Abstract
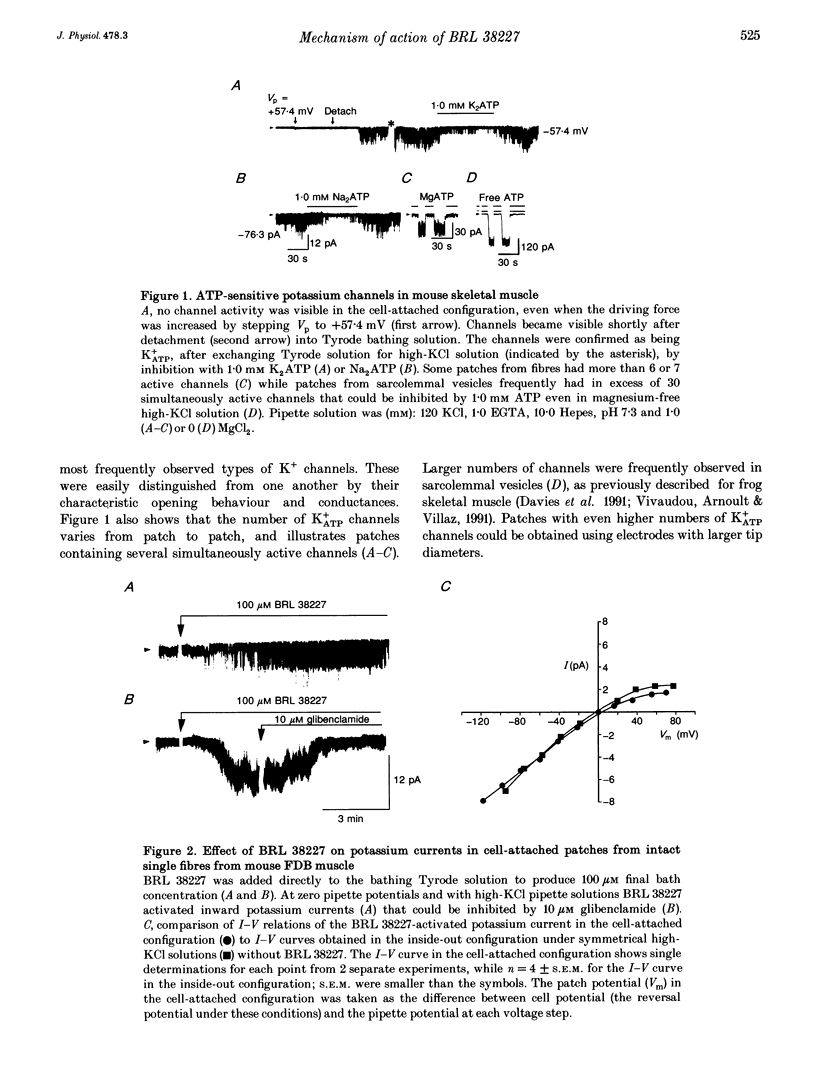
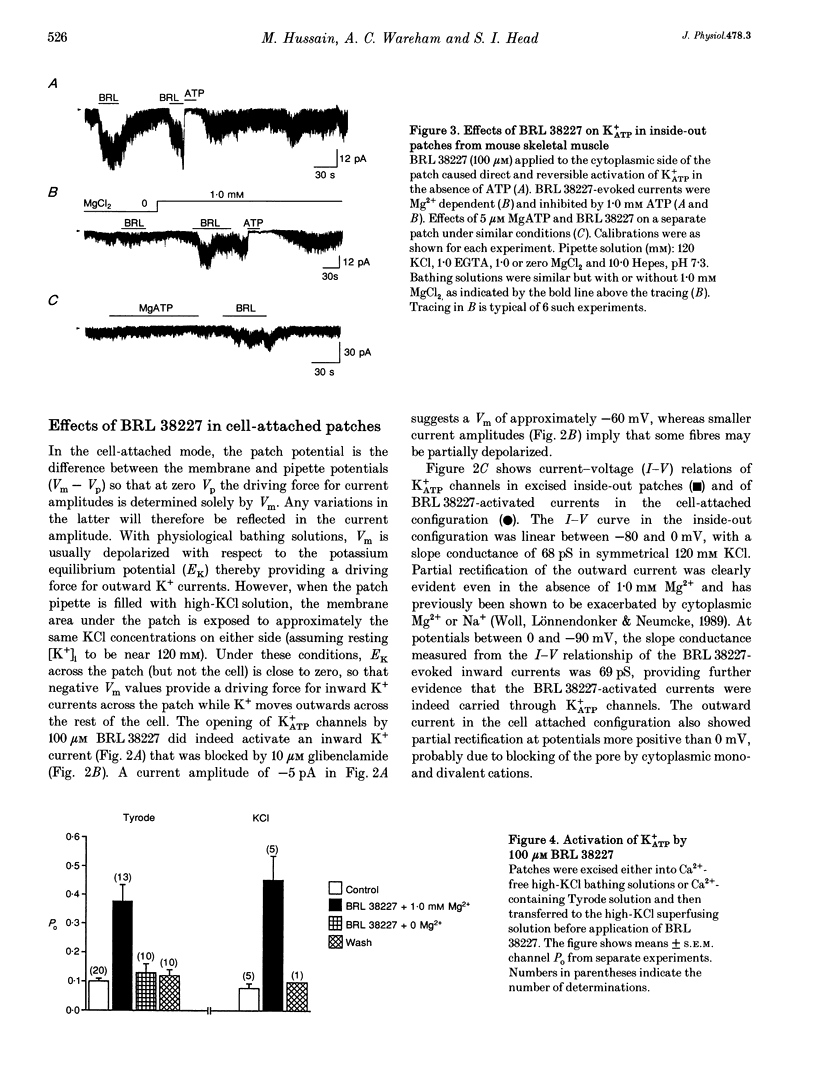
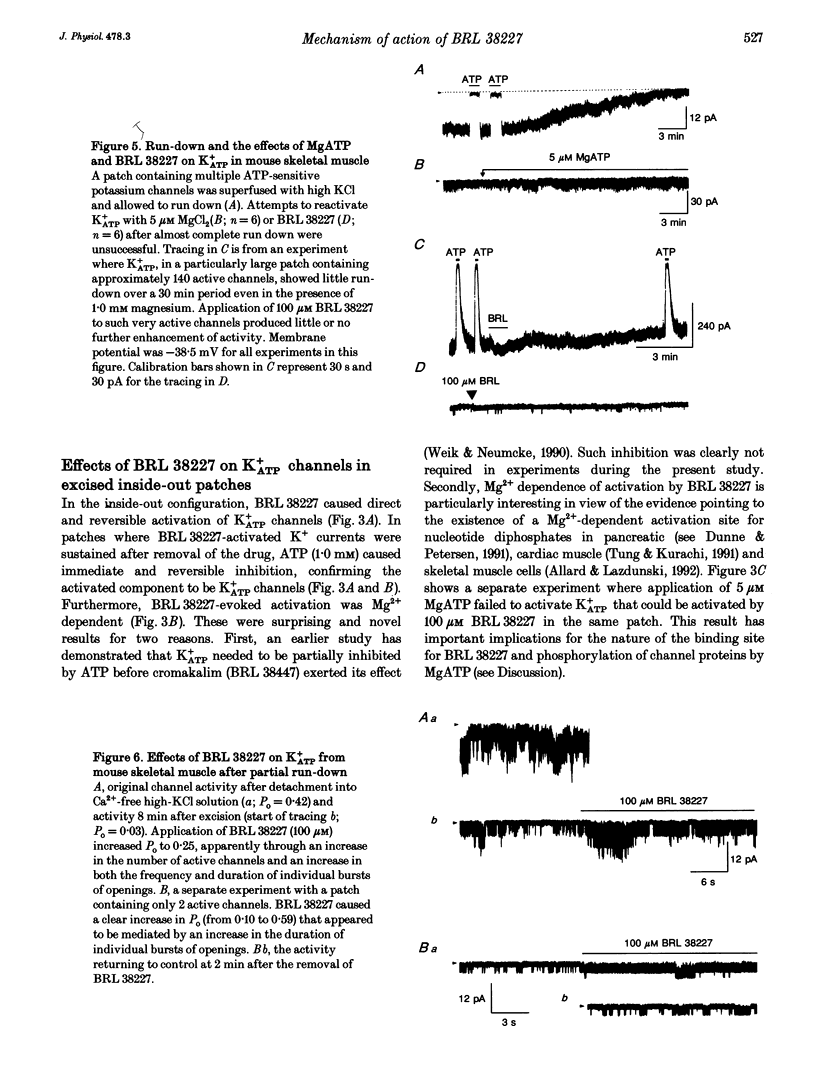
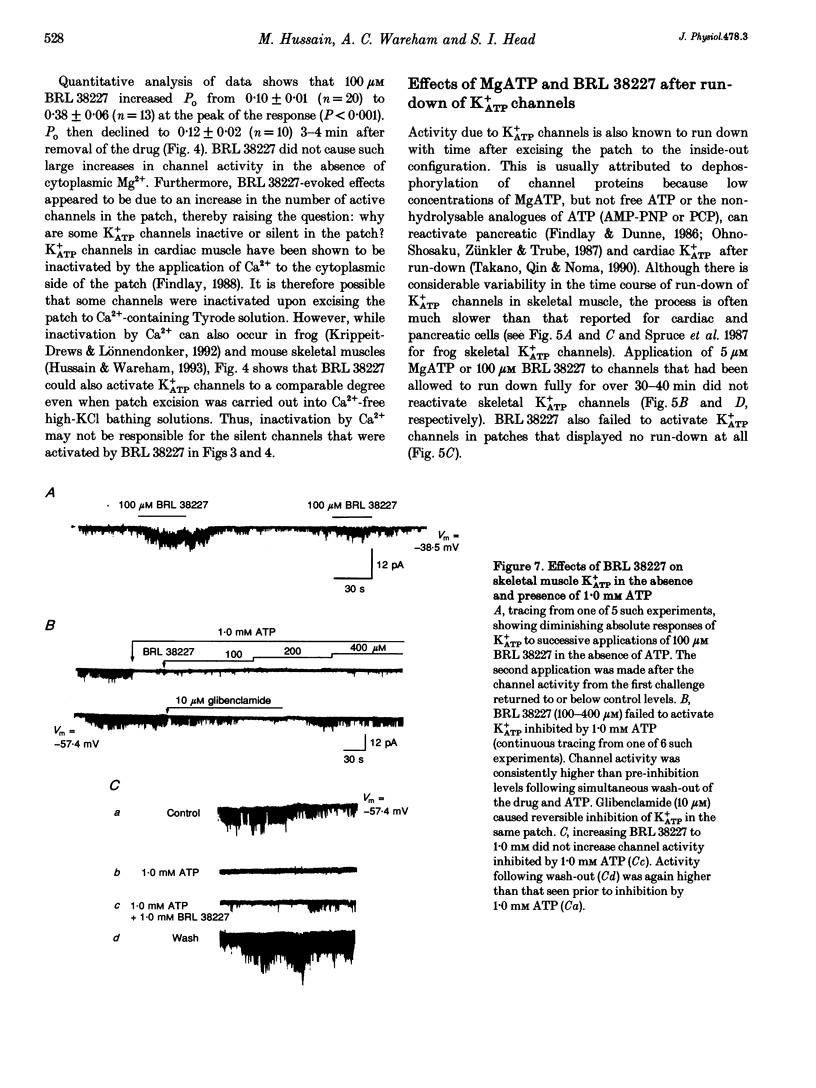
1. Investigations were made into the effects of BRL 38227, a potassium channel activator, on ATP-sensitive potassium channels (K+ATP channels) in single fibres dissociated from the flexor digitorum brevis muscle of C57BL/6J mice. 2. In cell-attached patches BRL 38227 (100 microM) caused activation of a glibenclamide-sensitive potassium current. Linear slope conductance of the inward current, partial rectification of the outward current and glibenclamide sensitivity indicate that K+ATP channels are the site of action of BRL 38227. 3. In the absence of ATP at the cytoplasmic side of excised inside-out patches, BRL 38227 caused direct and magnesium-dependent activation of K+ATP channels. The degree of activation diminished with successive applications of BRL 38227. 4. BRL 38227 also caused activation of K+ATP channels in the presence of low (< 100 microM) but not high (1.0 mM) ATP, particularly in patches containing large numbers of channels. 5. BRL 38227 and 5 microM MgATP failed to activate channels following complete run-down. 6. Results show that BRL 38227 caused direct activation of K+ATP in skeletal muscle and that this was mediated through a magnesium-dependent binding site rather than alleviation of inhibition by competitive displacement of ATP from the inhibitory site.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard B., Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflugers Arch. 1992 Nov;422(2):185–192. doi: 10.1007/BF00370419. [DOI] [PubMed] [Google Scholar]
- Arena J. P., Kass R. S. Activation of ATP-sensitive K channels in heart cells by pinacidil: dependence on ATP. Am J Physiol. 1989 Dec;257(6 Pt 2):H2092–H2096. doi: 10.1152/ajpheart.1989.257.6.H2092. [DOI] [PubMed] [Google Scholar]
- Black J. L., Barnes P. J. Potassium channels and airway function: new therapeutic prospects. Thorax. 1990 Mar;45(3):213–218. doi: 10.1136/thx.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A., Bowen S., Gelband C. H., Sanders K. M., Hume J. R. Cromakalim and lemakalim activate Ca(2+)-dependent K+ channels in canine colon. Pflugers Arch. 1992 May;421(1):67–76. doi: 10.1007/BF00374735. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G. Effect of channel blockers on potassium efflux from metabolically exhausted frog skeletal muscle. J Physiol. 1987 Feb;383:31–43. doi: 10.1113/jphysiol.1987.sp016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Potassium selective ion channels in insulin-secreting cells: physiology, pharmacology and their role in stimulus-secretion coupling. Biochim Biophys Acta. 1991 Mar 7;1071(1):67–82. doi: 10.1016/0304-4157(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Escande D., Thuringer D., Leguern S., Cavero I. The potassium channel opener cromakalim (BRL 34915) activates ATP-dependent K+ channels in isolated cardiac myocytes. Biochem Biophys Res Commun. 1988 Jul 29;154(2):620–625. doi: 10.1016/0006-291x(88)90184-2. [DOI] [PubMed] [Google Scholar]
- Fan Z., Nakayama K., Hiraoka M. Multiple actions of pinacidil on adenosine triphosphate-sensitive potassium channels in guinea-pig ventricular myocytes. J Physiol. 1990 Nov;430:273–295. doi: 10.1113/jphysiol.1990.sp018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Calcium-dependent inactivation of the ATP-sensitive K+ channel of rat ventricular myocytes. Biochim Biophys Acta. 1988 Aug 18;943(2):297–304. doi: 10.1016/0005-2736(88)90561-5. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J. ATP maintains ATP-inhibited K+ channels in an operational state. Pflugers Arch. 1986 Aug;407(2):238–240. doi: 10.1007/BF00580683. [DOI] [PubMed] [Google Scholar]
- Forestier C., Vivaudou M. Modulation by Mg2+ and ADP of ATP-sensitive potassium channels in frog skeletal muscle. J Membr Biol. 1993 Feb;132(1):87–94. doi: 10.1007/BF00233054. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hong S. J., Chang C. C. Hyperpolarization of denervated skeletal muscle by lemakalim and its antagonism by glybenclamide and tolbutamide. J Pharmacol Exp Ther. 1991 Nov;259(2):932–938. [PubMed] [Google Scholar]
- Hood J. S., McMahon T. J., Kadowitz P. J. Influence of lemakalim on the pulmonary vascular bed of the cat. Eur J Pharmacol. 1991 Sep 4;202(1):101–104. doi: 10.1016/0014-2999(91)90260-w. [DOI] [PubMed] [Google Scholar]
- Ito S., Kajikuri J., Itoh T., Kuriyama H. Effects of lemakalim on changes in Ca2+ concentration and mechanical activity induced by noradrenaline in the rabbit mesenteric artery. Br J Pharmacol. 1991 Sep;104(1):227–233. doi: 10.1111/j.1476-5381.1991.tb12411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippeit-Drews P., Lönnendonker U. Dual effects of calcium on ATP-sensitive potassium channels of frog skeletal muscle. Biochim Biophys Acta. 1992 Jul 8;1108(1):119–122. doi: 10.1016/0005-2736(92)90122-3. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Arakawa C., Yokoyama T., Shigenobu K., Tanaka S. The antihypertensive property of NIP-121, a novel potassium channel opener in rats. J Cardiovasc Pharmacol. 1991 Aug;18(2):190–197. doi: 10.1097/00005344-199108000-00003. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Post J. M., Stevens R. J., Sanders K. M., Hume J. R. Effect of cromakalim and lemakalim on slow waves and membrane currents in colonic smooth muscle. Am J Physiol. 1991 Feb;260(2 Pt 1):C375–C382. doi: 10.1152/ajpcell.1991.260.2.C375. [DOI] [PubMed] [Google Scholar]
- Quasthoff S., Spuler A., Spittelmeister W., Lehmann-Horn F., Grafe P. K+ channel openers suppress myotonic activity of human skeletal muscle in vitro. Eur J Pharmacol. 1990 Sep 4;186(1):125–128. doi: 10.1016/0014-2999(90)94068-9. [DOI] [PubMed] [Google Scholar]
- Rowe I. C., Wareham A. C., Whittle M. A. Potassium channel activity in sarcolemmal vesicles formed from skeletal muscle fibres of normal and dystrophic mice. J Neurol Sci. 1990 Aug;98(1):51–61. doi: 10.1016/0022-510x(90)90181-l. [DOI] [PubMed] [Google Scholar]
- Sauviat M. P., Ecault E., Faivre J. F., Findlay I. Activation of ATP-sensitive K channels by a K channel opener (SR 44866) and the effect upon electrical and mechanical activity of frog skeletal muscle. Pflugers Arch. 1991 Apr;418(3):261–265. doi: 10.1007/BF00370524. [DOI] [PubMed] [Google Scholar]
- Shen W. K., Tung R. T., Machulda M. M., Kurachi Y. Essential role of nucleotide diphosphates in nicorandil-mediated activation of cardiac ATP-sensitive K+ channel. A comparison with pinacidil and lemakalim. Circ Res. 1991 Oct;69(4):1152–1158. doi: 10.1161/01.res.69.4.1152. [DOI] [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuler A., Lehmann-Horn F., Grafe P. Cromakalim (BRL 34915) restores in vitro the membrane potential of depolarized human skeletal muscle fibres. Naunyn Schmiedebergs Arch Pharmacol. 1989 Mar;339(3):327–331. doi: 10.1007/BF00173587. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M., Noma A. Selective modulation of the ATP-sensitive K+ channel by nicorandil in guinea-pig cardiac cell membrane. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):592–597. doi: 10.1007/BF00169050. [DOI] [PubMed] [Google Scholar]
- Takano M., Qin D. Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am J Physiol. 1990 Jan;258(1 Pt 2):H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol Pharmacol. 1989 Dec;36(6):897–902. [PubMed] [Google Scholar]
- Tung R. T., Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J Physiol. 1991 Jun;437:239–256. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M. B., Arnoult C., Villaz M. Skeletal muscle ATP-sensitive K+ channels recorded from sarcolemmal blebs of split fibers: ATP inhibition is reduced by magnesium and ADP. J Membr Biol. 1991 Jun;122(2):165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- Weik R., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: characterization of the ATP-binding site. J Membr Biol. 1989 Sep;110(3):217–226. doi: 10.1007/BF01869152. [DOI] [PubMed] [Google Scholar]
- Weik R., Neumcke B. Effects of potassium channel openers on single potassium channels in mouse skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 1990 Sep;342(3):258–263. doi: 10.1007/BF00169435. [DOI] [PubMed] [Google Scholar]
- Woll K. H., Lönnendonker U., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: different modes of blockage by internal cations, ATP and tolbutamide. Pflugers Arch. 1989 Sep;414(6):622–628. doi: 10.1007/BF00582126. [DOI] [PubMed] [Google Scholar]


