M, Sterling J, et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018;90(20):e1789-e1798. doi: 10.1212/wnl.0000000000005527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorans KS, Bazzano LA, Qi L, et al. Effects of a low-carbohydrate dietary intervention on hemoglobin A1c: a randomized clinical trial. JAMA Netw Open. 2022;5(10):e2238645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.*