Abstract
Background:
Evaluating the risk factors for intracerebral hemorrhage is indispensable for primary prevention. However, the pathogenesis varies depending on the bleeding site, and few prospective studies have explored risk factors in detail for each site.
Participants and methods:
The Japan Public Health Center-based Prospective Study is a prospective study comprising a population-based sample of Japanese adults in 1990 (Cohort I) and in 1993 (Cohort II). A total of 34,137 participants (11,907 men and 22,230 women) were enrolled in this study and followed up until 2009 for Cohort I and until 2012 for Cohort II. The association between risk factors (age, sex, blood pressure, serum cholesterol, triglycerides, blood glucose, body mass index, smoking, and drinking status) and intracerebral hemorrhage by its bleeding site (lobes, putamen, thalamus, cerebellum, and brainstem) was assessed using Cox proportional hazards analysis.
Results:
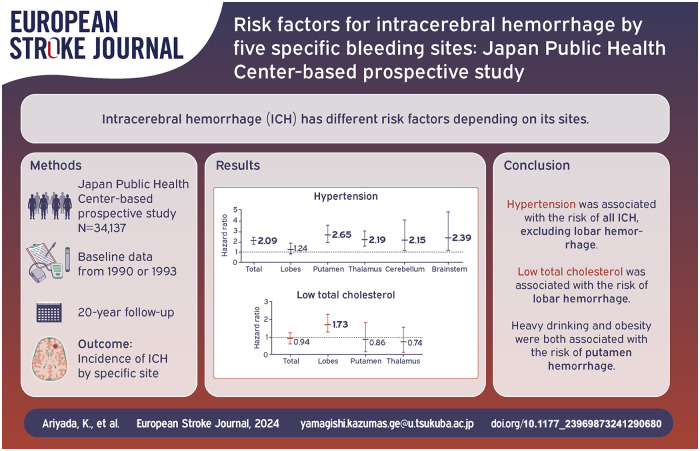
During a median 20-year follow-up, 571 intracerebral hemorrhage events occurred. Hypertension was associated with an increased risk of total intracerebral hemorrhage, but not lobar hemorrhage. The multivariable hazard ratio (95% confidence intervals) was 2.09 (1.75–2.50) for total intracerebral hemorrhage. In contrast, a low serum total cholesterol level was associated only with lobar hemorrhage (1.73 (1.01–2.96)). Heavy drinking was associated with the risk of total and putamen hemorrhage, and obesity was associated with the risk of putamen hemorrhage.
Discussion and conclusion:
The present study identified different risk factors depending on the bleeding site of intracerebral hemorrhage.
Keywords: Intracerebral hemorrhage, hypertension, serum cholesterol, amyloid angiopathy, risk factors, epidemiology
Graphical abstract.
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a life-threatening illness because effective treatments are limited depending on its size and site. Although ICH accounts for less than 30% of all strokes,1,2 the case fatality rate and the rate of resulting disabilities are very high; ICH fatality at 1 month was about 40%, and the rate of functional independence ranged from 12% to 39%. 3 To deal with healthcare and welfare problems associated with ICH, evaluating risk factors for primary prevention is a priority on a global scale. 4
The major risk factors for ICH include age, hypertension, high alcohol intake, and obesity.3,5–11 Of these, hypertension is the most important risk factor for etiology of ICH.5–7 Low serum total cholesterol levels are another potential risk factor for ICH. 12 However, the pathogenesis, prognosis, and operative procedure differ among bleeding sites, and few prospective studies have characterized the risk factors for site-specific ICH, beyond the broad classification into two categories: deep and lobar hemorrhages. 13 Unlike previous studies, we decided to examine risk factors for ICH by detailed anatomic site of hemorrhage with different responsible vessels as this may be important in the search for more targeted ICH prevention measures.
To assess the risk factors of ICH, we explored the data from the Japan Public Health Center-based Prospective (JPHC) study, which had a large registry of stroke cases in a single cohort of middle-aged Japanese men and women since the 1990s. In countries where the incidence of ICH is low, such as in the United States and Europe, such studies are difficult to conduct, and we consider that it would be significant to examine this topic in the Japanese population, where ICH is more prevalent. 14 We sought to investigate the association of several risk factors with five site-specific ICH in a large cohort of the Japanese population.
Participants and methods
Study design and population
The JPHC study is a prospective study comprising a population-based sample of 140,420 Japanese adults (68,722 men and 71,698 women) in 1990 (Cohort I) and 1993 (Cohort II). 15 Although the coverage was mainly restricted to rural areas, its large scale, with coverage across various regions throughout Japan, is a notable feature of this study. Cohort I was initiated in 5 public health center areas (Iwate, Akita, Tokyo, Nagano, and Okinawa-Chubu), with 61,595 participants aged 40–59, and Cohort II was started in 6 public health center areas (Ibaraki, Niigata, Osaka, Kochi, Nagasaki, and Okinawa-Miyako), with 78,825 participants aged 40–69. Two areas (Tokyo and Osaka) were excluded from the present study because stroke incidence data were unavailable. The participants of the two cohorts were followed up until December 31, 2009, and December 31, 2012, respectively. Of these, 34,295 participants (12,010 men and 22,285 women) who had complete health check-up data (i.e. body height, weight, and blood pressure) were enrolled in the present analysis. We excluded 158 participants (42 in Cohort I and 116 in Cohort II) with a history of stroke. In the end, a total of 34,137 persons were included in this study.
Baseline measurements
A self-administered baseline questionnaire on lifestyle and medical history was presented to participants in 1990 for Cohort I and in 1993 and 1994 for Cohort II. The questionnaire included questions on smoking habits, alcohol consumption, past medical history, and prescriptions.
Smoking status was defined as never smoking (reference), past smoking, smoking less than 20 cigarettes per day, and smoking 20 or more cigarettes per day. The weekly ethanol consumption was estimated by considering the frequency of drinking, the average quantity consumed per occasion, and the types of alcoholic beverages consumed. Drinking status was defined as non-drinkers (reference), occasional drinkers (1–3 times per week), persons who drank less than 150 g per week, those drinking between 150 g and 300 g per week, and those drinking 300 g or more per week. Blood pressure was measured in the right arm of the seated participant using a standard mercury sphygmomanometer, after resting for at least 5 min. Hypertension was defined as systolic blood pressure (BP) equal to or greater than 140 mmHg, diastolic BP equal to or greater than 90 mmHg, and/or the use of antihypertensive medication. Body mass index (BMI) was calculated as the weight (kg) divided by the square of height (m2). BMI was categorized as less than 18.5 kg/m2, between 18.5 kg/m2 and 25 kg/m2 (reference), and equal to or greater than 25 kg/m2.
Serum total cholesterol (n = 34,075) and triglycerides (n = 25,251) were measured using conventional enzyme-based methods in 23 laboratories. According to the Osaka Medical Center for Health Science and Promotion, a member of the Cholesterol Reference Method Laboratory Network,16,17 the precision and accuracy of the lipid measurements in all laboratories were satisfactory. Total cholesterol level was categorized as less than 160, between 160 and 240 (reference), and equal to or greater than 240 mg/dL. High total cholesterol was defined as equal to or greater than 240 mg/dL and/or the use of dyslipidemia medication. The cut-off values were based on scientific data indicating a higher risk of ICH. 12 Fasting was defined as abstaining from all food and drink, except water, for a period of more than 8 h. The cut-off points for fasting triglycerides and non-fasting triglycerides were 150 mg/dL and 175 mg/dL, respectively. 18 Serum glucose levels were obtained from 28,714 participants. Diabetes mellitus was defined as a fasting glucose level equal to or greater than 126 mg/dL, a non-fasting glucose level equal to or greater than 200 mg/dL, and/or the use of diabetes medication.
Confirmation of stroke
Stroke events were registered in nine public health centers. A total of 81 hospitals were included in this study as the major medical institutions in these public health center areas. Nearly all hospitals were equipped with imaging technologies, including computed tomography and magnetic resonance imaging scanners and had the capacity to provide treatment during the acute stage of stroke. After extracting cases with potential stroke onset according to information provided by the hospitals, physicians, hospital workers, or investigators, who were blinded to the patients’ lifestyle data, we reviewed the patients’ medical records and created onset registration forms. For cases screened based on the discharge diagnosis, the physicians confirmed the diagnosis of stroke based on the criteria outlined in the National Survey of Stroke, 19 which require the presence of sudden or rapid-onset focal neurologic deficits lasting at least 24 h, or until death. Only first-ever onsets were recorded as incidents of stroke and classified as subarachnoid hemorrhage, cerebral infarction, or ICH with bleeding sites (lobes, putamen, thalamus, cerebellum, brainstem, or multiple hemorrhage sites). We defined as unclassifiable cases those cases with large and multiple ICH making it difficult to identify the source of bleeding as well as ICH with inaccessible image data. To examine each ICH-specific risk factor, we analyzed the ICH records by bleeding site. Unclassifiable cases (n = 31) were included in the total ICH but were not considered in the site-specific analysis.
Statistical analysis
Age-adjusted mean values or prevalence of baseline health checkups were compared between participants with ICH events and those without using analysis of covariance or logistic regression analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) for ICH and site-specific ICH incidence were calculated with the reference group, except for age, using Cox proportional hazards models. Person-years were calculated as the sum of individual follow-up durations until stroke event, death, emigration, or end of the follow-up, whichever occurred first. For subarachnoid hemorrhage and cerebral infarction, follow-up was censored at the time of their onset. We adjusted for age and sex in the first model to calculate HRs, and the second model was further adjusted for systolic blood pressure, the use of antihypertensive medication, low total cholesterol, high total cholesterol, hypertriglyceridemia, diabetes, body weight, smoking, and drinking status in the multivariable-adjusted model. When hypertension was considered the explanatory variable, systolic blood pressure and the use of antihypertensive medication were excluded from the models. Since there was no regional difference in the associations between risk factors and any outcomes, and the numbers of cases were limited for some outcomes, community was not included in the formal models. Of note, including community in the models did not change the results for any outcomes.
All statistical tests were two-sided, and p values less than 0.05 were considered statistically significant. SAS (version 9.4, SAS Institute, Cary, NC, USA) was used for all analyses.
Results
During a 20-year median follow-up and the 615,542 person-years, there were 571 ICH events including 106 lobar hemorrhages, 194 putamen hemorrhages, 163 thalamic hemorrhages, 43 cerebellar hemorrhages, 34 brainstem hemorrhages, and 31 unclassifiable hemorrhages. Of these, 9.4%, 11.3%, 7.4%, 25.6%, 38.2%, and 41.9% cases of lobar, putamen, thalamic, cerebellar, brainstem, and unclassifiable hemorrhage, respectively, died within 28 days of onset.
Table 1 shows the age-adjusted means and prevalence of baseline characteristics for total ICH events and their sites, and for non-cases. The mean values of systolic and diastolic blood pressure, and the prevalence of hypertension were higher among men and women with total ICH than among those without stroke. The mean values of BMI and the prevalence of obesity and ethanol consumption of less than 150 g/week and equal to or greater than 300 g/week was higher only among men with total ICH. In terms of the supratentorial brain, men and women with ICH had a significantly higher prevalence of hypertension than subjects without stroke apart from ICH located in the lobes. As for the infratentorial brain, there was a higher prevalence of hypertension only among men with ICH located in the brainstem.
Table 1.
Age-adjusted baseline characteristics among participants who did or did not develop incident intracerebral hemorrhage, JPHC.
| Clinical characteristics | Intracerebral hemorrhage | Bleeding site | Noncases | ||||
|---|---|---|---|---|---|---|---|
| Lobes | Putamen | Thalamus | Cerebellum | Brainstem | |||
| Men (n = 11,907) | |||||||
| No. of cases | 283 | 43 | 107 | 80 | 18 | 24 | 10,962 |
| 28-day mortality, % | 14.8 | 14.0 | 8.4 | 10.0 | 16.7 | 37.5 | |
| Age*, years | 56.6 # | 60.3 # | 54.7 | 55.9 § | 59.9 || | 56.8 | 54.2 |
| Systolic BP, mmHg | 141.7 # | 135.4 | 141.9 # | 141.2 # | 144.3 || | 149.7 # | 132.3 |
| Diastolic BP, mmHg | 85.3 # | 81.9 | 86.3 # | 85.7 # | 84.7 | 88.9 # | 80.0 |
| Hypertension † , % | 64.3 # | 53.5 | 68.2 # | 63.8 # | 66.7 | 66.7 || | 42.2 |
| Total cholesterol, mg/dL | 192.6 | 184.2 | 195.9 | 192.6 | 184.9 | 200.3 | 193.3 |
| <160 mg/dL, % | 12.0 | 23.3 | 9.4 § | 11.3 | 5.6 | 8.3 | 15.5 |
| ⩾240 mg or dyslipidemia treatment, % | 8.1 | 9.3 | 10.3 | 7.5 | 0 | 8.3 | 9.9 |
| Triglyceride, mg/dL | 140.6 | 113.4 | 141.1 | 157.0 § | 154.5 | 109.6 | 136.3 |
| ⩾150 mg/dL (fasting) or ⩾175 mg/dL (non-fasting), % | 17.3 | 16.3 | 20.6 | 18.8 | 5.6 | 4.2 | 18.2 |
| Diabetes mellitus ‡ , % | 5.7 | 11.6 § | 4.7 | 5.0 | 0 | 4.2 | 4.2 |
| BMI, kg/m2 | 23.9 || | 23.3 | 24.5 # | 23.9 | 23.2 | 23.4 | 23.5 |
| ⩾25 kg/m2, % | 32.2 || | 25.6 | 42.1 # | 28.8 | 27.8 | 20.8 | 27.2 |
| <18.5 kg/m2, % | 2.8 | 7.0 | 0.9 | 2.5 | 5.6 | 4.2 | 2.4 |
| Drinking status | |||||||
| Occasional, % | 5.0 § | 7.0 | 6.5 | 2.5 § | 5.6 | 4.2 | 8.4 |
| <150 g/week, % | 11.3 || | 14.0 | 8.4 || | 15.0 | 0 | 20.8 | 19.2 |
| 150–300 g/week, % | 21.9 | 16.3 | 22.4 | 21.3 | 50.0 || | 12.5 | 20.2 |
| ⩾300 g/week, % | 33.9 # | 20.9 | 42.1 # | 31.3 | 22.2 | 37.5 | 25.4 |
| Smoking status | |||||||
| Past, % | 9.5 | 7.0 | 9.4 | 11.3 | 5.6 | 12.5 | 11.8 |
| <20 cigarettes/day, % | 12.4 | 7.0 | 12.2 | 13.8 | 16.7 | 16.7 | 13.3 |
| ⩾20 cigarettes/day, % | 27.6 | 27.9 | 27.1 | 30.0 | 27.8 | 20.8 | 30.9 |
| Women (n = 22,230) | |||||||
| No. of cases | 288 | 63 | 87 | 83 | 25 | 10 | 21,194 |
| 28-day mortality, % | 13.5 | 6.4 | 14.9 | 4.8 | 32.0 | 40.0 | |
| Age*, years | 57.9 # | 58.3 # | 57.9 # | 57.3 # | 59.1 # | 52.8 | 53.8 |
| Systolic BP, mmHg | 139.5 # | 135.0 | 142.9 # | 139.3 # | 140.8 || | 136.1 | 129.2 |
| Diastolic BP, mmHg | 82.5 # | 81.0 || | 83.7 # | 82.0 # | 83.9 || | 81.2 | 76.9 |
| Hypertension † , % | 59.7 # | 47.6 | 66.7 # | 59.0 || | 64.0 § | 50.0 | 35.7 |
| Total cholesterol, mg/dL | 210.3 | 205.4 | 210.6 | 208.1 | 224.2 § | 186.5 § | 205.5 |
| <160 mg/dL, % | 7.6 | 11.1 § | 9.2 | 4.8 | 4.0 | 20.0 | 8.2 |
| ⩾240 mg or dyslipidemia treatment, % | 22.9 | 12.7 | 25.3 | 20.5 | 44.0 || | 0 | 17.2 |
| Triglyceride, mg/dL | 121.6 | 121.2 | 116.1 | 120.7 | 131.1 | 107.0 | 114.6 |
| ⩾150 mg/dL (fasting) or ⩾175 mg/dL (non-fasting), % | 12.9 | 9.5 | 11.5 | 15.7 | 12.0 | 0 | 11.6 |
| Diabetes mellitus ‡ , % | 2.4 | 0 | 3.5 | 3.6 | 0 | 10.0 | 2.14 |
| BMI, kg/m2 | 24.0 | 23.9 | 24.0 | 23.8 | 24.2 | 24.0 | 23.6 |
| ⩾25 kg/m2, % | 34.4 | 25.4 | 36.8 | 30.1 | 40.0 | 40.0 | 29.9 |
| <18.5 kg/m2, % | 1.0 § | 0 | 2.3 | 0 | 0 | 0 | 2.8 |
| Drinking status | |||||||
| Occasional, % | 7.3 | 9.5 | 2.3 | 12.1 § | 8.0 | 10.0 | 8.6 |
| <150 g/week, % | 4.2 | 1.6 | 5.8 | 3.6 | 4.0 | 10.0 | 6.4 |
| 150–300 g/week, % | 0.7 | 1.6 | 1.2 | 0 | 0 | 0 | 0.9 |
| ⩾300 g/week, % | 0.4 | 1.6 | 0 | 0 | 0 | 0 | 0.5 |
| Smoking status | |||||||
| Past, % | 0.7 | 0 | 2.3 || | 0 | 0 | 0 | 0.6 |
| <20 cigarettes/day, % | 2.4 | 3.2 | 1.2 | 1.2 | 4.0 | 20.0 || | 2.2 |
| ⩾20 cigarettes/day, % | 1.0 | 1.6 | 1.2 | 0 | 0 | 0 | 0.7 |
Values indicate means or prevalence, adjusted for age.
Unadjusted.
Systolic blood pressure ⩾ 140 mmHg, diastolic blood pressure ⩾ 90 mmHg, or antihypertensive medication use.
Fasting glucose level ⩾ 126 mg/dL, non-fasting glucose level ⩾ 200 mg/dL, or the use of diabetes medication.
p < 0.1, ||p < 0.05, #p < 0.001 (difference from noncases).
The age- and sex-adjusted HRs for total ICH and its sites in the presence of each risk factor are shown in Supplemental Table S1. Men had a higher risk of total ICH, putamen, thalamic, and brainstem hemorrhages than women. Hypertension was significantly associated with the risk of all ICH, excluding lobar hemorrhage. In contrast, low total cholesterol was associated only with the risk of lobar hemorrhage. Obesity and ethanol consumption equal to or greater than 300 g per week were associated with a risk of total ICH and putamen hemorrhage, and ethanol consumption of between 150 and 300 g per week was associated with cerebellar hemorrhage. No significant association was detected between smoking status and the risk of ICH with any hemorrhage site.
After further adjustment for cardiovascular risk factors (Table 2), the associations were shown to be largely similar: Men had a higher risk of total ICH (multivariable HR = 1.88 (1.47–2.40)), putamen hemorrhage (2.12 (1.38–3.26)), thalamic hemorrhage (1.85 (1.18–2.91)), and brainstem hemorrhage (4.21 [1.54–11.47]) than women. The multivariable HRs (95% CI) for hypertension were 2.09 (1.75–2.50) for total ICH, 2.65 (1.94–3.63) for putamen hemorrhage, 2.19 (1.57–3.04) for thalamic hemorrhage, 2.15 (1.12–4.12) for cerebellar hemorrhage, and 2.39 (1.16–4.93) for brainstem hemorrhage. Low total cholesterol was only associated with the risk of lobar hemorrhage (1.73 (1.01–2.96)), and the HR did not change materially when the participants with dyslipidemia treatment (n = 690) were excluded (1.70 (0.98–2.94)). Obesity was associated with the risk of putamen hemorrhage (1.39 (1.03–1.88)). As for drinking status, ethanol consumption equal to or greater than 300 g per week was associated with the risk of total ICH (1.36 (1.02–1.82)) and putamen hemorrhage (1.94 (1.20–3.13)), and ethanol consumption of between 150 and 300 g per week was associated with the risk of cerebellar hemorrhage (3.01 (1.04–8.70)).
Table 2.
Multivariate-adjusted HRs and 95% CIs for risk factors in relation to intracerebral hemorrhage locations, JPHC.
| Clinical characteristics | No. at risk | Person-years | Intracerebral hemorrhage (n = 571) | Bleeding site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lobes (n = 106) | Putamen (n = 194) | Thalamus (n = 163) | Cerebellum (n = 43) | Brainstem (n = 34) | ||||||||||
| No. of cases | Multivariable HR (95% CI)* | No. of cases | Multivariable HR (95% CI)* | No. of cases | Multivariable HR (95% CI)* | No. of cases | Multivariable HR (95% CI)* | No. of cases | Multivariable HR (95% CI)* | No. of cases | Multivariable HR (95% CI)* | |||
| Age | 1.05 (1.04–1.06) | 1.10 (1.07–1.14) | 1.03 (1.00–1.05) | 1.03 (1.01–1.06) | 1.10 (1.05–1.16) | 1.03 (0.97–1.08) | ||||||||
| Sex | ||||||||||||||
| Women | 22,230 | 408,744 | 288 | 1 (Reference) | 63 | 1 (Reference) | 87 | 1 (Reference) | 83 | 1 (Reference) | 25 | 1 (Reference) | 10 | 1 (Reference) |
| Men | 11,907 | 206,798 | 283 | 1.88 (1.47–2.40) | 43 | 1.53 (0.88–2.66) | 107 | 2.12 (1.38–3.26) | 80 | 1.85 (1.18–2.91) | 18 | 1.17 (0.44–3.12) | 24 | 4.21 (1.54–11.47) |
| Blood pressure | ||||||||||||||
| Nonhypertension | 20,749 | 380,453 | 217 | 1 (Reference) | 53 | 1 (Reference) | 63 | 1 (Reference) | 63 | 1 (Reference) | 15 | 1 (Reference) | 13 | 1 (Reference) |
| Hypertension † | 13,388 | 235,089 | 354 | 2.09 (1.75–2.50) | 53 | 1.24 (0.83–1.84) | 131 | 2.65 (1.94–3.63) | 100 | 2.19 (1.57–3.04) | 28 | 2.15 (1.12–4.12) | 21 | 2.39 (1.16–4.93) |
| Total cholesterol | ||||||||||||||
| 160–240 mg/dL | 19,655 | 355,651 | 321 | 1 (Reference) | 59 | 1 (Reference) | 104 | 1 (Reference) | 98 | 1 (Reference) | 20 | 1 (Reference) | 22 | 1 (Reference) |
| <160 mg/dL | 3,638 | 64,540 | 56 | 0.94 (0.71–1.24) | 17 | 1.73 (1.01–2.96) | 18 | 0.86 (0.52–1.41) | 13 | 0.74 (0.41–1.31) | 2 | 4 | – | |
| ⩾240 mg or dyslipidemia treatment | 5,068 | 91,482 | 89 | 0.99 (0.78–1.24) | 12 | 0.70 (0.38–1.30) | 33 | 1.10 (0.75–1.62) | 23 | 0.85 (0.54–1.34) | 11 | 1.77 (0.87–3.60) | 2 | – |
| Triglyceride | ||||||||||||||
| <150 mg/dL (fasting) or <175 mg/dL (non-fasting) | 20,470 | 366,915 | 337 | 1 (Reference) | 69 | 1 (Reference) | 110 | 1 (Reference) | 93 | 1 (Reference) | 28 | 1 (Reference) | 18 | 1 (Reference) |
| ⩾150 mg/dL (fasting) or ⩾175 mg/dL (non-fasting) | 4,781 | 84,517 | 86 | 0.92 (0.72–1.17) | 13 | 0.87 (0.47–1.59) | 32 | 0.92 (0.62–1.39) | 28 | 1.10 (0.71–1.70) | 4 | – | 1 | – |
| Blood glucose | ||||||||||||||
| Nondiabetes | 27,669 | 500,385 | 448 | 1 (Reference) | 80 | 1 (Reference) | 152 | 1 (Reference) | 131 | 1 (Reference) | 33 | 1 (Reference) | 28 | 1 (Reference) |
| Diabetes ‡ | 1,045 | 16,986 | 23 | 1.05 (0.69–1.61) | 5 | 1.42 (0.57–3.54) | 8 | 1.04 (0.51–2.12) | 7 | 1.11 (0.51–2.39) | 0 | – | 2 | – |
| Boby weight | ||||||||||||||
| 18.5–25 kg/m2 | 23,240 | 418,899 | 370 | 1 (Reference) | 76 | 1 (Reference) | 114 | 1 (Reference) | 113 | 1 (Reference) | 27 | 1 (Reference) | 24 | 1 (Reference) |
| ⩾25 kg/m2 | 9,994 | 180,983 | 190 | 1.08 (0.90–1.29) | 27 | 0.85 (0.54–1.33) | 77 | 1.39 (1.03–1.88) | 48 | 0.84 (0.59–1.18) | 15 | 1.21 (0.63–2.32) | 9 | 0.79 (0.36–1.74) |
| <18.5 kg/m2 | 903 | 15,660 | 11 | 0.83 (0.46–1.52) | 3 | – | 3 | – | 2 | – | 1 | – | 1 | – |
| Drinking status (ethanol) | ||||||||||||||
| None | 21,710 | 394,064 | 329 | 1 (Reference) | 72 | 1 (Reference) | 99 | 1 (Reference) | 94 | 1 (Reference) | 26 | 1 (Reference) | 14 | 1 (Reference) |
| Occasional | 2,873 | 52,818 | 35 | 0.87 (0.61–1.24) | 9 | 1.19 (0.58–2.43) | 9 | 0.69 (0.34–1.39) | 12 | 0.99 (0.53–1.83) | 3 | – | 2 | – |
| <150 g/week | 3,658 | 65,990 | 44 | 0.65 (0.46–0.91) | 7 | 0.53 (0.24–1.21) | 14 | 0.66 (0.36–1.20) | 15 | 0.74 (0.41–1.33) | 1 | – | 6 | 1.20 (0.41–3.52) |
| 150–300 g/week | 2,631 | 45,831 | 64 | 1.07 (0.78–1.47) | 8 | 0.73 (0.32–1.65) | 25 | 1.33 (0.78–2.27) | 17 | 0.93 (0.51–1.69) | 9 | 3.01 (1.04–8.70) | 3 | - |
| ⩾300 g/week | 3,164 | 55,036 | 97 | 1.36 (1.02–1.82) | 10 | 0.83 (0.38–1.81) | 45 | 1.94 (1.20–3.13) | 25 | 1.13 (0.65–1.96) | 4 | - | 9 | 1.47 (0.52–4.18) |
| Smoking status | ||||||||||||||
| None | 26,402 | 481,725 | 417 | 1 (Reference) | 85 | 1 (Reference) | 137 | 1 (Reference) | 117 | 1 (Reference) | 33 | 1 (Reference) | 20 | 1 (Reference) |
| Past | 1,510 | 27,692 | 29 | 0.82 (0.54–1.23) | 3 | – | 12 | 0.76 (0.40–1.43) | 9 | 0.95 (0.45–2.00) | 1 | – | 3 | – |
| <20 cigarettes/day | 2,082 | 35,310 | 42 | 0.98 (0.70–1.38) | 5 | 0.66 (0.26–1.71) | 14 | 0.86 (0.48–1.54) | 12 | 1.05 (0.55–1.98) | 4 | – | 6 | 1.75 (0.64–4.77) |
| ⩾20 cigarettes/day | 3,886 | 66,352 | 81 | 0.97 (0.73–1.27) | 13 | 1.02 (0.52–2.02) | 30 | 0.84 (0.54–1.33) | 24 | 1.07 (0.64–1.79) | 5 | 1.13 (0.38–3.37) | 5 | 0.68 (0.23–2.01) |
Adjusted for age, sex, systolic blood pressure, antihypertensive medication use, low total cholesterol, high total cholesterol, low HDL cholesterol, hypertriglyceridemia, diabetes, body weight, smoking, and drinking status. When hypertension was considered as the explanatory variable, systolic blood pressure and antihypertensive medication use were excluded from the models.
Systolic blood pressure ⩾140 mmHg, diastolic blood pressure ⩾90 mmHg, and/or antihypertensive medication use.
Fasting glucose level ⩾126 mg/dL, non-fasting glucose level ⩾200 mg/dL, and/or the use of diabetes medication.
HRs were calculated only when there were five cases or more.
Discussion
In this large and long-term cohort study of the general Japanese population with a high incidence of ICH, we found distinct risk factors for ICH across the five specific bleeding sites. Hypertension was associated with the risk of ICH in non-lobar sites and low total cholesterol with the risk of lobar hemorrhage.
Hypertension is strongly associated with ICH located in the putamen, thalamus, cerebellum, and brainstem supplied by small penetrating arteries. The mechanism of bleeding due to arteriolosclerosis of the perforating arteries has already been reported.20,21 Our results were consistent with the pathological conditions indicating an important role of hypertension for non-lobar hemorrhage often referred to as “deep hemorrhage.” Although previous studies have already shown that hypertension is a more common risk factor for deep than for lobar hemorrhage,22,23 our results clearly highlighted the significant association between hypertension and each bleeding site except for the lobes by classifying the bleeding sites more rigorously in a cohort design with a sufficient number of ICH cases.
Cerebral amyloid angiopathy, another cause of ICH which is characterized by amyloid beta-peptide deposits in the cortical and leptomeningeal blood vessel walls, is a common cause of lobar hemorrhage especially in older adults.24–26 Aging and genotype are well-known risk factors for cerebral amyloid angiopathy, but other risk factors remain unclear. The association between hypertension and cerebral amyloid angiopathy-related hemorrhage is also controversial.5,27–30 In the present study, no statistically significant association was observed between hypertension and the risk of lobar hemorrhage, which may support the hypothesis that the infiltration of cortical vessels by amyloid-β protein rather than hypertension mainly leads to vascular fragility, resulting in subcortical hemorrhage. Notably, the association between cerebral amyloid angiopathy and lobar hemorrhage as a causative factor was not assessed in this study population.
To our knowledge, this is the first study to assess the association between low total cholesterol levels and the specific risk of lobar ICH separately from other bleeding sites. Our previous study, based on lipid data collected 5 years after the baseline in Cohort I and at the baseline in Cohort II, showed that lower non-high-density lipoprotein cholesterol levels were associated with an increased risk of ICH, particularly of lobar hemorrhage including cerebellar hemorrhages. 31 Many previous studies have reported an inverse association between blood total cholesterol or low-density lipoprotein cholesterol levels and the risk of ICH.12,32–35 It has been considered that low total cholesterol levels may promote endothelium fragility and smooth muscle cell necrosis in the arterial medial layer, which contribute to the development of hemorrhage. 33 Our study showed that low total cholesterol levels may contribute to lobar hemorrhage often caused by cerebral amyloid angiopathy. The association between low cholesterol levels and a higher risk of deep hemorrhage, more apparent with higher blood pressure, 36 may have become less apparent due to the lowering of blood pressure levels in the Japanese population over the past decades. 37
Heavy alcohol intake has been reported to be strongly associated with ICH. 10 The association is considered to be mediated by high blood pressure, reduced platelet aggregation, and enhanced fibrinolysis. 38 A meta-regression analysis showed a significant linear relationship between alcohol consumption and the risk of hemorrhagic stroke. 10 We found associations between ethanol consumption and the risk of total ICH and putamen hemorrhage. Although the analysis may be less reliable especially for infratentorial hemorrhages due to the low number of cases, it is noteworthy that the point estimates of all deep hemorrhages indicated a positive association. Despite the robust relationship between hypertension and alcohol consumption, 39 these associations remained statistically significant even when adjusting for systolic blood pressure and the use of antihypertensive medication; the multivariable HRs without and with adjustment for systolic blood pressure and the use of antihypertensive medication were 1.57 (1.18–2.10) and 1.36 (1.02–1.82), respectively, for total ICH, 2.27 (1.42–3.64) and 1.94 (1.20–3.13), respectively, for putamen hemorrhage.
Higher BMI was associated with an elevated risk of stroke.40,41 Higher BMI and obesity are correlated with other stroke risk factors such as hypertension. 9 In the present study, when adjusting for systolic blood pressure and the use of antihypertensive medication, the association between being overweight and the risk of putamen hemorrhage was attenuated but remained statistically significant; multivariable HRs without and with adjustment for systolic blood pressure and the use of antihypertensive medication were 1.60 (1.19–2.15) and 1.39 (1.03–1.88), respectively. The large difference in BMI distribution between the Western and Japanese populations should be noted; the respective prevalence of BMI equal to or greater than 30 was approximately 20% within the Organization for Economic Co-operation and Development, which predominantly includes Western countries, and 4% in Japan (in the present study, the prevalence was 3%). 42
As reported previously, 43 men have a higher risk of deep hemorrhages. Low triglyceride levels were reported as a risk factor for ICH due to their structural role in cell membranes, similar to that of cholesterol. 44 However, this study found no significant association with the risk of ICH. The association between diabetes or smoking, and the risk of ICH has been controversial,8,11,45,46 and we did not find a significant association between them.
Strengths and limitations
The strengths of this study are its large-scale implementation and the long-term follow-ups. A large number of participants across Japan were recruited and then followed up for 20 years. The cohort setting in Japan, a country with a high incidence of ICH, allowed for the analysis of bleeding sites.
This study has several potential limitations. First, although cerebral amyloid angiopathy is a well-known major cause of lobar hemorrhage, we did not identify ICH based on a diagnosis of cerebral amyloid angiopathy that required full autopsy. 47 At the beginning of the follow-up period (1990s), the concept of cerebral amyloid angiopathy had not yet been established, and diagnoses of ICH relied exclusively on CT. However, since the purpose of this study was to investigate risk factors by anatomical sites of bleeding, this would not affect the results. Second, we did not have data on anticoagulant therapy status and other coagulation abnormalities, which are suggested to be associated with specific bleeding sites. 48 However, this is unlikely to have confounded the results because anticoagulants were less prevalent during the follow-up period than they are today, and their use is unlikely to have affected the cardiovascular risk factors examined in this study. Third, the generalizability should be considered with caution. Although the incidence rate of ICH of Asian population was notably higher than other ethnic groups due to lifestyle and an inherited susceptibility of the intracranial arteries to atherosclerosis,3,49,50 almost all risk factors for ICH are common across all races. The evidence in a population with high incidence of ICH could provide the basis for extrapolation not only to Asians but also to other races who are reported to have an increased risk of ICH. 14 Fourth, because participation in the community health checkups was voluntary, the healthy participant effect was unavoidable, especially as women were generally more health-conscious. Furthermore, men were often already covered by workplace health checkups in Japan at the time of baseline (1990s). Last, we only used the data of each risk factor measured at baseline which may have weakened the associations with ICH due to dilution bias. Further research in diverse populations is necessary to enhance generalizability and the exploration of genetic and lifestyle factors in greater detail.
Conclusion
The present study found that hypertension was associated with an increased risk of ICH in the putamen, the thalamus, the cerebellum, and the brainstem, but not in the lobes. In contrast, low total cholesterol levels were associated with a higher risk of lobar hemorrhage. Heavy drinking and obesity were both associated with the risk of hemorrhage in the putamen.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241290680 for Risk factors for intracerebral hemorrhage by five specific bleeding sites: Japan Public Health Center-based Prospective Study by Kenichi Ariyada, Kazumasa Yamagishi, Tomomi Kihara, Isao Muraki, Hironori Imano, Yoshihiro Kokubo, Isao Saito, Hiroshi Yatsuya, Hiroyasu Iso, Shoichiro Tsugane and Norie Sawada in European Stroke Journal
Acknowledgments
The authors wish to thank Florescu Mihail Cosmin, Medical English Communications Center, University of Tsukuba, for English language editing.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the National Cancer Center Research and Development Fund (23-A-31 [toku], 26-A-2, 29-A-4, 2000-J-4 and 2023-J-4) since 2011, and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan between 1989–2010.
Ethics approval: The protocol for this study was approved by the institutional human ethics review boards of the National Cancer Center, Japan (approval number: 2001-021 and 2015-085) and the University of Tsukuba (approval number: 66-14).
Informed consent: Participants were informed of the objectives of the study, and that completion of the survey questionnaire was regarded as providing consent to participate. Formal informed consent was not obtained for the JPHC Study because the JPHC Study was initiated before the ethical guidelines were established in Japan.
Guarantor: KY
Contributorship: KY and KA contributed to the study conception and design. Material preparation and data collection were performed by all authors. After formal analysis, the first draft of the manuscript was written by KA and all authors commented on the manuscript. All authors read and approved the final manuscript.
ORCID iDs: Kazumasa Yamagishi  https://orcid.org/0000-0003-3301-5519
https://orcid.org/0000-0003-3301-5519
Yoshihiro Kokubo  https://orcid.org/0000-0002-0705-9449
https://orcid.org/0000-0002-0705-9449
Supplemental material: Supplemental material for this article is available online.
References
- 1. Steiner T, Al-Shahi Salman R, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9: 840–855. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8: 355–369. [DOI] [PubMed] [Google Scholar]
- 3. van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 4. Pandian JD, Gall SL, Kate MP, et al. Prevention of stroke: a global perspective. Lancet 2018; 392: 1269–1278. [DOI] [PubMed] [Google Scholar]
- 5. Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke 2002; 33: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 6. Woo D, Haverbusch M, Sekar P, et al. Effect of untreated hypertension on hemorrhagic stroke. Stroke 2004; 35: 1703–1708. [DOI] [PubMed] [Google Scholar]
- 7. Zia E, Hedblad B, Pessah-Rasmussen H, et al. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke 2007; 38: 2681–2685. [DOI] [PubMed] [Google Scholar]
- 8. O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 9. Pezzini A, Grassi M, Paciaroni M, et al. Obesity and the risk of intracerebral hemorrhage: the multicenter study on cerebral hemorrhage in Italy. Stroke 2013; 44: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 10. Reynolds K, Lewis B, Nolen JD, et al. Alcohol consumption and risk of stroke: a meta-analysis. JAMA 2003; 289: 579–588. [DOI] [PubMed] [Google Scholar]
- 11. Larsson SC, Chen J, Gill D, et al. Risk factors for intracerebral hemorrhage: genome-wide association study and mendelian randomization analyses. Stroke 2024; 55: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iso H, Jacobs DR, Jr, Wentworth D, et al. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med 1989; 320: 904–910. [DOI] [PubMed] [Google Scholar]
- 13. Jolink WMT, Wiegertjes K, Rinkel GJE, et al. Location-specific risk factors for intracerebral hemorrhage: systematic review and meta-analysis. Neurology 2020; 95: e1807–e1818. [DOI] [PubMed] [Google Scholar]
- 14. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013; 1: e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol 2014. ;44: 777–782. [DOI] [PubMed] [Google Scholar]
- 16. Iida M, Sato S, Nakamura M. Standardization of laboratory test in the JPHC study. Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Diseases. J Epidemiol 2001; 11: S81–S86. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura M, Sato S, Shimamoto T. Improvement in Japanese clinical laboratory measurements of total cholesterol and HDL-cholesterol by the US Cholesterol Reference Method Laboratory Network. J Atheroscler Thromb 2003; 10: 145–153. [DOI] [PubMed] [Google Scholar]
- 18. Okamura T, Tsukamoto K, Arai H, et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. J Atheroscler Thromb 2024; 31: 641–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981; 12: I13–I44. [PubMed] [Google Scholar]
- 20. Garcia JH, Ho KL. Pathology of hypertensive arteriopathy. Neurosurg Clin N Am 1992; 3: 497–507. [PubMed] [Google Scholar]
- 21. Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol 2002; 12: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jackson CA, Sudlow CL. Is hypertension a more frequent risk factor for deep than for lobar supratentorial intracerebral haemorrhage? J Neurol Neurosurg Psychiatry 2006; 77: 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martini SR, Flaherty ML, Brown WM, et al. Risk factors for intracerebral hemorrhage differ according to hemorrhage location. Neurology 2012; 79: 2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012; 83: 124–137. [DOI] [PubMed] [Google Scholar]
- 25. Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol 2011; 70: 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenberg SM, Edgar MA. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 22-1996. Cerebral hemorrhage in a 69-year-old woman receiving warfarin. N Engl J Med 1996; 335: 189–196. [DOI] [PubMed] [Google Scholar]
- 27. Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015; 314: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferreiro JA, Ansbacher LE, Vinters HV. Stroke related to cerebral amyloid angiopathy: the significance of systemic vascular disease. J Neurol 1989; 236: 267–272. [DOI] [PubMed] [Google Scholar]
- 29. Broderick J, Brott T, Tomsick T, et al. Lobar hemorrhage in the elderly. The undiminishing importance of hypertension. Stroke 1993; 24: 49–51. [DOI] [PubMed] [Google Scholar]
- 30. Massaro AR, Sacco RL, Mohr JP, et al. Clinical discriminators of lobar and deep hemorrhages: the Stroke Data Bank. Neurology 1991; 41: 1881–1885. [DOI] [PubMed] [Google Scholar]
- 31. Saito I, Yamagishi K, Kokubo Y, et al. Non-high-density lipoprotein cholesterol and risk of stroke subtypes and coronary heart disease: the Japan Public Health Center-Based Prospective (JPHC) Study. J Atheroscler Thromb 2020; 27: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui R, Iso H, Toyoshima H, et al. Serum total cholesterol levels and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis 2007; 194: 415–420. [DOI] [PubMed] [Google Scholar]
- 33. Konishi M, Iso H, Komachi Y, et al. Associations of serum total cholesterol, different types of stroke, and stenosis distribution of cerebral arteries. The Akita Pathology Study. Stroke 1993; 24: 954–964. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Dong Y, Qi X, et al. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke 2013; 44: 1833–1839. [DOI] [PubMed] [Google Scholar]
- 35. Tirschwell DL, Smith NL, Heckbert SR, et al. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology 2004; 63: 1868–1875. [DOI] [PubMed] [Google Scholar]
- 36. Satoh M, Ohkubo T, Asayama K, et al. Combined effect of blood pressure and total cholesterol levels on long-term risks of subtypes of cardiovascular death: evidence for cardiovascular prevention from observational cohorts in Japan. Hypertension 2015; 65: 517–524. [DOI] [PubMed] [Google Scholar]
- 37. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019; 42: 1235–1481. [DOI] [PubMed] [Google Scholar]
- 38. Iso H, Baba S, Mannami T, et al. Alcohol consumption and risk of stroke among middle-aged men: the JPHC Study Cohort I. Stroke 2004; 35: 1124–1129. [DOI] [PubMed] [Google Scholar]
- 39. Di Federico S, Filippini T, Whelton PK, et al. Alcohol intake and blood pressure levels: a dose-response meta-analysis of nonexperimental cohort studies. Hypertension 2023; 80: 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu Y, Hajifathalian K, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 2014; 383: 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nuamah HG, Li Y, Yatsuya H, et al. The effect of age on the relationship between body mass index and risks of incident stroke subtypes: the JPHC study. J Stroke Cerebrovasc Dis 2024; 33: 107486. [DOI] [PubMed] [Google Scholar]
- 42. OECD. Obesity update 2017, https://www.oecd.Org/els/health-systems/Obesity-Update-2017.pdf (2017, accessed 30 April 2024).
- 43. Foschi M, D’Anna L, Gabriele C, et al. Sex differences in the epidemiology of intracerebral hemorrhage over 10 years in a population-based stroke registry. J Am Heart Assoc 2024; 13: e032595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sturgeon JD, Folsom AR, Longstreth WT, Jr, et al. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007; 38: 2718–2725. [DOI] [PubMed] [Google Scholar]
- 45. Larsson SC, Burgess S, Michaelsson K. Smoking and stroke: a mendelian randomization study. Ann Neurol 2019; 86: 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boulanger M, Poon MT, Wild SH, et al. Association between diabetes mellitus and the occurrence and outcome of intracerebral hemorrhage. Neurology 2016; 87: 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Charidimou A, Boulouis G, Frosch MP, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 2022; 21: 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Seiffge DJ, Curtze S, Dequatre-Ponchelle N, et al. Hematoma location and morphology of anticoagulation-associated intracerebral hemorrhage. Neurology 2019; 92: e782–e791. [DOI] [PubMed] [Google Scholar]
- 49. Toyoda K, Koga M, Hayakawa M, et al. Acute reperfusion therapy and stroke care in Asia after successful endovascular trials. Stroke 2015; 46: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 50. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014; 371: 624–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241290680 for Risk factors for intracerebral hemorrhage by five specific bleeding sites: Japan Public Health Center-based Prospective Study by Kenichi Ariyada, Kazumasa Yamagishi, Tomomi Kihara, Isao Muraki, Hironori Imano, Yoshihiro Kokubo, Isao Saito, Hiroshi Yatsuya, Hiroyasu Iso, Shoichiro Tsugane and Norie Sawada in European Stroke Journal