Abstract
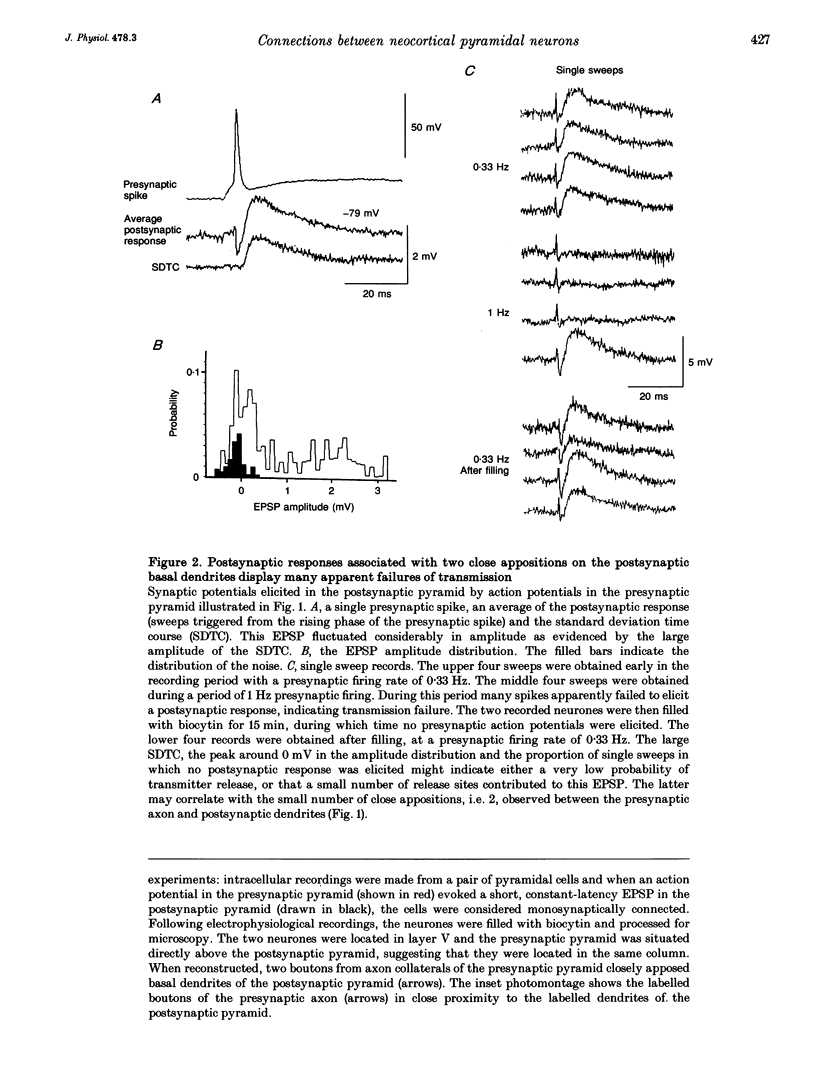
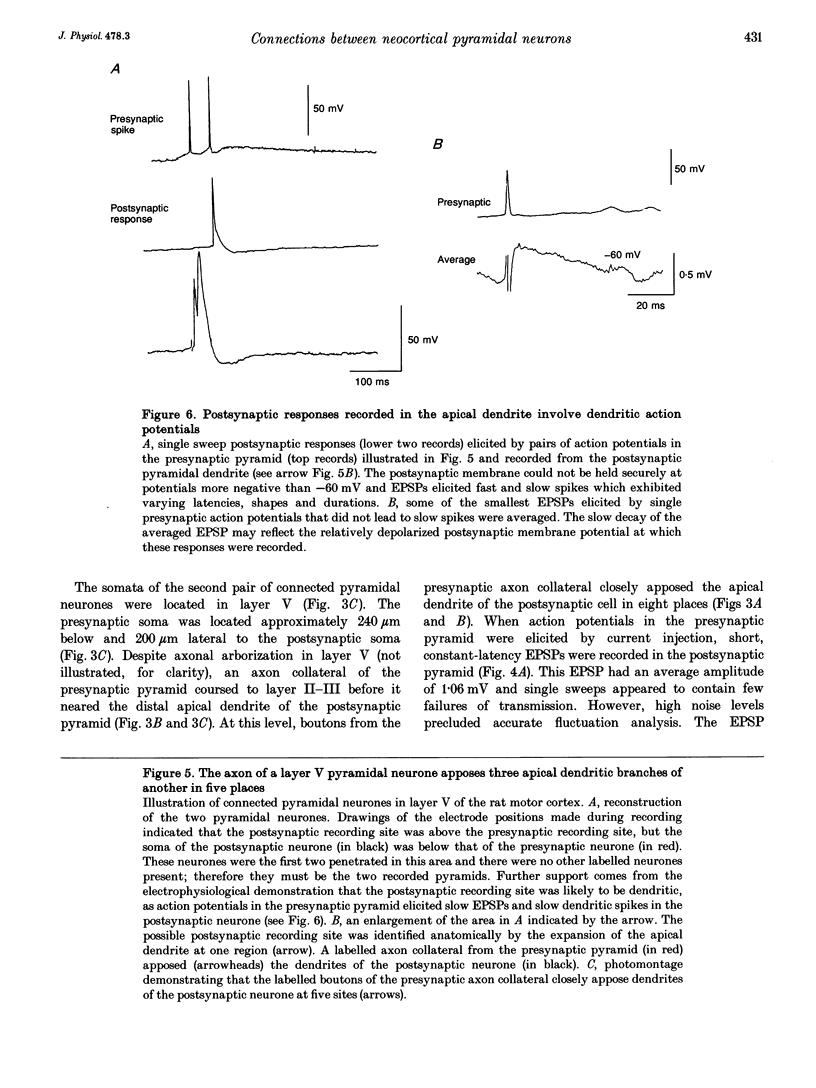
1. Double intracellular recordings were made from 1163 pairs of pyramidal neurones in layer V-VI of the rat somatomotor cortex in vitro using sharp electrodes filled with biocytin. Monosynaptically connected pairs of cells were identified when an action potential in one could elicit a constant latency excitatory postsynaptic potential (EPSP) in the other and the cells were filled with biocytin. Labelled cells were subsequently identified histologically with avidin-horseradish peroxidase. 2. Thirty-four pairs of cells were found to be monosynaptically connected. Fifteen of these pairs were sufficiently stable for electrophysiological recordings and three of these were recovered sufficiently to permit full morphological reconstruction. 3. The EPSP recorded between the first pair of pyramids varied in amplitude between 0 and 3 mV (mean 1.33 +/- 1.06 mV) and fluctuated considerably (coefficient of variation, 0.796). This was largely due to a high incidence of apparent failures of transmission. On reconstruction two boutons from the presynaptic pyramid axon were in close apposition to the proximal portions of basal dendrites of the postsynaptic cell. 4. In the second pair of pyramids the EPSP had a mean amplitude of 1.06 mV, and displayed a 10-90% rise time of 2.8 ms and a width at half-amplitude of 23 ms. This EPSP did not alter significantly with changes in membrane potential at the soma. The presynaptic axon closely apposed the distal apical dendrite of the postsynaptic cell in eight places. 5. In the third pair of pyramids, the EPSPs, recorded at a relatively depolarized membrane potential, were long lasting and could elicit slow dendritic spikes with long and variable latencies. These slow spikes suggested that the postsynaptic recording site was dendritic and on reconstruction a possible location was identified on the apical dendrite. A total of five presynaptic boutons closely apposed three separate, proximal branches of the postsynaptic apical dendrite. 6. These results provide the first illustration of a morphological basis for variations in functional properties of pyramid-pyramid connections in the neocortex.
Full text
PDF


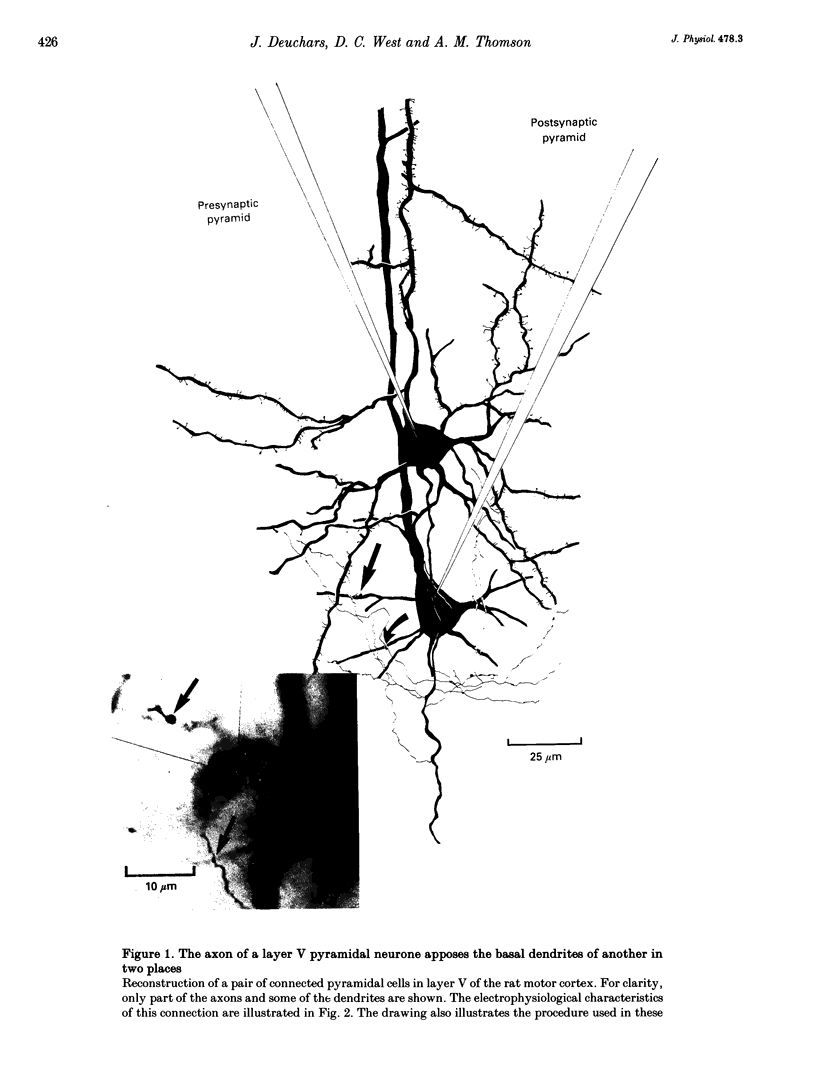
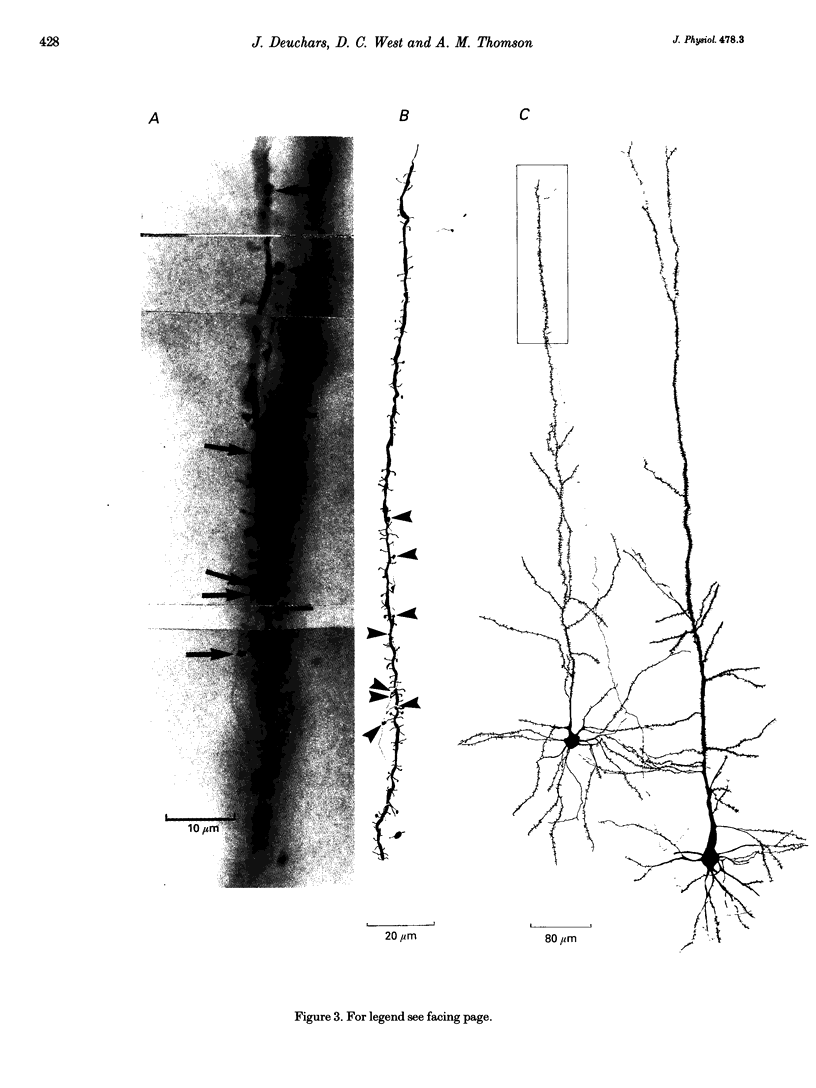
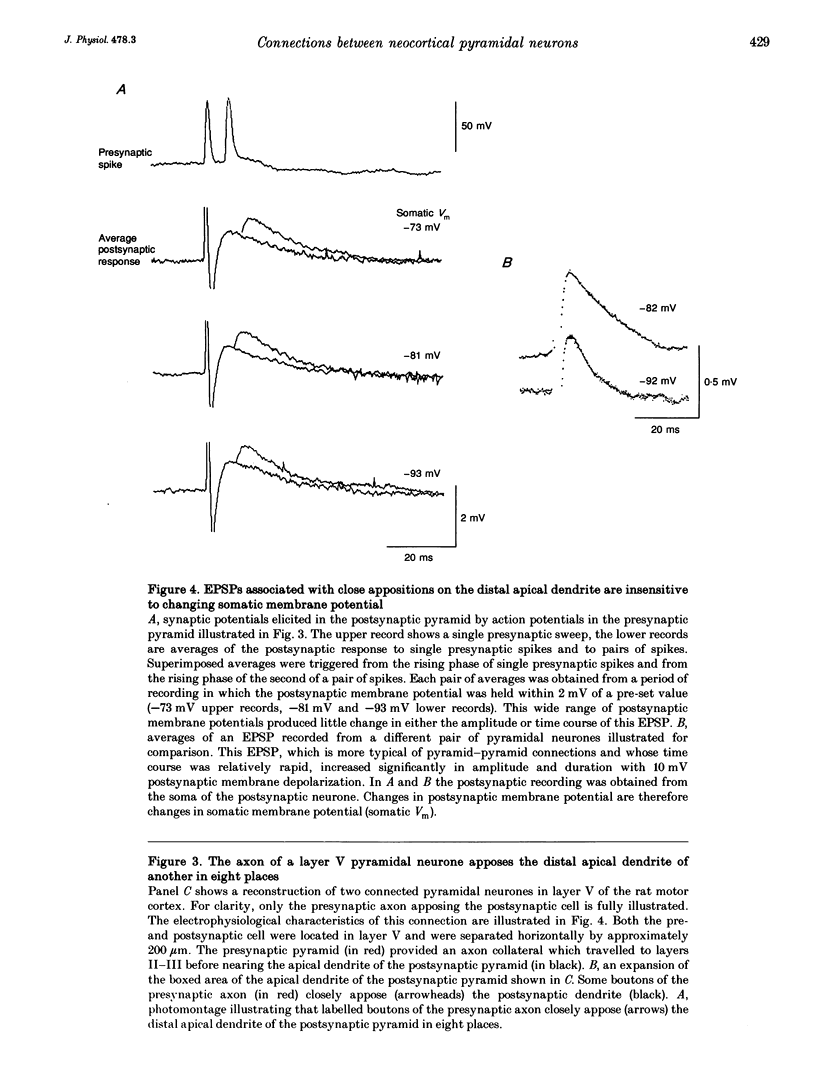




Images in this article
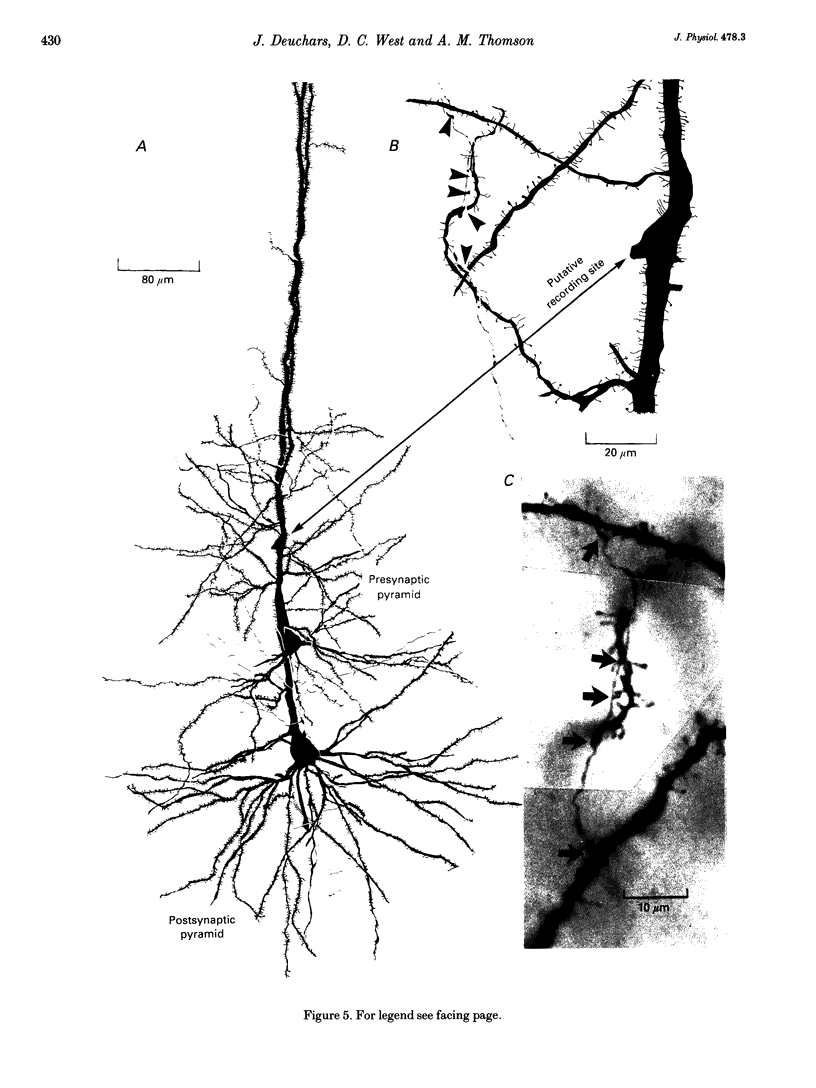
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amitai Y., Friedman A., Connors B. W., Gutnick M. J. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993 Jan-Feb;3(1):26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- DeFelipe J., Fariñas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992 Dec;39(6):563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- Gabbott P. L., Martin K. A., Whitteridge D. Connections between pyramidal neurons in layer 5 of cat visual cortex (area 17). J Comp Neurol. 1987 May 15;259(3):364–381. doi: 10.1002/cne.902590305. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvárday Z. F., Eysel U. T. Cellular organization of reciprocal patchy networks in layer III of cat visual cortex (area 17). Neuroscience. 1992;46(2):275–286. doi: 10.1016/0306-4522(92)90050-c. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Freund T. F., Maglóczky Z., Whitteridge D., Somogyi P. Synaptic targets of HRP-filled layer III pyramidal cells in the cat striate cortex. Exp Brain Res. 1986;64(3):541–552. doi: 10.1007/BF00340492. [DOI] [PubMed] [Google Scholar]
- Kojima M., Iwatsuki N., Data E. S., Villegas C. D., Uritani I. Changes of cyanide content and linamarase activity in wounded cassava roots. Plant Physiol. 1983 May;72(1):186–189. doi: 10.1104/pp.72.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. Form, function and intracortical projections of spiny neurones in the striate visual cortex of the cat. J Physiol. 1984 Aug;353:463–504. doi: 10.1113/jphysiol.1984.sp015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Nicoll A., Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci. 1991 Jan;11(1):72–84. doi: 10.1523/JNEUROSCI.11-01-00072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGuire B. A., Gilbert C. D., Rivlin P. K., Wiesel T. N. Targets of horizontal connections in macaque primary visual cortex. J Comp Neurol. 1991 Mar 15;305(3):370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Noda T., Yamamoto T. Response properties and morphological identification of neurons in the cat motor cortex. Brain Res. 1984 Jul 23;306(1-2):197–206. doi: 10.1016/0006-8993(84)90369-x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pockberger H. Electrophysiological and morphological properties of rat motor cortex neurons in vivo. Brain Res. 1991 Jan 25;539(2):181–190. doi: 10.1016/0006-8993(91)91619-c. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Holmes W. R., Jack J. J., Redman S. J., Segev I. Matching dendritic neuron models to experimental data. Physiol Rev. 1992 Oct;72(4 Suppl):S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- SHOLL D. A. The organization of the visual cortex in the cat. J Anat. 1955 Jan;89(1):33–46. [PMC free article] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. THE USE OF DEGENERATION METHODS IN THE INVESTIGATION OF SHORT NEURONAL CONNEXIONS. Prog Brain Res. 1965;14:1–32. [PubMed] [Google Scholar]
- Szentágothai J. The 'module-concept' in cerebral cortex architecture. Brain Res. 1975 Sep 23;95(2-3):475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., Deuchars J., West D. C. Large, deep layer pyramid-pyramid single axon EPSPs in slices of rat motor cortex display paired pulse and frequency-dependent depression, mediated presynaptically and self-facilitation, mediated postsynaptically. J Neurophysiol. 1993 Dec;70(6):2354–2369. doi: 10.1152/jn.1993.70.6.2354. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Fluctuations in pyramid-pyramid excitatory postsynaptic potentials modified by presynaptic firing pattern and postsynaptic membrane potential using paired intracellular recordings in rat neocortex. Neuroscience. 1993 May;54(2):329–346. doi: 10.1016/0306-4522(93)90256-f. [DOI] [PubMed] [Google Scholar]