Abstract
Background:
The Canadian health sector’s carbon footprint is among the highest in the world and is responsible for 4.6% of Canada’s total greenhouse gas emissions, a quarter of which is linked to pharmaceuticals, with metered-dose inhalers (MDIs) contributing disproportionally high amounts.
Objectives:
To describe MDI prescribing, dispensing, use and waste patterns at a Canadian tertiary care academic hospital.
Methods:
In a retrospective point-prevalence cohort study, 100 consecutive patients discharged from medical and surgical services who were prescribed at least 1 MDI during their admission were included. Data were collected to describe patient demographics, MDI prescribing, dispensing, use and waste patterns. Use and waste data were applied to annual purchasing data to estimate annual usage and waste. Financial cost was computed using local purchasing estimates and carbon cost was calculated using published estimates.
Results:
In 100 consecutively discharged patients, 315 MDIs were dispensed in total, of which 96 were unused. This represents 61,440 actuations dispensed, with 56,773 (92%) of doses unused or wasted. Waste data were applied to annual estimates, with a calculated annual carbon footprint of 315.8 tons of carbon dioxide equivalent (tCO2e). We estimate that a 20% waste reduction would result in carbon savings of 68.5 tCO2e. If 20% of salbutamol prescriptions were switched to the dry powder inhaler alternative, terbutaline, a 14% reduction in waste would be required to offset the additional monetary cost.
Conclusions:
This study suggests that 92% of MDI doses are unused and wasted. Opportunities for waste reduction exist and would be associated with both financial and carbon savings.
Knowledge into Practice.
Pharmacy services and pharmaceutical products account for approximately 25% of the carbon footprint attributed to the health care sector.
Medications delivered by metered-dose inhalers (MDIs) are disproportionately greater contributors to the carbon footprint attributed to medications compared to other drugs.
This study quantifies the use and waste of MDIs in a Canadian academic hospital and estimates its environmental impact.
Pharmacists can play a significant role in environmental stewardship by reducing MDI waste to achieve both financial and carbon savings.
Introduction
Canada’s health sector has 1 of the highest carbon footprints in the world and is responsible for 4.6% of Canada’s total greenhouse gas emissions. 1 The United States’ health sector accounts for approximately 8.5% of national carbon emissions. 2 Many countries are aiming to reduce health care-related greenhouse gas emissions, yet health care emissions continue to increase. 1 Many organizations, including the United Nations and the United Kingdom’s National Health Services (NHS), have identified pharmaceuticals and their production as a major contributor to greenhouse gas emissions. 3 A quarter of the health sector’s greenhouse gas emissions have been linked to medicines and chemicals, with 5% represented by anesthetic gases and inhalers alone. 3 There are several recommendations from large organizations for decreasing greenhouse gas emissions in health care; however, uptake has been slow, potentially due to a lack of awareness of the climate effects of health care practice.
Metered-dose inhalers (MDIs) are commonly prescribed medications for several respiratory disorders, such as asthma and chronic obstructive pulmonary disease (COPD). These types of inhalers use liquefied gas propellants to allow medication delivery through inhalation. However, despite a switch to more ozone-friendly hydrofluorocarbons (HFCs), they still act as greenhouse gases and thus contribute to the health care sector’s overall carbon footprint. 4 The National Institute for Health and Care Excellence (NICE) identified that a 100-dose MDI would have the carbon footprint equivalent of driving a car almost 300 km. 5 Just 1 actuation from a salbutamol MDI pump is equivalent to 141 g of carbon dioxide equivalent emissions (gCO2e) compared with an actuation of an alternative such as terbutaline dry powder inhaler (DPI), which is approximately 4.1 gCO2e. 4
The Canadian Thoracic Society recently released a position statement stating that providers can prioritize DPIs over MDIs, considering patient preference, similar efficacy and decreased carbon footprint. 6 In addition to being highly polluting, inhalers are particularly prone to waste in the hospital setting.7,8 Reports from a Canadian acute care hospital describe that for ipratropium MDIs, 34% of all inhalers dispensed, and 87% of all doses dispensed, were wasted. 7 Another report from a large American acute care hospital similarly describes that among more than 500 patients with COPD or asthma admitted to hospital, 87% of MDI- and DPI-dispensed doses were wasted and associated costs were over $85,000 (USD). 8 Although inappropriate prescribing may be part of the problem, variables that contribute to unnecessary waste are likely also a product of inefficiencies related to dispensing and distribution, as well as a lack of awareness of the environmental harms of inhalers. 9 Audits such as these are important to describe the problem but also to identify opportunities to minimize waste and improve appropriate prescribing.
Mise En Pratique Des Connaissances.
Les services et les produits pharmaceutiques représentent environ 25 % de l’empreinte carbone attribuée au secteur de la santé.
Par rapport aux autres médicaments, ceux administrés par des aérosols-doseurs contribuent de façon disproportionnée à l’empreinte carbone attribuée aux médicaments.
Cette étude quantifie l’utilisation et le gaspillage des aérosols-doseurs dans un hôpital universitaire canadien et estime leurs répercussions sur l’environnement.
Les pharmaciens peuvent jouer un rôle important dans la gestion de l’environnement en réduisant les déchets d’aérosols-doseurs afin de réaliser des économies financières et de réduire l’empreinte carbone.
The Ottawa Hospital is a university-affiliated, tertiary care centre with 2 inpatient campuses comprised of more than 1100 beds. The pharmacy department established an Environmental Stewardship Committee in 2022, with a mandate to identify opportunities within pharmacy to reduce the hospital’s carbon footprint and increase awareness of the impact hospital processes have on climate change. MDIs were identified as significant contributors of greenhouse gases and there is opportunity to reduce waste. This study has 3 main aims: 1) to describe local MDI prescribing, dispensing, use and waste patterns; 2) to estimate the monetary and carbon cost of current local practice and the potential benefits and costs of switching formulary MDIs to similar DPIs; and 3) to identify opportunities to reduce waste and the associated carbon footprint.
Methods
Study design, population and sample size
This was a retrospective, point-prevalence study. The Ottawa Hospital Research Ethics Board approved this study as a quality improvement project.
One hundred consecutive, eligible patients discharged from medical and surgical services at 2 campuses of the Ottawa Hospital were identified from health records at 3 predefined timepoints, 1 month apart. The 100-patient sample was chosen a priori based on convenience due to resources available and our objectives. The 3 timepoints used for this study were predefined by study investigators and these dates were selected to minimize the risk of including the same patients more than once throughout the study period.
Eligibility criteria were 1) age greater than 18 years; 2) admitted to a general medicine or surgical ward and discharged or deceased up to 21 days prior to each audit day; 3) prescribed 1 or more MDIs (salbutamol, ipratropium or ciclesonide) during their hospital stay. Patients were excluded if they were admitted for elective surgeries, admitted to an intensive care unit (ICU) or did not have a discharge summary.
Data collection
Electronic medical records were reviewed, and data related to demographics, patient trajectory during hospital stay (e.g., transfers between wards or services), MDI prescribing, dispensing, use and waste were collected by 1 author (C.C.) using an electronic case report form. The form was piloted in 5 patients by 2 study investigators (C.C. and S.K.) after which revisions were made to improve efficiency and accuracy. After the form was finalized, all data were collected by 1 investigator (C.C.); 10% of included cases were randomly selected and audited for accuracy by a second investigator (C.C., S.S., O.D. or K..B). Discrepancies were resolved via discussion.
Prescribing referred to an actual order documented in the medical record whereas a dispensing event may have been the result of a new order or a nursing request for a missing dose (i.e., redispensed). Patients often received multiple orders for the same MDI over the course of their hospital stay (i.e., MDIs may be reordered on transfer from 1 unit to another, which triggers a redispense). Use was identified as doses recorded in the medication administration record as administered or self-administered by the patient. Waste was defined as remaining unused doses from a device as well as completely unused devices. At the time of this study, the hospital did not have a policy or procedure to send partially used inhalers home with the patient at discharge, nor was there a policy or procedure for redeploying unused inhalers. The existing policy at the time was that unused or partially used MDIs were returned to the pharmacy and sent to a waste management facility for incineration where propellants within the canisters were released into the environment. We were informed by the waste management facility that the plastic inhaler itself is recycled and the canister is incinerated but this process could not be verified. It is also possible that some unused or partially used inhalers were not returned to pharmacy and were rather thrown in the garbage on the unit, in which case both the plastic inhaler and the canister would end up in the landfill. Annual MDI usage and cost data across both hospitals were obtained from pharmacy purchasing reports for 1 year, ending October 2022. Carbon costs for relevant MDIs and DPIs were obtained from the CASCADES Detailed Inhaler Comparison Chart. 10
Analytical plan
Data collected regarding MDI prescribing, dispensing, use and waste were described per patient and in aggregate using measures of central tendency and variance and presented in tables without comparative statistics. Waste was described as the proportion of unused doses divided by the number of doses dispensed in the 100-patient cohort. This proportion was then applied to the annual MDI use obtained from purchasing reports to estimate the annual waste. The monetary cost of MDIs obtained from purchasing records was applied to the annual use rates for each MDI to estimate annual costs. Similarly, the carbon costs of estimated annual MDI use and waste were calculated. Using the monetary and carbon estimates for annual use and waste, the carbon emission savings were calculated for a hypothetical reduction in waste (10%, 20%, 30%, 40% and 50%) and presented graphically. A formulary switch from MDI to equivalent DPI may be appropriate for some patients. This hypothetical switch would incur a monetary cost but savings in carbon emissions, which we present in a graph describing the magnitude of waste reduction required to make the incremental formulary switches cost-neutral. The proposed MDI to DPI switches included salbutamol MDI to terbutaline DPI, ipratropium MDI to tiotropium DPI, and ciclesonide MDI to fluticasone DPI.
Results
Data were collected for 100 eligible medical or surgical patients between January and June 2023. Of the 100 patients, 60% were female and 90% were admitted to medicine wards (10% from surgical wards). The median length of stay was 7 days (range, 1–47 days). Patients were transferred between units a median of 4 times throughout admission (range, 0–11). The most common inpatient diagnoses were respiratory tract infections (43%) and exacerbations of COPD (28%). Sixty-two percent of participants were prescribed salbutamol MDIs at home prior to admission (Table 1).
Table 1.
Baseline characteristics
| Characteristics* | All patients, n = 100 | |
|---|---|---|
| Age (years) | 69.4 ± 15.9 | |
| Sex (female) | 60/100 (60) | |
| Smoking status | Current smoker | 25/100 (25) |
| Past smoker | 45/100 (45) | |
| Nonsmoker | 30/100 (30) | |
| Comorbidities (percentage) | COPD diagnosis | 43/100 (43) |
| COPD requiring oxygen use at home | 5/100 (5) | |
| Asthma | 40/100 (40) | |
| Metered-dose inhalers prescribed at home | Salbutamol | 62/100 (62) |
| Ipratropium | 6/100 (6) | |
| Fluticasone | 6/100 (6) | |
| Ciclesonide | 1/100 (1) | |
| Fluticasone/salmeterol | 4/100 (4) | |
| Mometasone/formoterol | 7/100 (7) | |
| Inpatient diagnoses | Asthma exacerbation | 7/100 (7) |
| COPD exacerbation | 28/100 (28) | |
| Respiratory tract infection | 43/100 (43) | |
| Shortness of breath related to heart failure | 14/100 (14) | |
| Pulmonary embolism | 5/100 (5) | |
| Bronchiectasis | 9/100 (9) | |
| Pulmonary fibrosis | 8/100 (8) | |
| Sarcoidosis | 1/100 (1) | |
| Admission service | Medicine | 90/100 (90) |
| Surgery | 10/100 (10) | |
| Physical transfers per patient between units during admission | 4 (0–11) | |
| Hospital length of stay (days) | 7 (1–47) | |
Data presented as mean ± standard deviation, proportion (%) or median (range). COPD, chronic obstructive pulmonary disease.
Patients had a median of 4 MDI prescriptions documented throughout their admission (range, 1–15). Multiple prescriptions for the same MDI were encountered on transfer between units or when dosing regimens were changed. One hundred percent of patients received at least 1 salbutamol MDI (range, 1–7), 50% of patients received at least 1 ipratropium MDI (range, 1–7) and 7% of patients received at least 1 ciclesonide MDI (range, 1–2). In terms of number of actuations dispensed, a median of 200 (range, 200–1400), 400 (range, 200–1400) and 30 (range, 30–60) actuations were dispensed for salbutamol, ipratropium and ciclesonide, respectively. For salbutamol, a median of 5 actuations (range, 0–232) were used per patient during their hospital stay. For ipratropium, a median of 26 actuations were used per patient, and for ciclesonide, a median of 0 actuations (range, 0–8) were used per patient (Table 2). Approximately 30% of dispensed inhalers were not used at all. Considering all actuations dispensed, 93%, 92% and 73% were unused and presumed wasted for salbutamol, ipratropium and ciclesonide, respectively.
Table 2.
Dispensing, use and waste of MDIs prescribed in hospital
| Salbutamol | Ipratropium | Ciclesonide | Aggregate of all 3 MDIs | ||||
|---|---|---|---|---|---|---|---|
| Per patient | Aggregate (n = 100) | Per patient | Aggregate (n = 100) | Per patient | Aggregate (n = 100) | Aggregate (n = 100) |
|
| MDI prescriptions | 1 | 100 | 0.5 | 50 | 0.07 | 7 | NA |
| No. of MDIs dispensed | 1 (1–7) | 191 | 2 (1–7) | 116 | 1 (1–2) | 8 | 315 |
| No. of MDIs with no use | 1 (0–3) | 66 | 0 (0–6) | 29 | 0 (0–1) | 1 | 96 |
| No.of actuations dispensed | 200 (200–1400) | 38,200 | 400 (200–1400) | 23,200 | 30 (30–60) | 240 | 61,440 |
| No. of actuations used | 5 (0–232) | 2656 | 26 (0–244) | 1947 | 8 (0–35) | 64 | 4667 |
| No. of actuations wasted | 200 (44–1322) | 35,544 | 372 (44–1394) | 21,053 | 25 (20–30) | 176 | 56,773 |
| No. of actuations used/ dispensed |
2656/38,200 (7%) | 1947/23,200 (8%) | 64/240 (27%) | 4667/61,440 (8%) | |||
| Estimated annual MDIs dispensed from purchasing data | 9240 | 4931 | 819 | 14,990 | |||
| Estimated annual carbon cost of wasted actuations (tCO2e) | 242.3 | 66.2 | 7.3 | 315.8 | |||
Aggregate refers to the entire study cohort of 100 patients. Data presented as actual counts, median (range) or proportion (percentage). MDI, metered-dose inhaler; NA, not applicable; tCO2e, tons of carbon dioxide equivalents.
Using annual purchasing reports, we estimated that 14,990 formulary MDIs (9240 salbutamol, 4931 ipratropium and 819 ciclesonide) were used throughout a 1-year period, ending in October 2022. By applying wastage data from our 100-patient sample, we estimate that the carbon cost of wasted doses is 315.8 tons of carbon dioxide equivalent (tCO2e) (Table 2).
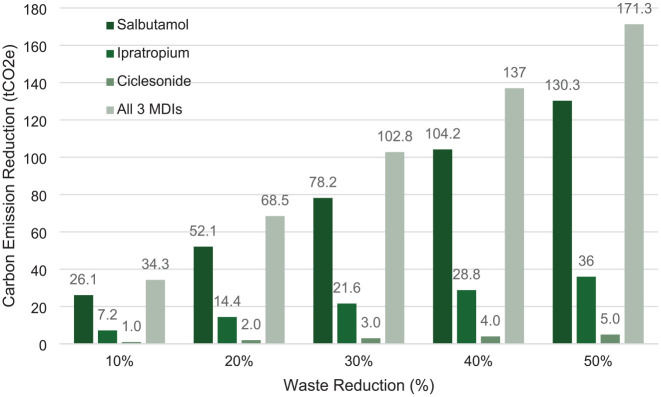
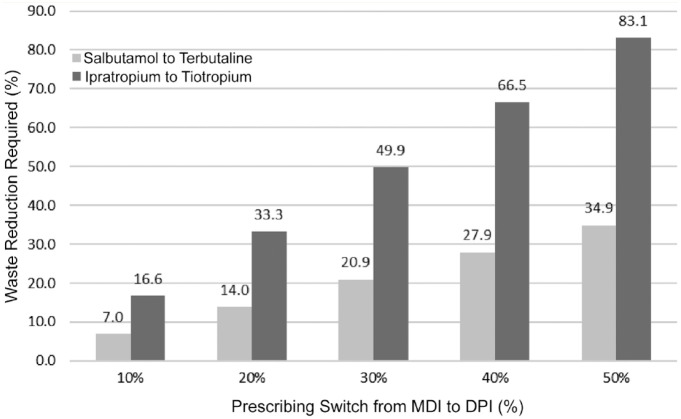
We then calculated that a 20% reduction in waste of formulary MDIs would result in a reduction of carbon emissions by 68.5 tCO2e (Figure 1). For example, reducing salbutamol waste by 14% would result in a cost savings that would allow for 20% of salbutamol MDI prescriptions to be changed to terbutaline DPI prescriptions without increasing net drug acquisition costs (Figure 2). Opportunities for waste reduction include decreasing the number of unnecessary prescriptions, decreasing the number of repeat dispenses from pharmacy, switching/adding DPI formulary alternatives, choosing the lowest strength MDI as formulary preference, and developing a procedure whereby unused MDIs returned to pharmacy are redeployed or sent home with the patient.
Figure 1.
Potential carbon emission savings from MDI waste reduction
Interpretation example: A 20% reduction in salbutamol MDI waste correlates with a carbon emission reduction of 52.1 tCO2e, whereas a 20% reduction in all MDI waste correlates with a carbon emission reduction of 68.5 tCO2e. MDI, metered-dose inhaler; tCO2e, tons of carbon dioxide equivalent.
Figure 2.
How much waste reduction is required to make a formulary switch from MDI to DPI cost-neutral?
Data provided using annual inventory purchasing reports. Ciclesonide MDI to fluticasone DPI not represented, as cost per device of fluticasone DPI is less than ciclesonide MDI. Interpretation example: This graph depicts the percentage in waste reduction (number of inhalers not prescribed and wasted) required to allow for different percentages of MDIs to be switched to their equivalent DPIs in a cost-neutral way. For example, a 14% reduction in salbutamol waste (14% less salbutamol actuations wasted) would be required in order to make a cost-neutral formulary switch from salbutamol MDI to terbutaline DPI in 20% of patients. DPI, dry powder inhaler; MDI, metered-dose inhaler.
Discussion
This study aimed to describe local MDI prescribing, dispensing, use and waste patterns. For the 100 patients included in our cohort, 315 MDIs were prescribed and dispensed in total, specifically 191 salbutamol, 116 ipratropium and 8 ciclesonide MDIs. We established that of these 315 MDIs or 61,440 actuations, 4667 were used and 56,773 actuations were wasted. The wasted doses accounted for 92% of dispensed doses. The secondary objective was to then estimate the costs of current local practices. When the results were applied to our local annual inventory and purchasing history (approximately 14,990 MDIs used in the previous year), we established that MDI waste contributes 315.8 tCO2e to our hospital’s greenhouse gas emissions. This is comparable to driving a car around the circumference of the planet 31 times.
Our study results are in accordance with previous studies looking at waste of MDIs, such as the studies previously cited that established 87% waste individually.7,8 The study by Sakaan et al., 8 however, did not look at the environmental cost in comparison to our study. In the study by Aeng et al., 7 the approximate cost of wasted inhalers for their sample population of 336 patients was $2156. They determined that the most common reasons for waste was no doses being administered after an inhaler was dispensed and extra dispenses of inhalers due to changes in prescribed dosing. 7 In comparison, our study established that for our 100-patient sample, there would be an associated economic cost of approximately $3056. Other studies have established input and output analyses 11 ; however, none to our knowledge have calculated total formulary MDI waste associated in the hospital setting and suggest how waste reduction could offset the costs associated with adding more environmentally friendly DPI alternatives to formulary. Although not evaluated in our study, Drummond et al. 12 also identified that 12.5% of all salbutamol and ipratropium inhalers were withdrawn unnecessarily from their automated dispensing cabinets, which may be a future direction in quality improvement initiatives.
Many studies have shown the importance of trying to mitigate the environmental impact of inhalers, such as a study by GlaxoSmithKline (GSK) identifying their HFC inhalers were 17 times higher in terms of global warming potential as compared with their DPIs. 13 In our current study, we identified that by switching 20% of formulary MDIs to DPI alternatives, we could reach cost-neutrality, and therefore benefit from having further DPI alternatives on our formulary. It must be acknowledged, however, that a switch from MDI to DPI is not appropriate for all patients. Some patients may not have the capacity or inspiratory strength to use a DPI. In acute settings such as for asthma exacerbations, an MDI may have a faster onset of action and can be nebulized or delivered to mechanically ventilated patients whereas DPIs cannot. 14 Regardless, we estimate that at least 20% of current MDI prescriptions would be appropriate for switching to DPI and thus this is our goal for future endeavours, which is supported by recent recommendations from the Canadian Thoracic Society. 6
Another article reported that by switching 1 in 10 MDIs prescribed in England for DPIs, 58 ktCO2e could be saved annually. 15 In our present study, we identified that we could reduce our hospital’s carbon emissions by 68.5 tCO2e annually by decreasing waste of all 3 formulary MDIs by 20%. The authors of this UK-based article also discussed that for each MDI switched to a DPI, there could be a reduction of carbon dioxide emissions of 150 to 400 kg per year, which would roughly be equivalent to an individual removing meat from their diet for the same time frame. 15 More recently, in 2019, an article discussed a switch from 1 MDI to 2 DPIs (therapeutic alternative combination), which resulted in an annual carbon footprint reduction of 422 kg of carbon dioxide emissions per patient. 16
Many organizations have identified inhaler waste as being a major contributor to the health care sector’s overall carbon footprint, and this study aims to add to the bank of knowledge and identify opportunities for our hospital and others to approach sustainable carbon cost-neutrality in the hospital setting. In our organization, we identified that fluticasone DPIs were in fact cheaper than the ciclesonide MDI we currently had on formulary, and that if we were to make a 50% switch, we could help cover the costs of introducing terbutaline to the formulary. We also established that if we substitute ciclesonide MDI from 200 µg/actuation to the less concentrated strength of 100 µg/actuation, we would further limit waste with regards to inhaled corticosteroids in addition to cost savings. We demonstrated that by decreasing waste of all 3 MDIs on formulary by 20%, we would reduce carbon emissions from our site by approximately 68.5 tCO2e, which is comparable to driving the circumference of the planet 6.8 times.
Other potential solutions to explore would be to purchase or lobby for smaller package sizes, in which inhalers would have fewer actuations and thus less waste. We found that on average, patients used 5 or less actuations from their salbutamol inhalers (which contain 200 actuations) and 26 actuations or less of the ipratropium MDI (which contain 200 actuations). Ciclesonide was also most often used as a scheduled maintenance treatment, whereas salbutamol and ipratropium were often used scheduled for a few doses (i.e., 2–4 inhalations every 4 hours × 3 doses), then switched to “as-needed” use only. This meant that for salbutamol and ipratropium, there were often a minimum of 2 prescriptions each, 1 for scheduled dosing and 1 for “as-needed” dosing. Every subsequent order (i.e., when patients are transferred between units) would result in an additional MDI being sent to the ward unless a team member changed the option in the electronic health record to “do not dispense,” which may have occurred but is not common practice. This could be an additional opportunity to reduce unnecessary prescribing and dispensing. One hundred percent of patients included in our cohort received at least 1 salbutamol inhaler; however, a median of only 5 actuations were used and almost 1 in 3 dispensed MDIs were not used at all.
Indication for use was not formally evaluated in this study; however, we hypothesize that the low use may suggest that the prescription was not actually needed, though this would have to be confirmed. Many patients were prescribed and dispensed the same MDIs several times, particularly when patients were being transferred between units or from room to room. Opportunities to ensure that medications, including inhalers, are transferred with the patient should be explored to minimize redispenses of MDIs.
One of the assumptions we made in this study is that unused doses and unused MDIs are all returned to pharmacy and discarded (incinerated at a local facility). It is possible that unused MDIs that were still in the box and unopened could be redeployed, but that is not our routine practice. MDIs are typically dispensed with the label on the device itself and then are not able to be reused as it is impossible to know whether actuations have been discharged. Partially used MDIs are always discarded and incinerated. Opportunities to minimize waste would be to change labelling processes such that the box gets labelled or the device is delivered in a tamper-evident container that might allow for redeployment of unused and returned devices. Furthermore, inhaler recycling programs should be identified and used as an alternative to throwing out partially used MDIs or sending for incineration.
Limitations
We anticipated and acknowledge some limitations to our methods. Although we attempted to obtain equal representation of medical and surgical patients, our recruitment strategy yielded primarily medicine patients. This may be because MDIs are prescribed more frequently in medicine patients and the fact that we excluded patients admitted for elective surgeries and those with less than 24-hour admissions. Next, we assumed that the proportion of waste identified in our cohort could be applied to the entire hospital. We acknowledge that this may be an inappropriate assumption. It is likely that use and waste vary from unit to unit. For example, it is likely that there is less waste in the ICU where mechanically ventilated patients receive 8 actuations of salbutamol and ipratropium per dose in a scheduled manner. Another limitation may be the assumption that unused inhalers or partially used MDIs were all returned to the pharmacy and discarded (incinerated at a local facility) as per hospital policy, whereas certain patients may have kept partially used inhalers or the inhalers may have been discarded in alternative ways on the wards. The estimates for carbon emissions used in our calculations were obtained from the CASCADES Detailed Inhaler Comparison Chart. 10 Carbon emissions vary between manufacturers, and our calculations are based on the brands used during the audit; however, we acknowledge that brands, particularly for generic products, may vary at other hospitals and likely within our hospital between purchase contracts.6,10 It is possible that we have overestimated or underestimated use and waste between units or in the hospital as a whole but it is very unlikely that opposite conclusions would be drawn had we increased the scope of the audit to the entire hospital. Another limitation is the accuracy of doses administered. Inhalers prescribed for self-administration may not have been recorded in the medication administration records, potentially amplifying our estimates of waste and revealing a common disadvantage of the retrospective nature of our data collection. Finally, we did not collect the indications for MDI prescriptions. If we had, we might be better equipped to identify opportunities to reduce unnecessary prescribing. A prospective audit might be better suited to accurately describe this information.
Conclusion
There are many opportunities to improve prescribing and dispensing of MDIs to reduce waste in the hospital setting. Our study identified that 92% of all MDI actuations are wasted and almost one-third of all MDIs dispensed are unused. By reducing waste, there would be inherent cost and carbon savings. Monetary savings could support the added costs of adding environmentally friendlier alternative inhalers and other quality improvement initiatives. Pharmacy professionals are central in helping to combat climate change through various initiatives within operations, research, education and advocacy. The health sector can make small changes that can have large effects regarding the reduction of carbon emissions and the improvement of climate change awareness. This project identifies areas for reduction of overall waste and reduction of unnecessary prescribing, which may lead to carbon emission savings in a cost-efficient manner. ■
Footnotes
Author Contributions: Each author contributed to the study design, interpretation of findings and review of the final draft. CC was responsible for data collection and the initial drafts of the protocol and final manuscript. SK was responsible for project supervision.
None declared by any author.
Funding and Financial Acknowledgments: None.
ORCID iD: Salmaan Kanji  https://orcid.org/0000-0003-0594-0360
https://orcid.org/0000-0003-0594-0360
Contributor Information
Carolanne Caron, Hôpital Montfort, Ottawa, ON; The Ottawa Hospital, Ottawa, ON.
Shellyza Sajwani, The Ottawa Hospital, Ottawa, ON.
Katherine Bateman, The Ottawa Hospital, Ottawa, ON.
Owen Degenhardt, The Ottawa Hospital, Ottawa, ON.
Mathilde Gaudreau-Simard, The Ottawa Hospital, Ottawa, ON; The Ottawa Hospital Research Institute, Ottawa, ON.
Smita Pakhale, The Ottawa Hospital, Ottawa, ON; The Ottawa Hospital Research Institute, Ottawa, ON.
Salmaan Kanji, The Ottawa Hospital, Ottawa, ON; The Ottawa Hospital Research Institute, Ottawa, ON.
References
- 1. Canada’s health system is among the least green. CMAJ News [Internet]. 2019. Available: https://cmajnews.com/2019/11/13/healthcare-emissions-1095834/ (accessed Nov. 14, 2022).
- 2. Dzau VJ, Levine R, Barrett G, Witty A. Decarbonizing the U.S. health sector—a call to action. N Engl J Med 2021;385(23):2117-9. [DOI] [PubMed] [Google Scholar]
- 3. Drugs & Devices. Peach Health Ontario [Internet]. Available: https://www.peachhealthontario.com/drugs-and-devices (accessed Nov. 14, 2022).
- 4. Primer–Inhalers . Cascades Canada [Internet]. 2021. Available: https://cascadescanada.ca/resources/all-topics/inhalers/primer-inhalers/ (accessed Nov. 14, 2022).
- 5. Asthma inhalers as bad for the environment as 180-mile car journey, health chiefs say [Internet]. The Telegraph, 2019. Available: https://www.telegraph.co.uk/news/2019/04/08/asthma-inhalers-bad-environment-180-mile-car-journey-health/ (accessed Nov. 14, 2022).
- 6. Gupta S, Couillard S, Digby G, et al. Canadian thoracic society position statement on climate change and choice of inhalers for patients with respiratory disease. Can J Respir Crit Care Sleep Med 2023;7(5):232-9. [Google Scholar]
- 7. Aeng ESY, McDougal KC, Allegretto-Smith EM, Tejani AM. Hidden costs of multiple-dose products: quantifying Ipratropium inhaler wastage in the hospital setting. Can J Hosp Pharm [Internet] 2021;74(2):117. Available: https://www.cjhp-online.ca/index.php/cjhp/article/view/3098 (accessed Sep. 25, 2023). [PMC free article] [PubMed] [Google Scholar]
- 8. Sakaan S, Ulrich D, Luo J, Finch CK, Self TH. Inhaler use in hospitalized patients with chronic obstructive pulmonary disease or asthma: assessment of wasted doses. Hosp Pharm 2015;50(5):386-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Good for you, good for us, good for everybody: a plan to reduce overprescribing to make patient care better and safer, support the NHS, and reduce carbon emissions [Internet]. Department of Health and Social Care, 2021. Available: https://assets.publishing.service.gov.uk/media/614a10fed3bf7f05ab786551/good-for-you-good-for-us-good-for-everybody.pdf (accessed Sep. 25, 2023). [Google Scholar]
- 10. Green S, Bursque G, Chang B, et al. Climate conscious inhaler prescribing in outpatient care version 3.0. CASCADES (Creating a Sustainable Canadian Health System in a Climate Crisis). 2023. Available://cascadescanada.ca/resources/sustainable-inhaler-prescribing-in-primary-care-playbook/ (accessed Aug. 25, 2023).
- 11. Steenmeijer MA, Rodrigues JFD, Zijp MC, Waaijers-van der Loop SL. The environmental impact of the Dutch health-care sector beyond climate change: an input-output analysis. Lancet Planet Health 2022;6(12):e949-57. [DOI] [PubMed] [Google Scholar]
- 12. Drummond I, Aeng ESY, Yeh P, Chen C, Tejani AM. Hiding in plain sight: quantifying salbutamol and ipratropium inhaler wastage in hospitals. Can J Hosp Pharm 2023;76(4):314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carbon Trust. GSK Carbon Trust Certification. 2014. Available: https://networks.sustainablehealthcare.org.uk/sites/default/files/media/GSK%20Carbon%20Trust%20Certification%202014.pdf (accessed Nov. 20, 2022).
- 14. Clark AR, Weers JG, Dhand R. The confusing world of dry powder inhalers: it is all about inspiratory pressures, not inspiratory flow rates. J Aerosol Med Pulm Drug Deliv 2020;33(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilkinson AJK, Braggins R, Steinbach I, Smith J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019;9(10):e028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janson C, Henderson R, Löfdahl M, Hedberg M, Sharma R, Wilkinson AJK. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax 2020;75(1):82-4. [DOI] [PMC free article] [PubMed] [Google Scholar]