Abstract
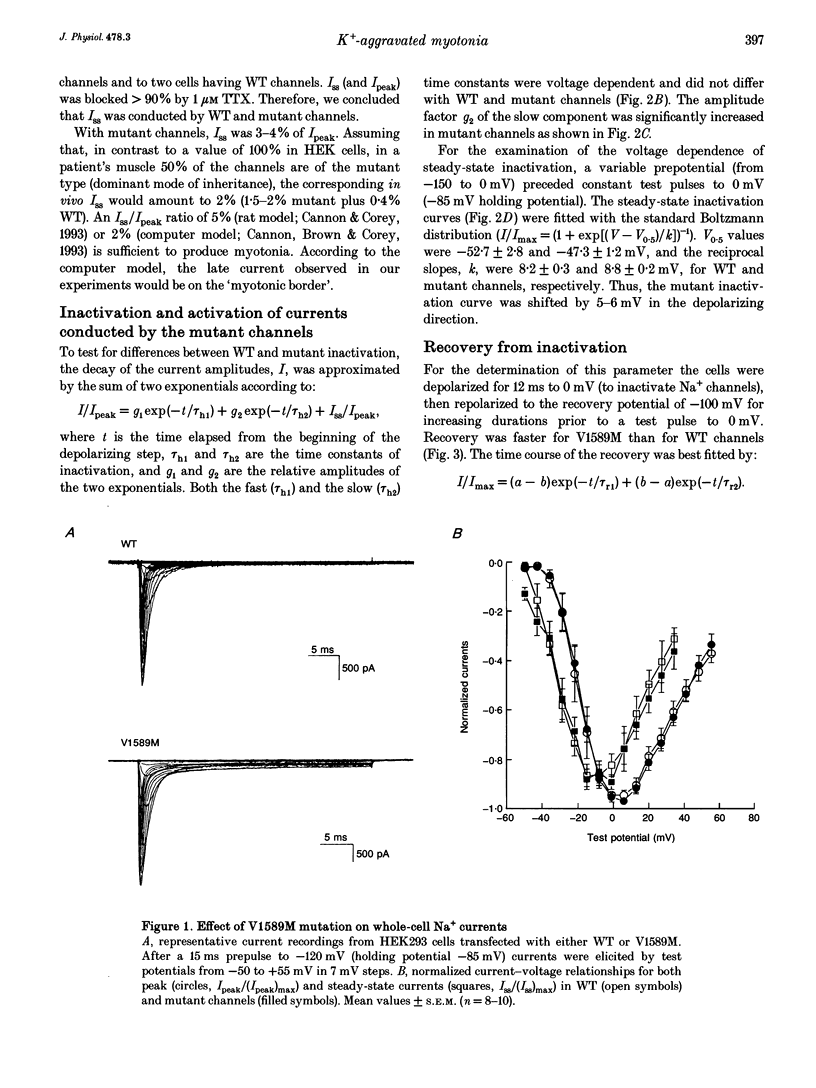
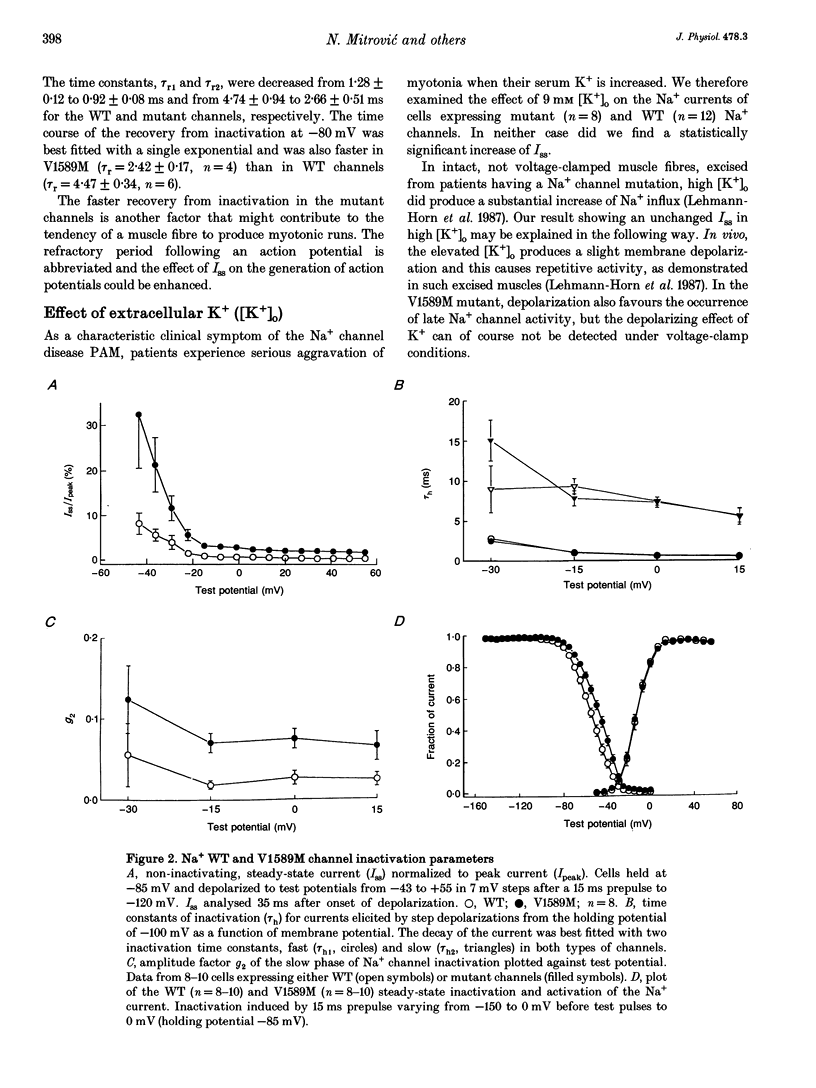
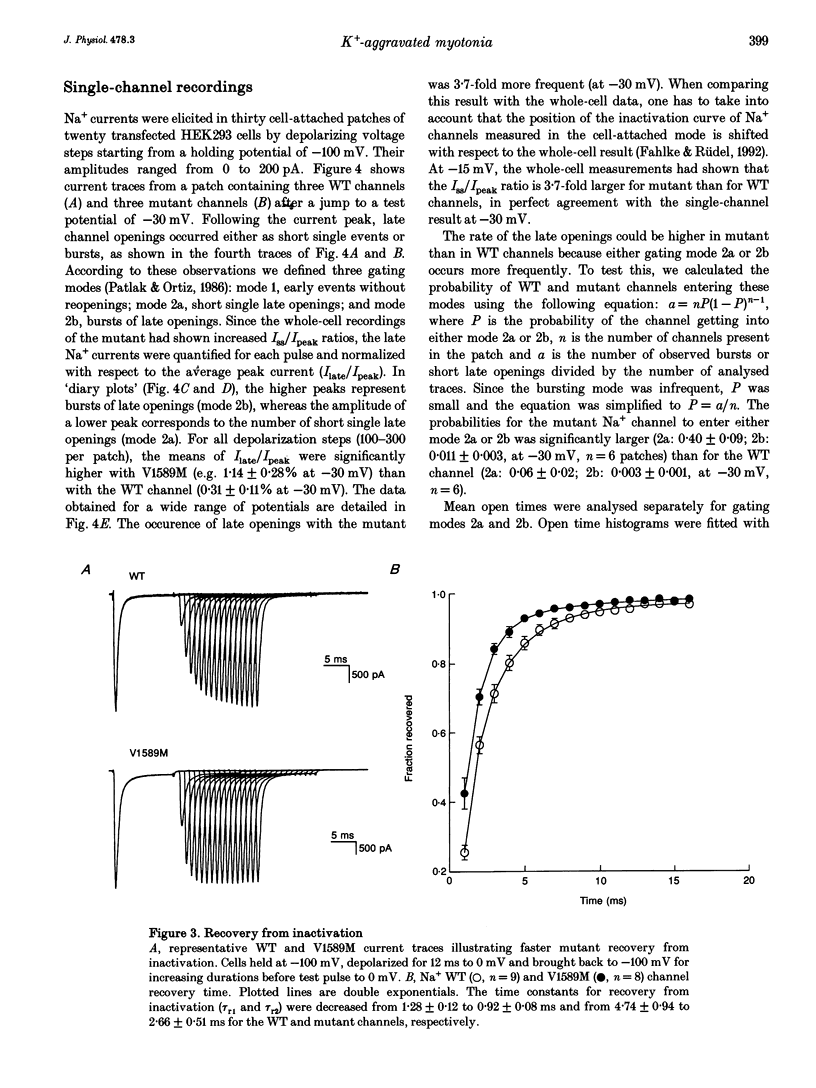
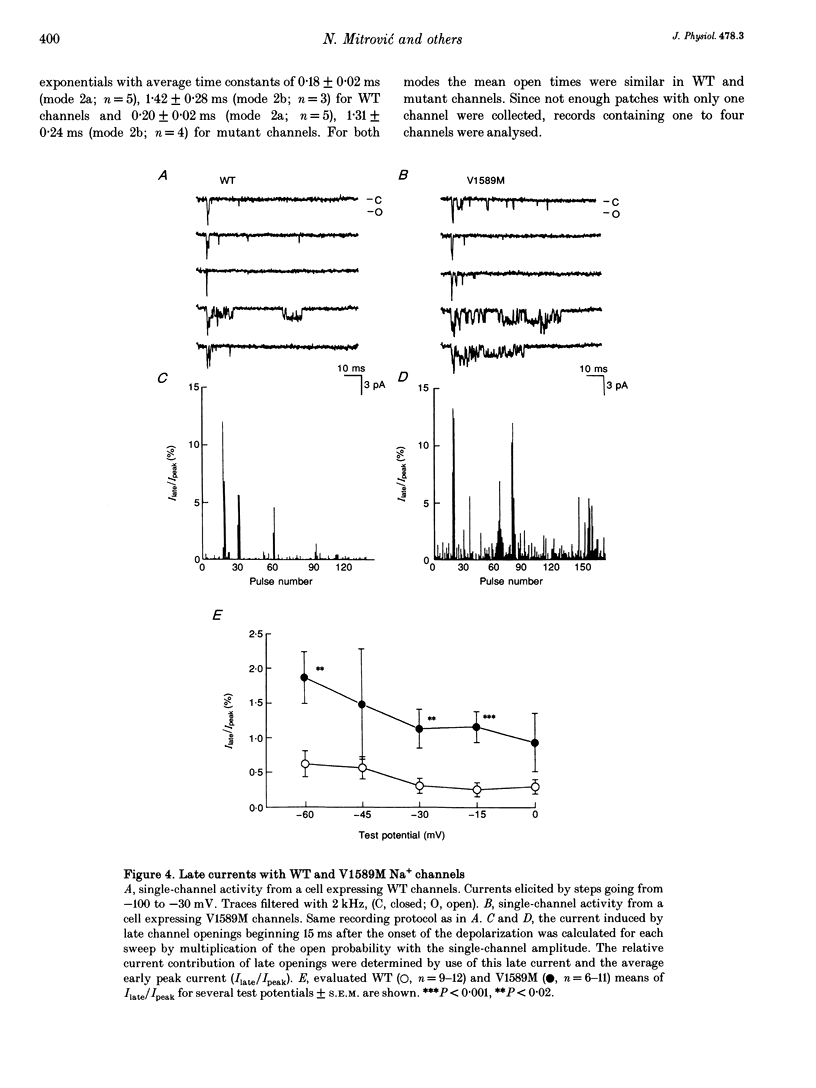
1. Wild type (WT) and V1589M channels were expressed in human embryonic kidney (HEK293) cells for the study of the pathophysiology of the V1589M muscle Na+ channel mutation leading to K(+)-aggravated myotonia. 2. In comparison to WT, whole-cell recordings with V1589M channels showed an increased Na+ steady-state to peak current ratio (Iss/Ipeak) (3.15 +/- 0.70 vs. 0.87 +/- 0.10%, at -15 mV) and a significantly faster recovery from inactivation. The recovery time constants, tau r1 and tau r2, were decreased from 1.28 +/- 0.12 to 0.92 +/- 0.08 ms and from 4.74 +/- 0.94 to 2.66 +/- 0.51 ms for the WT and mutant channels, respectively. 3. Single-channel recordings with mutant channels showed higher probability of short isolated late openings (0.40 +/- 0.09 vs. 0.06 +/- 0.02, at -30 mV) and bursts of late openings (0.011 +/- 0.003 vs. 0.003 +/- 0.001, at -30 mV) compared to WT. 4. These results suggest that the mutation increases the probabilities for channel transitions from the inactivated to the closed and the opened states. 5. Increased extracellular concentrations of K+ had no effects on either V1589M or WT currents in HEK293 cells. The aggravation of myotonia seen in patients during increased serum K+ may arise from the associated membrane depolarization which favours the occurrence of late openings in the mutant channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. A sodium channel defect in hyperkalemic periodic paralysis: potassium-induced failure of inactivation. Neuron. 1991 Apr;6(4):619–626. doi: 10.1016/0896-6273(91)90064-7. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J. 1993 Jul;65(1):270–288. doi: 10.1016/S0006-3495(93)81045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Corey D. P. Loss of Na+ channel inactivation by anemone toxin (ATX II) mimics the myotonic state in hyperkalaemic periodic paralysis. J Physiol. 1993 Jul;466:501–520. [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Strittmatter S. M. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993 Feb;10(2):317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Chahine M., George A. L., Jr, Zhou M., Ji S., Sun W., Barchi R. L., Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994 Feb;12(2):281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Cummins T. R., Zhou J., Sigworth F. J., Ukomadu C., Stephan M., Ptácek L. J., Agnew W. S. Functional consequences of a Na+ channel mutation causing hyperkalemic periodic paralysis. Neuron. 1993 Apr;10(4):667–678. doi: 10.1016/0896-6273(93)90168-q. [DOI] [PubMed] [Google Scholar]
- Fahlke C., Rüdel R. Giga-seal formation alters properties of sodium channels of human myoballs. Pflugers Arch. 1992 Mar;420(3-4):248–254. doi: 10.1007/BF00374454. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Komisarof J., Kallen R. G., Barchi R. L. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann Neurol. 1992 Feb;31(2):131–137. doi: 10.1002/ana.410310203. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heine R., Pika U., Lehmann-Horn F. A novel SCN4A mutation causing myotonia aggravated by cold and potassium. Hum Mol Genet. 1993 Sep;2(9):1349–1353. doi: 10.1093/hmg/2.9.1349. [DOI] [PubMed] [Google Scholar]
- Horn R., Vandenberg C. A. Statistical properties of single sodium channels. J Gen Physiol. 1984 Oct;84(4):505–534. doi: 10.1085/jgp.84.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom L. L., De Jongh K. S., Patton D. E., Reber B. F., Offord J., Charbonneau H., Walsh K., Goldin A. L., Catterall W. A. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science. 1992 May 8;256(5058):839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Iaizzo P. A., Hatt H., Franke C. Altered gating and conductance of Na+ channels in hyperkalemic periodic paralysis. Pflugers Arch. 1991 Apr;418(3):297–299. doi: 10.1007/BF00370530. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Küther G., Ricker K., Grafe P., Ballanyi K., Rüdel R. Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve. 1987 May;10(4):363–374. doi: 10.1002/mus.880100414. [DOI] [PubMed] [Google Scholar]
- Lerche H., Heine R., Pika U., George A. L., Jr, Mitrovic N., Browatzki M., Weiss T., Rivet-Bastide M., Franke C., Lomonaco M. Human sodium channel myotonia: slowed channel inactivation due to substitutions for a glycine within the III-IV linker. J Physiol. 1993 Oct;470:13–22. doi: 10.1113/jphysiol.1993.sp019843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986 Feb;87(2):305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Ricker K., Lehmann-Horn F. Genotype-phenotype correlations in human skeletal muscle sodium channel diseases. Arch Neurol. 1993 Nov;50(11):1241–1248. doi: 10.1001/archneur.1993.00540110113011. [DOI] [PubMed] [Google Scholar]


