Abstract
Purpose
This study aimed to establish a combined histological assessment system of neo-cartilage outcomes and to evaluate variations in an established rat defect model treated with human juvenile cartilage-derived chondrocyte (JCC) sheets fabricated from various donors.
Methods
JCCs were isolated from the polydactylous digits of eight patients. Passage 2 (P2) JCC sheets from all donors were transplanted into nude rat chondral defects for 4 weeks (27 nude rats in total). Defect-only group served as control. Histological samples were stained for safranin O, collagen 1 (COL1), and collagen 2 (COL2). (1) All samples were scored, and correlation coefficients for each score were calculated. (2) Donors were divided into “more effective” and “less effective” groups based on these scores. Then, differences between each group in each category of modified O’Driscoll scoring were evaluated.
Results
(1) Modified O’Driscoll scores were negatively correlated with %COL1 area, and positively correlated with %COL2 area and COL2/1 ratio. (2) Four of 8 donors exhibited significantly higher modified O’Driscoll scores and %COL2 areas. JCC donors were divided into two groups by average score values. Significant differences between the two groups were observed in modified O’Driscoll categories of “Nature of predominant tissue,” “Reconstruction of subchondral bone,” and “Safranin O staining.”
Conclusion
The combined histological evaluation method is useful for detailed in vivo efficacy assessments of cartilage defect regeneration models. Variations in histological scores among juvenile cartilage-derived chondrocyte donors were correlated to the quality of regenerated cartilage hyaline structure and subchondral bone remodeling observed in the nude rat defect model.
Keywords: osteoarthritis, cell sheet transplantation, cartilage regeneration, donor variation, subchondral bone
Introduction
Chondral defects in human knees are prevalent in both general and athletic populations from multiple etiologies. Frequently incurred with osteological disorders from acute trauma, increasing participation in athletic activities is especially associated with increasing prevalence.1,2 Articular cartilage defects have a limited capacity to regenerate after injury and may lead to accelerated wear, worsening pain, and potential arthritis progression.3-5 Many methods have been described to treat chondral defects in the knee: microfracture or drilling,6-8 osteochondral autograft transfer (OAT),9-11 osteochondral allograft (OCA),12-14 autologous chondrocyte implantation (ACI), matrix-assisted autologous chondrocyte implantation (MACI),15-17 and particulated juvenile allograft.18-20 However, fibrocartilage after microfracture, 21 fragmentation and resorption of allografts after OAT and OCA, and disturbed fusion of the regenerative cartilage and the healthy surrounding cartilage after ACI and MACI have been reported, while low product scalability is another problem. 22
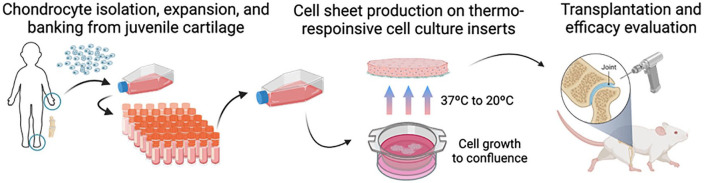
Scaffold-free cell-dense implants, termed “cell sheets,” are routinely fabricated using temperature-responsive cell culture surfaces ( Fig. 1 ).23–26 These sheets preserve intercellular interactions and extracellular matrix that facilitate intimate tissue site integration, overcoming engraftment issues often reported in other tissue-and cell-based approaches.27,28 To enable scalable, allogeneic implants, we have established a human juvenile cartilage-derived chondrocyte (JCC) bank, and demonstrated the safety and cartilage regenerative capacity of JCC sheets in nude rat chondral defects. 29 A small cohort human clinical research study also recently demonstrated safety and efficacy of JCC sheets transplanted into patients presenting with Outerbridge Grade III or IV defects accompanied by an instrumented high tibia osteotomy/microfracture cartilage repair model. 30
Figure 1.
Schema of the experiment. Chondrocytes were expanded from an established JCC bank, cultured in thermo-responsive cell culture inserts, and transplanted into nude rats. Created with BioRender.com.
Allogenic cell source variability is known to affect cell-based therapeutic results. 31 Differences in secreted paracrine regenerative and immuno-modulatory factors from mesenchymal stromal cell (MSC) and JCC sheets derived from different human donors have been reported.30,32,33 These data suggest that JCC sheet characteristics relevant to their regenerative potency are affected by variations in donors. To improve JCC sheet therapeutic efficacy and reliability, extents to which therapeutic effects vary with human cell sourcing must be determined, and key factors that influence this donor variation must be elucidated to optimize donor selection criteria using relevant animal models.
Histological analysis of cartilage tissue to assess structural and biomolecular changes in animal defect models remains a primary strategy to describe regenerative progress in injured cartilage during treatment and attempt to extrapolate treatment results to clinical feasibility. O’Driscoll scoring was established to assess cartilage repair in rabbit models. 34 This score has frequently been used in modified versions 33 ( Table 1 ) and remains among the few scoring systems in which integration of the repair tissue with native cartilage is assessed. 36 Structural characteristics, for example, subchondral bone assessments, are also included in one of O’Driscoll score categories. Separate analyses of in situ changes of key collagen expressions by immunohistochemistry are more sensitive and accurate for specific healing-related markers. 37 Therefore, combinations of these assessments are expected to better stratify in vivo JCC sheet implantation results based on multi-component assessments of cartilage regeneration.
Table 1.
Modified O’Driscoll Score.
| I. Nature of Predominant | |
| Hyaline cartilage | 4 |
| Mostly hyaline cartilage | 3 |
| Mixed hyaline and fibrocartilage | 2 |
| Mostly fibrocartilage | 1 |
| Some fibrocartilage, mostly nonchondrocytic cells | 0 |
| II. Structural Characteristics | |
| A. Surface regularity | |
| Smooth and intact | 3 |
| Superficial horizontal lamination | 2 |
| Fissures | 1 |
| Severe disruption, including fibrillation | 0 |
| B. Structural integrity, homogeneity | |
| Normal | 2 |
| Slight disruption | 1 |
| Severe disintegration, disruptions | 0 |
| C. Thickness | |
| 100% of adjacent cartilage | 2 |
| 50-100% of normal cartilage | 1 |
| 0-50% of normal cartilage | 0 |
| D. Bonding to adjacent cartilage | |
| Bonded at both ends of graft | 2 |
| Bonded at one end or partially at both ends | 1 |
| Not bonded | 0 |
| III. Freedom from Cellular Changes of Degeneration | |
| A. Hypocellularity | |
| Normal cellularity | 2 |
| Slight hypocellularity | 1 |
| Moderate hypocellularity or hypercellularity | 0 |
| B. Chondrocyte clustering | |
| No clusters | 2 |
| <25% of the cells | 1 |
| 25-100% of the cells | 0 |
| IV. Freedom from degenerative changes in adjacent cartilage | |
| Normal cellularity, no clusters, normal staining | 3 |
| Normal cellularity, mild clusters, moderate staining | 2 |
| Mild or moderate hypo/hypercellularity, slight staining | 1 |
| Sever hypocellularity, poor or no staining | 0 |
| V. Subchondral bone | |
| A. Reconstruction of subchondral bone | |
| Normal | 3 |
| Reduced subchondral bone reconstruction | 2 |
| Minimal subchondral bone reconstruction | 1 |
| No subchondral bone reconstruction | 0 |
| B. Inflammatory response in subchondral bone region | |
| None/mild | 2 |
| Moderate | 1 |
| Severe | 0 |
| VI. Safranin O staining | |
| Normal or near normal | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| Total maximum score: | 28 |
The purpose of this study was 3-fold: (1) to stratify observed human JCC sheet in vivo therapeutic effects from an established nude rat cartilage defect-implant model using modified O’Driscoll scoring and specific collagen immunohistochemistry (IHC), (2) to examine relationships between these in vivo scores, and (3) to identify specific characteristics that define significant in vivo differences in cartilage regeneration using different human donors.
Materials and Methods
Cartilage Sourcing
Primary human cartilage cells were isolated from 8 amputated polydactylous fingers and a toe from 8 juvenile patients aged between 6 and 30 months (5 males, 3 females; 14.6±6.9 months; Caucasian; Suppl. Table S1). These patients underwent polydactyly surgery, and tissue discards from these surgeries were collected at Intermountain Primary Children’s Hospital (Salt Lake City, USA). Institutional Review Board oversight from the University of Utah and Intermountain Primary Children’s Hospital was waived due to the use of de-identified routine surgical discards for research purposes.
Fabrication of Juvenile Chondrocyte Sheets
Cartilage tissues from polydactyly surgical discards were dissected, enzymatically digested, and cultured for cryogenic cell banking, and JCC sheets were fabricated according to methods described by Kondo et al. 27 and as summarized below. Briefly, human cartilage tissue was cut into <4 mm2 pieces by scalpel and incubated with 5 mg/mL Type 1 collagenase (LS004197, Worthington Biochemical, Lakewood, USA) at 37°C for 1.5-3.0 h. Resulting cells were filtered through a 100-µm cell strainer, washed with saline, and then resuspended in chondrocyte culture medium (DMEM-F12, 11320082, ThermoFisher Scientific, Waltham, USA) containing 1% antibiotic-antimycotic (15240062, ThermoFisher) and 20% fetal bovine serum (FBS) (16000044, ThermoFisher). Isolated chondrocytes were seeded at 5,000–10,000 cells/cm2 on polystyrene dishes (CELLTREAT, Pepperell, USA) in chondrocyte culture medium. At the first medium change on day 4, 100 μg/mL L-ascorbic acid phosphate magnesium salt n-hydrate (013-19641, Fujifilm Wako Pure Chemical, Osaka, Japan) was added and the medium was replaced. Cells were passaged with this medium thereafter. Sub-confluent cells were suspended in STEM-CELLBANKER GMP grade media (Zenoaq, Fukushima, Japan) and cryopreserved at the end of P0. Serial subculture was performed with thawed cells at an initial density of 10,000 cells/cm2 passaged every 3-5 days. To create passage 2 (P2) JCC sheets, P1 thawed cryopreserved cells were seeded and sub-confluent P1 cells were collected, then seeded at a density of 10,000 cells/cm2 on 23-mm diameter temperature-responsive cell culture inserts (CellSeed, Tokyo, Japan). Cell culture medium was changed every 2-4 days. After 2 weeks of culture, confluent cell sheets were harvested with forceps after incubation at room temperature. P2 JCC sheets from all human donors were used for in vivo transplantation as described below.
Nude Rat Cartilage Defect Model and Human Cell Sheet Transplantation
All in vivo implant procedures were approved by the Institutional Animal Care & Use Committee (IACUC, University of Utah, assigned ID: 20-12001). A total of 30 nude rats (6-week-old), both male and female, were purchased from Charles River Laboratories (Wilmington, USA). After a week of acclimatization at the animal facility, rats were randomized to each experimental condition by comparable body weights. Under anesthesia using isoflurane and O2 gas, medial parapatellar incision was made on the right knee, and the patella was laterally dislocated. A focal chondral defect (diameter 2 mm; depth 300-400 μm) was created on the patellofemoral groove using an electric grinder with minimal damage to the subchondral bone. We have previously published histological findings immediately after defect creation, confirming the accuracy of this technique. 29 Freshly prepared JCC sheets were washed with saline, then cut in half with a sterile razor blade, and single sheet halves were transplanted into the defect ( Fig. 2A ). Sheets attached spontaneously without suturing to the defect surface. The patella was anatomically repositioned, and the quadriceps femoris muscle and skin were sutured. All groups received surgery with comparable duration regardless of the treatment conditions. Only one implant was placed in one chondral defect per animal. Animals were sacrificed after 4 weeks for further histological evaluations. The employed animal number was determined by a significance level of 5 and 80% power using pilot study results. Results from one rat were excluded due to defect site positioning outside of the desired rat patellofemoral groove anatomical area.
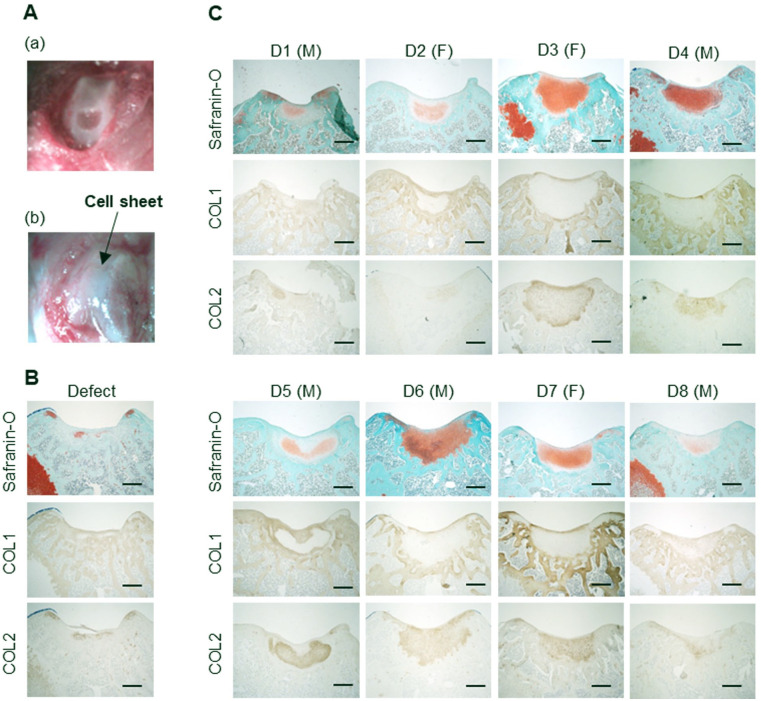
Figure 2.
Transplantation of human juvenile cartilage-derived chondrocyte (JCC) sheets on nude rat knee cartilage defect model. (A) Macroscopic image of transplantation. (a) Osteochondral defect on the patellofemoral groove and (b) patellofemoral groove after cell sheet transplantation on the defect. (B) Representative histological sample in defect group. (C) Representative histological images of the rat knees at 4 weeks after JCC sheet transplantation. D represents donor. The gender of each donor is Indicated after the donor number. M: male, F: female, Bars: 500μm.
Harvested rat knee tissue was fixed in 4% paraformaldehyde for 4 days and decalcified in RapidCal Immuno (BBC Biochemical, Mount Vernon, USA) for 1 day at RT. Samples were embedded in paraffin blocks and then cut into 5-µm transverse sections with a microtome. P2 JCC cell sheets from donor 1 were implanted in 3 female rats, cell sheets from donor 2 into 2 male and 1 female rat, cell sheets from donor 3 into 3 female rats, cell sheets from donor 4 into 3 male rats, cell sheets from donor 5 into 3 male and 3 female rats each, and cell sheets from donor 6, 7, and 8 into 3 male rats. Defect models served as controls using 3 male rats.
Histological Evaluation of Harvested Cartilage
Allocation and assessment were done by multiple investigators for randomization. Histological samples from each rat defect site were prepared separately for safranin O, collagen type 1 (COL1), collagen type 2 (COL2), and human-specific vimentin (hVIM) staining. Slides were deparaffinized by baking in an oven at 65°C and subsequent washes with xylene and ethanol. Safranin O staining was conducted using standard methods. 38 Briefly, samples were stained for 5 min with Weigert’s Iron Hematoxylin (MilliporeSigma), 5 min with 0.5 g/L Fast Green (MilliporeSigma), and 5 min with 0.1% Safranin O (MilliporeSigma). After safranin O staining, slides were randomized and scored with modified O’Driscoll scoring, and the observer was blinded regarding JCC donor information.
For immunostaining, histology samples were rehydrated and protease K (S3020, Agilent Technologies, Santa Clara, USA) was used for antigen retrieval for COL2 staining. Peroxidase blocking was performed with 3% hydrogen peroxide (216763, MilliporeSigma). After blocking with 5% donkey serum and 0.1% Triton-X in PBS for 1 h, samples were treated with primary antibodies to COL1, COL2, and hVIM at 4°C overnight. Polyclonal goat anti-COL1 (1:200, SouthernBiotech, Birmingham, USA), monoclonal mouse anti-COL2 (1:200, 2B1.5, ThermoFisher, USA), and monoclonal rabbit anti-hVIM (1:200, SP20, Abcam) were used as primary antibodies. Normal goat IgG (1:50, NI02, MilliporeSigma), normal mouse IgG2a (1:100, X0943, Agilent), or normal rabbit IgG (1:1,500, X0903, Agilent) were used as isotype controls. Horseradish peroxidase (HRP)-conjugated donkey anti-goat antibody (1:1,000, 705-035-147, Jackson ImmunoResearch, West Grove, USA) was used for type 1 collagen. HRP-conjugated goat anti-mouse antibody (1:1,000, 115-035-166, Jackson) was used for type 2 collagen. HRP-conjugated goat anti-rabbit antibody (1:1,000, 111-035-144, Jackson) was used for hVIM staining. ImmPACT DAB Peroxidase (HRP) Substrate (SK-4105, Vector Laboratories, Burlingame, USA) was used as a chromogen. Brightfield images were taken with a BX41 microscope and processed with AmScope Software. For quantitative IHC, the positive cell area across the entire cell sheet was measured and expressed as percent using ImageJ software (NIH) (%COL1 and %COL2 areas, Fig. 3 ). 39 Safranin O staining and hVIM staining of the serial sections were used as a reference. Ratios of COL1 to COL2 in both the total defect and regenerating areas (COL2/1 ratio) were also calculated.
Figure 3.
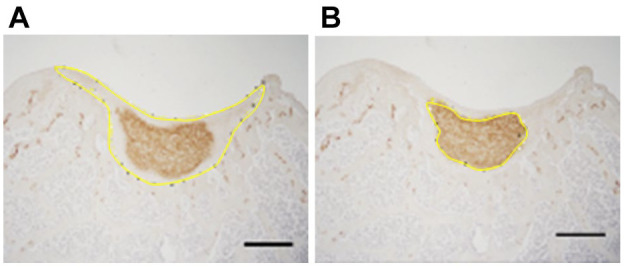
Analysis of image stained with anti-type 2 collagen antibody using Image J. The area of the entire implanted cell sheet regenerative area (A); and the positive cell area (B). % COL2 area is shown as the percentage of (B) to (A). Bars: 500 μm.
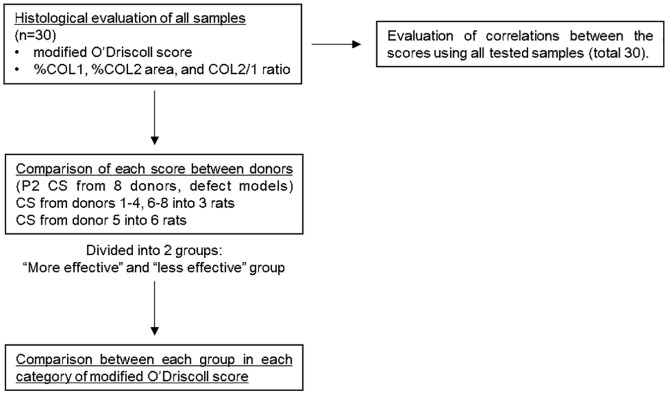
Correlation coefficients for each modified O’Driscoll score and %COL1 area, %COL2 area, and COL2/1 ratio were calculated. For donors treated with P2 JCC sheets, scores were compared between donors and the defect-only group and divided into “more effective” and “less effective” groups based on average scores. Significant differences between each group and the defect-only group in each category of modified O’Driscoll score were evaluated. A summary of the methodological process is described in Figure 4 .
Figure 4.
Study methodology. P2 cell sheets (CS) from donors 1-4, 6-8 were transplanted into 3 rats and CS from donor 5 into 6 rats. 3 defect models were also prepared as a control. Pearson’s correlation coefficient was used to evaluate the correlations between scores. ANOVA with post hoc Tukey-Kramer testing was used to compare each score between donors and each category of modified O’Driscoll score between groups.
Statistical analysis
Numerical results are expressed as a mean and standard deviation. Pearson’s correlation coefficient was used to identify significant relationships between modified O’Driscoll scores and %COL1 area, %COL2 area, and COL2/1 ratios. ANOVA with post hoc Tukey-Kramer testing was used to identify significant differences between donors in each score and between groups in each category of modified O’Driscoll score. All tests were performed at a significance level of p < 0.05. Statistical analysis was performed with statistical package R (version 4.2.0; http://www.r-project.org). O’Driscoll scores stratified and compared based on donor sex were insufficiently powered to produce any statistically validated conclusions-see Supplemental Information.
Results
Assessment of in vivo cartilage defect regeneration capacity is essential to improve cartilage regenerative medicine products. We employed a published nude rat knee cartilage defect model 29 as a platform to implant and test human juvenile chondrocyte sheets. JCC-regenerated histological samples were confirmed positive for hVIM immunostaining, 29 supporting that new cartilage originates from implanted JCC human cells (Suppl. Fig. S1). Representative histological samples are shown in Figure 2B and C.
Histological Scores and Their Correlations in the Cell Sheet Regeneration Model
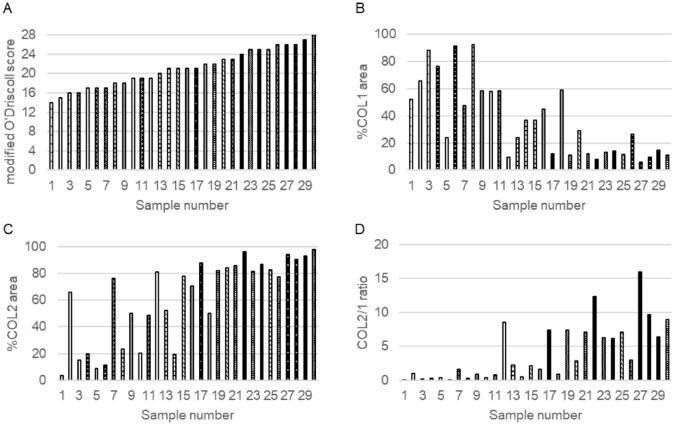
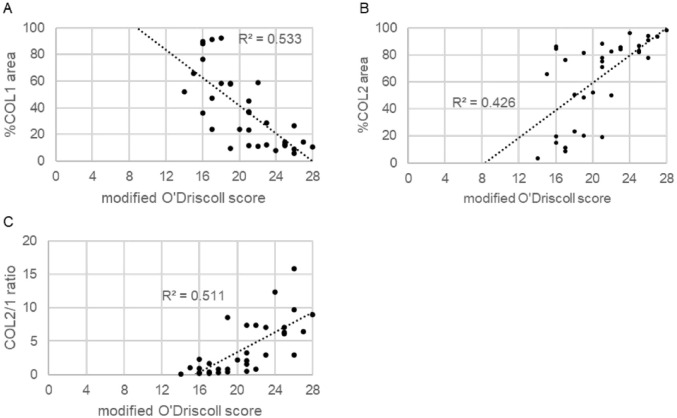
Modified O’Driscoll scores, %COL1 and %COL2 areas, and COL2/1 ratios varied among the 8 donor JCC sheet implant groups ( Fig. 5 ). The mean modified O’Driscoll score was 20.7±3.8. Mean percentages for %COL1 and %COL2 areas were 37.9±27.5 and 63.1±30.1, respectively. Mean COL2/1 ratio was 3.9±4.0. Compared to modified O’Driscoll scores, a negative correlation was observed for %COL1 area (R2=0.533, p<0.01) and a positive correlation for %COL2 area and COL2/1 ratio (R2=0.426, p<0.01 and R2=0.511, p<0.01) ( Fig. 6 ).
Figure 5.
Scores of each system in all samples. The scores were arranged in order of modified O’Driscoll score. Bars sharing the same pattern represent samples from the same donor. Sample numbered 1, 3, and 5 represent the defect groups. (A) modified O’Driscoll score, (B) %COL1 area, (C) %COL2 area, and (D) COL1/2 ratios.
Figure 6.
Scatter plot of the correlation between modified O’Driscoll score and %COL 1 area (A), %COL 2 area (B), and COL2/1 ratio (C). Modified O’Driscoll score correlated significantly with each score (P<0.01).
Comparisons of modified O’Driscoll score, %COL1 area, %COL2 area, and COL2/1 ratios between JCC donors
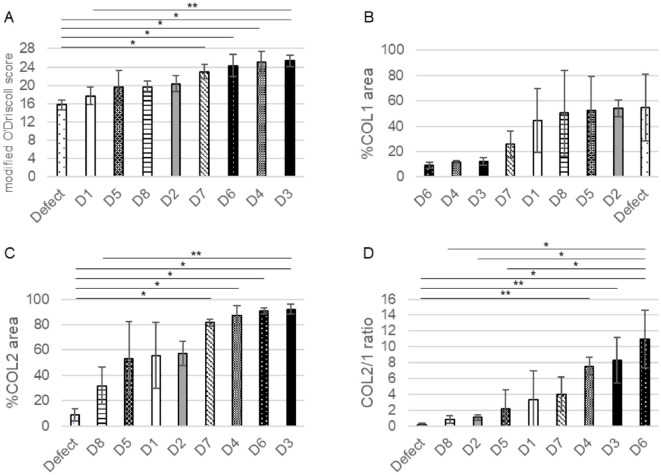
Four human donor-derived JCC sheet-transplanted groups showed significantly higher modified O’Driscoll scores compared to the defect-only group (p<0.01). %COL1 area was not significantly different from control due to high intrinsic variation within this assessment. %COL2 area of donor 3 was significantly higher than that of donor 8 and the defect-only group (p=0.047 and p<0.01, respectively), and those of donors 4, 6, and 7 were significantly higher than that of the defect-only group (p<0.01). The COL2/1 ratio has been used for assessing cartilage matrix quality in previous gene expression assays. 40 In our study, the COL2/1 ratio distinguishes donor matrix differences; for example, the ratio for donor 6 was significantly higher than that of donors 2, 5, 8, and the defect-only group (p<0.01); donor 3 and 4 were both significantly higher than that of the defect-only group (p<0.05). Using these assessments and histological scoring, JCC donors were divided into two groups: groups 3, 4, 6, and 7 (group “more effective”) and groups 1, 2, 5, and 8 (group “less effective”) ( Fig. 7 ).
Figure 7.
Comparison of each score between groups. (A) modified O’Driscoll score, (B) %COL1 area, (C) %COL2 area, (D) COL2/1 ratio. *<0.01, **<0.05.
Comparing each group in each modified O’Driscoll score evaluation category
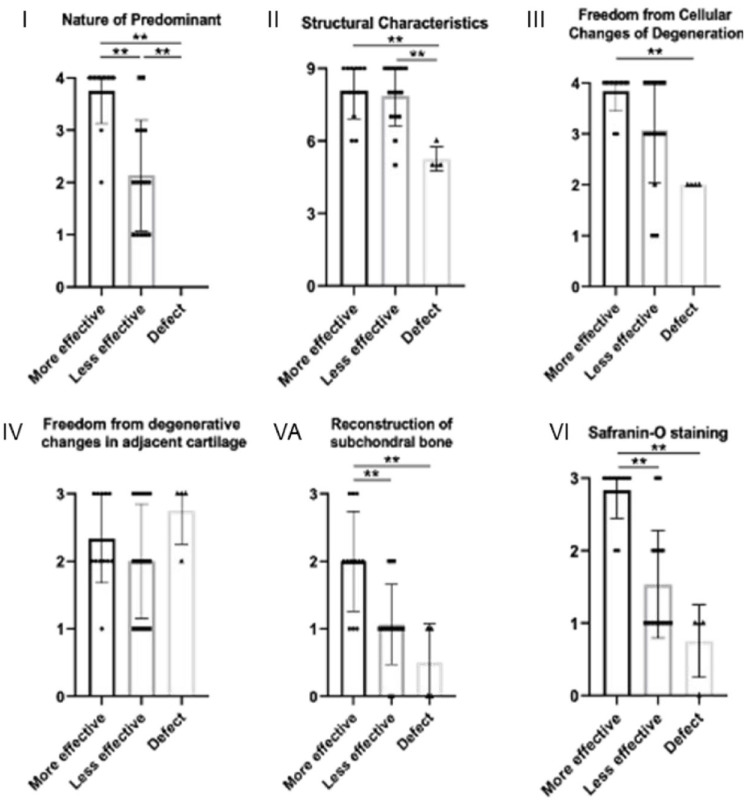
To further identify differential regenerative effects by JCC sheets from 8 donors in nude rat cartilage defects, modified O’Driscoll score subcategories were compared between knees bearing “more effective” sheet implants, “less effective” sheet implants, and defect-only group. Significant differences between group “more effective” and group “less effective” were observed in the modified O’Driscoll score categories of “Nature of predominant tissue” (I), “Reconstruction of subchondral bone” (V-A), and “Safranin O staining” (VI) (p<0.01). No significant differences were seen between these two groups in the categories “Structural characteristics” (II), “Freedom from cellular changes of degeneration” (III), and “Freedom from degenerative changes in adjacent cartilage” (IV) ( Fig. 8 , p=0.88, p=0.51, and p=0.49, respectively). Scores in both groups were significantly higher than the defect-only group control in the category “Structural characteristics” (II) (p<0.01). Since minor category “inflammatory response” (V-B) could not be confirmed, only “Reconstruction of subchondral bone” (V-A) in this O’Driscoll subcategory V was analyzed.
Figure 8.
Each category of modified O’Driscoll score in each group. (1) Nature of predominant, (II) Structural characteristics, (III) Freedom from cellular changes of degeneration, (IV) Freedom from degenerative changes in adjacent cartilage, (VA) Reconstruction of subchondral bone, and (VI) Safranin O staining. “More effective” group consisted of donors 3, 4, 6, and 7. “Less effective” group consisted of donors 1, 2, 5, and 8. *<0.01.
Discussion
Development of improved, validated, and scalable cartilage regeneration strategies remains an urgent clinical challenge. Assessment of reliable human cell-derived regenerative approaches is integral to assuring progress. The potential for human juvenile cartilage as an allogeneic cell source for regenerating cartilage is long recognized.41-43 Although multiple histological scoring systems have been introduced as part of regenerative outcomes analysis, 44 correlations of these systems to human cell-based approaches are not fully investigated. Modified O’Driscoll scoring is widely used to assess in vivo cartilage repair in defect models and has been reported to be superior to other scores in terms of coefficient of repeatability.34,45,46 This study showed variations in O’Driscoll scores, %COL1-and %COL2-positive stained defect histological section areas, and COL2/1 ratios for human JCC sheets derived from various donors in an established nude rat defect model. 29 Modified O’Driscoll scores correlated negatively with %COL1 areas and positively with %COL2 areas and COL2/1 ratios (see Fig. 6 ), linking the histological O’Driscoll cartilage scoring criteria to recognized cartilage-specific collagen matrix deposition. A previous in vivo study of particulated juvenile allograft cartilage in porcine articular cartilage defects showed that hyaline cartilage was found at only 20.1% abundance 4 weeks after transplantation. 42 Clinically, ACI outcomes report only 15% of patients exhibiting type 2 collagen-dominant cartilage. 47 Thus, clinical regeneration of hyaline cartilage is difficult and unreliable with currently available cell therapies.
In contrast, our in vivo data with human juvenile-sourced cell sheet treatment shows >80% COL2 and ~10% COL1 stained areas after 4 weeks of treatment with “more effective” donors in our model ( Fig. 7 ), suggesting high cartilage regenerative potential in defects using JCC sheets. Results also highlight variations in chondrogenic potential of JCC sheets from different donors ( Fig. 7 ). Our group has reported that a single juvenile polydactyly donor can theoretically produce 2-3 × 103 JCC sheets at P2. 27 To leverage this JCC scalability across allogenic donors, a reliable assessment system as proposed in this study is required for effective human donor cell screening.
Based on the scoring shown, juvenile donors could be divided into “more effective” and “less effective” groups ( Fig. 7 ). Between these groups, significant differences in donors were observed in scoring categories “Nature of predominant tissue,” “Reconstruction of subchondral bone,” and “Safranin O staining.” The significant difference in “Reconstruction of subchondral bone” scoring among JCC sheet donor sheets (see Fig. 8 ) is noteworthy. Subchondral bone absorbs most of the applied joint mechanical force and provides essential mechanical support for overlying articular cartilage 48 ; it may be an essential component for cartilage regeneration. Sato et al. 49 reported the importance of the union between the graft and subchondral bone in a rabbit model of costal osteochondral grafts, showing histological union between the graft and the subchondral bone at 6 weeks after transplantation. Qiu et al. 50 reported a dynamic relationship between articular cartilage and subchondral bone, suggesting that abnormality in either could disturb the homeostatic balance of the bone/cartilage unit. Hence, lack of subchondral bone repair/reconstruction potential might undermine cartilage regeneration in the “less effective” group.
Safranin O staining showed structures that appear to be blood vessels in the subchondral layer in the “less effective” group at the 4-week time point, not observed in the “more effective” group at 4 weeks (Suppl. Fig. S2A and B). Angiogenesis is a necessary process for bone remodeling, and its presence and staging in subchondral bone in joints with OA is recognized. 51 Thus, subchondral bone remodeling and associated neovascular dynamics post-injury may be completed earlier in the “more effective” group than in the “less effective” group as part of defect repair. While details to describe the defect healing process within 4 weeks remain tentative, at 2 weeks post-implantation with a “more effective donor,” blood vessels are found in the subchondral bone and subchondral bone remodeling is not yet completed (Suppl. Fig. S2C).
The scoring category “Structural characteristics” produced high scores in sheet-transplanted groups (see Fig. 8 ). A smooth regenerated tissue surface is required for low-friction articulation. 52 Implant integration with surrounding cartilage is critical for successful cartilage repair to enable normal stress distribution with weight-bearing and prevent tissue degeneration. 53 Thus, “Structural characteristics” is also an essential factor for reliable hyaline cartilage regeneration. Cell sheet implantation exhibited spontaneous sheet integration at the tissue site without suturing, producing the observed rapid hyaline cartilage structural regeneration reflected in the high scores in this category.
Analogous allogenic cell sheet transplantation studies can be performed in outbred animals, but cell properties, especially chondrogenic potential, are highly variable among different animal species. 54 Hence, predictive outcomes extrapolated to human potential will be difficult and speculative without direct correlations. The athymic rat model employed here enables the safety and efficacy testing of human cell products in the in vivo knee synovial articulating joint environment. A rabbit xenotransplantation model using immunosuppressing drugs is also reported for this purpose, although acute pharmacological immuno-suppression could affect natural healing cascades. 55 Future studies should consider intrinsic limitations of these xenogeneic models: biomechanical loading of the articulating surfaces and its influences on cartilage healing are distinct between species, healing processes are likely distinct from that of allogeneic transplants, and essential involvement of all host immune cells in cartilage repair is lacking in immune-incompetent models. Nonetheless, we believe that the nude rat cartilage implant model employed here provides useful mechanistic and potency screening of human cell transplantation products for cartilage regeneration. Host immune response to allogeneic JCC sheet transplantation in inflamed and post-surgery conditions to validate engraftment, safety, and efficacy of this potent human juvenile cell-based therapy is warranted.
Conclusions
This study establishes a combined histological assessment system for human cell products for cartilage repair in a nude rat knee cartilage defect model. 29 The system stratifies regenerative effects of human juvenile cartilage-derived chondrocyte (JCC) sheets derived from various juvenile donors. Scoring differences identified in histological evaluations of various juvenile JCC donor cartilage repair potentials are attributed to safranin O staining and subchondral bone remodeling. Most JCC sheet implant outcomes exhibit excellent “Structural characteristics” scoring in this model. The model outcomes extend and support previous work, 29 demonstrating the benefits of human juvenile chondrocyte sourcing to yield potent cell sheet constructs that spontaneously adhere to chondral defects and rapidly integrate to regenerate full thickness hyaline-like cartilage. While allogeneic transplantation studies are vital for evaluating immune response and rejection, leveraging this implant regenerative model to screen human cell sources for critical quality attributes is essential for product development. This process enables reliable cartilage regeneration and facilitates donor selection, banking, scaling, and achieving the desired cost-effectiveness for allogeneic living cartilage implants.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035241277946 for Regenerative Variability of Human Juvenile Chondrocyte Sheets From Different Cell Donors in an Athymic Rat Knee Chondral Defect Model by Keisuke Matsukura, Makoto Kondo, Nicolas F. Metzler, Adam J. Ford, Travis G. Maak, Douglas T. Hutchinson, Angela A. Wang, Masato Sato, David W. Grainger and Teruo Okano in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_19476035241277946 for Regenerative Variability of Human Juvenile Chondrocyte Sheets From Different Cell Donors in an Athymic Rat Knee Chondral Defect Model by Keisuke Matsukura, Makoto Kondo, Nicolas F. Metzler, Adam J. Ford, Travis G. Maak, Douglas T. Hutchinson, Angela A. Wang, Masato Sato, David W. Grainger and Teruo Okano in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by the University Technology Acceleration Grant (UTAG) from the Utah Science, Technology and Research (USTAR) program to T.O. and D.W.G. and American Orthopedic Society for Sports Medicine (AOSSM)-JRF Allograft Research Grant to M.K.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Teruo Okano is a shareholder of CellSeed, Inc. and is an inventor/developer designated on the patent for CellSeed’s commercialized thermos-responsive cultureware. Travis Maak discloses the following possible competing interest: Arthrex Inc. (paid speaker and consultant). All other authors declare no competing interests.
Ethical Approval: All in vivo implant procedures were approved by the Institutional Animal Care & Use Committee (IACUC) at the University of Utah (ID: 20-12001). For the use of human tissue, Institutional Review Board (IRB) oversight from both the University of Utah and Intermountain Primary Children’s Hospital was waived, as the research involved de-identified routine surgical discards.
Data Availability: Data sets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
ORCID iDs: Keisuke Matsukura  https://orcid.org/0009-0004-5296-0502
https://orcid.org/0009-0004-5296-0502
Makoto Kondo  https://orcid.org/0000-0002-5978-0043
https://orcid.org/0000-0002-5978-0043
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Coughlin RP, Gupta A, Sogbein OA, Shanmugaraj A, Kurz AZ, Simunovic N, et al. Cartilage restoration in the adolescent knee: a systematic review. Curr Rev Musculoskelet Med. 2019;12(4):486-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DiBartola AC, Wright BM, Magnussen RA, Flanigan DC. Clinical outcomes after autologous chondrocyte implantation in adolescents’ knees: a systematic review. Arthroscopy. 2016;32(9):1905-16. [DOI] [PubMed] [Google Scholar]
- 3. Cameron JI, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation of the femoral trochlea. Am J Sports Med. 2016;44(3):633-8. [DOI] [PubMed] [Google Scholar]
- 4. Hinckel BB, Thomas D, Vellios EE, Hancock KJ, Calcei JG, Sherman SL, et al. Algorithm for treatment of focal cartilage defects of the knee: classic and new procedures. Cartilage. 2021;13(1 suppl):473S-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 6. Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint–a golden standard? J Clin Orthop Trauma. 2016;7(3):145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medina J, Garcia-Mansilla I, Fabricant PD, Kremen TJ, Sherman SL, Jones K. Microfracture for the treatment of symptomatic cartilage lesions of the knee: a survey of International Cartilage Regeneration & Joint Preservation Society. Cartilage. 2021;13(1 suppl):1148S-1155S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber AE, Locker PH, Mayer EN, Cvetanovich GL, Tilton AK, Erickson BJ, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med. 2018;6(2):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsuura T, Hashimoto Y, Kinoshita T, Nishino K, Nishida Y, Takigami J, et al. Donor site evaluation after osteochondral autograft transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2019;47(12):2836-43. [DOI] [PubMed] [Google Scholar]
- 10. Solheim E, Hegna J, Inderhaug E. Long-term survival after microfracture and mosaicplasty for knee articular cartilage repair: a comparative study between two treatments cohorts. Cartilage. 2020;11(1):71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826-31. [DOI] [PubMed] [Google Scholar]
- 12. Riff AJ, Huddleston HP, Cole BJ, Yanke AB. Autologous chondrocyte implantation and osteochondral allograft transplantation render comparable outcomes in the setting of failed marrow stimulation. Am J Sports Med. 2020;48(4):861-70. [DOI] [PubMed] [Google Scholar]
- 13. Tírico LEP, McCauley JC, Pulido PA, Bugbee WD. Osteochondral allograft transplantation of the femoral condyle utilizing a thin plug graft technique. Am J Sports Med. 2019;47(7):1613-20. [DOI] [PubMed] [Google Scholar]
- 14. Tírico LEP, McCauley JC, Pulido PA, Demange MK, Bugbee WD. Is patient satisfaction associated with clinical outcomes after osteochondral allograft transplantation in the knee. Am J Sports Med. 2019;47(1):82-7. [DOI] [PubMed] [Google Scholar]
- 15. Ebert JR, Fallon M, Wood DJ, Janes GC. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2017;45(1):59-69. [DOI] [PubMed] [Google Scholar]
- 16. Pestka JM, Feucht MJ, Porichis S, Bode G, Südkamp NP, Niemeyer P. Return to sports activity and work after autologous chondrocyte implantation of the knee: which factors influence outcomes. Am J Sports Med. 2016;44(2):370-7. [DOI] [PubMed] [Google Scholar]
- 17. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid-to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farr J, Tabet SK, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014;42(6):1417-25. [DOI] [PubMed] [Google Scholar]
- 19. Grawe B, Burge A, Nguyen J, Strickland S, Warren R, Rodeo S, et al. Cartilage regeneration in full-thickness patellar chondral defects treated with particulated juvenile articular allograft cartilage: an MRI analysis. Cartilage. 2017;8(4):374-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tompkins M, Hamann JC, Diduch DR, Bonner KF, Hart JM, Gwathmey FW, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy. 2013;29(10):1661-70. [DOI] [PubMed] [Google Scholar]
- 21. Steaman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 suppl):S362-S369. [DOI] [PubMed] [Google Scholar]
- 22. Welton KL, Logterman S, Bartley JH, Vidal AF, McCarty EC. Knee cartilage repair and restoration: common problems and solutions. Clin Sports Med. 2018;37(2):307-30. [DOI] [PubMed] [Google Scholar]
- 23. Kondo M, Kameishi S, Grainger DW, Okano T. Novel therapies using cell sheets engineered from allogeneic mesenchymal stem/stromal cells. Emerg Top Life Sci. 2020;4(6):677-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thorp H, Kim K, Kondo M, Maak TG, Grainger DW, Okano T. Trends in articular cartilage tissue engineering: 3D mesenchymal stem cell sheets as candidates for engineered hyaline-like cartilage. Cells (2021) 10(3):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi J, Kikuchi A, Aoyagi T, Okano T. Cell sheet tissue engineering: cell sheet preparation, harvesting/manipulation, and transplantation. J Biomed Mater Res A. 2019;107(5):955-67. [DOI] [PubMed] [Google Scholar]
- 26. Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993;27(10):1243-51. [DOI] [PubMed] [Google Scholar]
- 27. Chen X, Lu M, Ma N, Yin G, Cui C, Zhao S. Dynamic tracking of injected mesenchymal stem cells after myocardial infarction in rats: a serial 7T MRI study. Stem Cells Int. 2016;2016:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wahl EA, Fierro FA, Peavy TR, Hopfner U, Dye JF, Machens HG, et al. In vitro evaluation of scaffolds for the delivery of mesenchymal stem cells to wounds. Biomed Res Int. 2015;2015:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kondo M, Kameishi S, Kim K, Metzler NF, Maak TG, Hutchinson DT, et al. Safety and efficacy of human juvenile chondrocyte-derived cell sheets for osteochondral defect treatment. NPJ Regen Med. 2021;6(1)65:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamahashi K, Toyoda E, Ishihara M, Mitani G, Takagaki T, Kaneshiro N, et al. Polydactyly-derived allogeneic chondrocyte cell-sheet transplantation with high tibial osteotomy as regenerative therapy for knee osteoarthritis. NPJ Regen Med. 2022;7(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318-26. [DOI] [PubMed] [Google Scholar]
- 32. Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toyoda E, Sato M, Takahashi T, Maehara M, Okada E, Wasai S, et al. Transcriptomic and proteomic analyses reveal the potential mode of action of chondrocyte sheets in hyaline cartilage regeneration. Int J Mol Sci. 2020;21(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(7):1017-35. [PubMed] [Google Scholar]
- 35. Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21(24):2561-74. [DOI] [PubMed] [Google Scholar]
- 36. Rutgers M, van Pelt MJP, Dhert WJA, Creemers LB, Saris DBF. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18(1):12-23. [DOI] [PubMed] [Google Scholar]
- 37. Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, et al. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage. 2012;20(6):476-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(suppl 3):S113-S116. [DOI] [PubMed] [Google Scholar]
- 39. Desando G, Grigolo B, Deangelles Pereira Florentino Á, Teixeira MW, Barbagallo F, Naro F, et al. Preclinical evidence of intra-articular autologous cartilage micrograft for osteochondral repair: evaluation in a rat model. Cartilage. 2021;13(2 suppl):1770S-1779S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mhanna R, Kashyap A, Palazzolo G, Vallmajo-Martin Q, Becher J, Möller S, et al. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng Part A. 2014;20(9):1454-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adkisson HD, 4th Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, et al. The potential of human allogeneic juvenile cartilage. Am J Sport Med. 2010;38(7):1324-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ao Y, Li Z, You Q, Zhang C, Yang L, Duan X. The use of particulated juvenile allograft cartilage for the repair of porcine articular cartilage defects. Am J Sports Med. 2019;47(10):2308-15. [DOI] [PubMed] [Google Scholar]
- 43. Cavalli E, Levinson C, Hertl M, Broguiere N, Brück O, Mustjoki S, et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci Rep. 2019;9(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orth P, Zurakowski D, Wincheringer D, Madry H. Reliability, reproducibility, and validation of five major histological scoring systems for experimental articular cartilage repair in the rabbit model. Tissue Eng Part C Methods. 2012;18(5):329-39. [DOI] [PubMed] [Google Scholar]
- 45. Bonasia DE, Marmotti A, Massa ADF, Ferro A, Blonna D, Castoldi F, et al. Intra-and inter-observer reliability of ten major histological scoring systems used for the evaluation of in vivo cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2484-93. [DOI] [PubMed] [Google Scholar]
- 46. Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res A. 2010;94(3):844-55. [DOI] [PubMed] [Google Scholar]
- 47. Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madry H. The subchondral bone: a new frontier in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):417-8. [DOI] [PubMed] [Google Scholar]
- 49. Sato K, Moy OJ, Peimer CA, Nakamura T, Howard C, Ko SH, et al. An experimental study on costal osteochondral graft. Osteoarthritis Cartilage. 2012;20(2):172-83. [DOI] [PubMed] [Google Scholar]
- 50. Qiu YS, Shahgaldi BF, Revell WJ, Heatley FW. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage. 2003;11(11):810-20. [DOI] [PubMed] [Google Scholar]
- 51. Zhu X, Chan YT, Yung PSH, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biol. 2021;8:607764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Espinosa MG, Otarola GA, Hu JC, Athanasiou KA. Cartilage assessment requires a surface characterization protocol: roughness, friction, and function. Tissue Eng Part C Methods. 2021;27(4):276-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. J Orthop Res. 2001;19(6):1105-12. [DOI] [PubMed] [Google Scholar]
- 54. Giannoni P, Crovace A, Malpeli M, Maggi E, Arbicò R, Cancedda R, et al. Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng. 2005;11(1):237-48. [DOI] [PubMed] [Google Scholar]
- 55. Takahashi T, Sato M, Toyoda E, Maehara M, Takizawa D, Maruki H, et al. Rabbit xenogeneic transplantation model for evaluating human chondrocyte sheets used in articular cartilage repair. J Tissue Eng Regen Med. 2018;12(10):2067-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035241277946 for Regenerative Variability of Human Juvenile Chondrocyte Sheets From Different Cell Donors in an Athymic Rat Knee Chondral Defect Model by Keisuke Matsukura, Makoto Kondo, Nicolas F. Metzler, Adam J. Ford, Travis G. Maak, Douglas T. Hutchinson, Angela A. Wang, Masato Sato, David W. Grainger and Teruo Okano in CARTILAGE
Supplemental material, sj-docx-2-car-10.1177_19476035241277946 for Regenerative Variability of Human Juvenile Chondrocyte Sheets From Different Cell Donors in an Athymic Rat Knee Chondral Defect Model by Keisuke Matsukura, Makoto Kondo, Nicolas F. Metzler, Adam J. Ford, Travis G. Maak, Douglas T. Hutchinson, Angela A. Wang, Masato Sato, David W. Grainger and Teruo Okano in CARTILAGE