Abstract
Background:
Conflicting research on retinal biomarkers of Alzheimer’s disease and related dementias (AD/ADRD) are likely related to limited sample sizes, study design, and protocol differences.
Objective:
The prospective Eye Adult Changes in Thought (Eye ACT) seeks to address these gaps.
Methods:
Eye ACT participants are recruited from ACT, an ongoing cohort of dementia-free, older adults followed biennially until AD/ADRD, and undergo visual function and retinal imaging assessment either in clinic or at home.
Results:
330 participants were recruited as of 03/2023. Compared to ACT participants not in Eye ACT (N=1868), Eye ACT participants (N=330) are younger (mean age: 70.3 vs. 71.2, p=0.014), newer to ACT (median ACT visits since baseline: 3 vs. 4, p<0.001), have more years of education (17.7 vs. 16.2, p<0.001) and had lower rates of visual impairment (12% vs. 22%, p<0.001). Compared to those seen in clinic (N=300), Eye ACT participants seen at home (N=30) are older (77.2 vs. 74.9, p=0.015), more frequently female (60% vs. 49%, p=0.026), and have significantly worse visual acuity (71.1 vs. 78.9 Early Treatment Diabetic Retinopathy Study letters, p<0.001) and contrast sensitivity (−1.9 vs. −2.1 mean log units at 3 cycles per degree, p=0.002). Cognitive scores and retinal imaging measurements are similar between the two groups.
Conclusion:
Participants assessed at home had significantly worse visual function than those seen in clinic. By including these participants, Eye ACT provides a unique longitudinal cohort for evaluating potential retinal biomarkers of dementia.
Keywords: Alzheimer’s disease, Adult Changes in Thought (ACT), Eye Adult Changes in Thought (Eye ACT), Ophthalmology, Prospective study, Visual acuity, Contrast sensitivity, Age-related macular degeneration, Cognitive Abilities Screening Instrument (CASI) score, Optical coherence measurement
INTRODUCTION
The concept of using the eye, often referred as the “window to the brain,” for understanding brain health has been explored for several decades. Remarkable recent advances in non-invasive retinal imaging and the marriage of Big Data and artificial intelligence (AI) have led to increasing enthusiasm for discovering eye-related biomarkers of Alzheimer’s disease and related dementias (AD/ADRD), either as early diagnostics, prognostics, or modifiable risk factors.
Since the 1986 optic nerve atrophy in AD report by Hinton et al,[1] additional associations between retinal imaging features and impaired cognition and/or AD have been reported, including structural changes such as thinning of the retinal nerve fiber layer (RNFL),[2–8] macular volume,[2,9] ganglion cell-inner plexiform layer (GC-IPL),[2,3,7,10,11] and choroidal volume.[12–14] These associations also include functional changes such as decreased blood flow[15,16] and attenuated vasculature.[17–23]
Studies to date have conflicting findings on retinal features and AD/ADRD risk[18–20] likely related to cross-sectional designs, small convenience samples, and the use of clinical rather than research-based AD/ADRD diagnoses.[24–29] Longitudinal studies have limited sample sizes (n=20),[30] and many imaging studies are not comparable due to differences in protocols and retinal imaging devices. Some promising studies rely on AD biomarker-positive cognitively intact younger adults at baseline, which may not be applicable to older adults.[31–34] Furthermore, most studies have excluded people with eye diseases, common in older people, and therefore preclude investigation of associations between eye diseases and cognitive functioning.[35–37]
The Eye Adult Changes in Thought study (“Eye ACT”) was designed to address these limitations by following a well-characterized community-based cohort and obtaining uniquely detailed eye data via repeated prospective eye imaging (performed in clinic or at home) accompanied by enriched eye clinical information abstracted from health records. Participants in Eye ACT are recruited from the parent ACT study,[38] an ongoing prospective study with a large sample and high retention rate (>90%). The ACT study provides extensive resources for Eye ACT to leverage: routine cognitive testing at the participant’s home or research clinic, research protocols to identify incident AD/ ADRD,[39] and state-of-the art neuropathology evaluation among deceased participants who volunteered for brain autopsy. The overall hypothesis is that visual function alterations and changes in the retinal structure and vasculature may serve as biomarkers of early AD/ADRD. In this paper, we provide the detailed Eye ACT research protocol and descriptive statistics from the first 330 Eye ACT participants who have been participating in prospective eye data collection since 2021.
METHODS
Study design and settings, the ACT study
The ACT study,[40] which began in 1994, is an ongoing prospective cohort study of older adults (≥ 65 years) who are dementia-free and community-dwelling at enrollment and followed for the onset of AD/ADRD (Supplementary Methods). Participants are randomly selected and recruited from Kaiser Permanente Washington (KPW) members, all of whom have access to comprehensive medical care, including ophthalmic care. KPW is an integrated health insurance and care delivery system in Washington State, serving over 650,000 members. The ACT study includes extensive medical records (both paper charts as well as electronic health records [EHR] starting in the early 2000s), pharmacy records, and laboratory datasets from participants.[38,41] ACT participants are extensively characterized not only with regard to cognitive status and dementia subtypes (Supplementary Methods) but also in terms of overall dementia-related comorbidities and risk factors.[42] Participants self-report any history of cardiovascular or cerebrovascular disease, hypertension, or smoking at each study visit. The ACT study has generated many seminal publications on aging and dementia to date.[43,44]
Participants undergo cognitive assessment with the item response theory-based Cognitive Abilities Screening Instrument (CASI-IRT)[45] at baseline and during biennial study visits. At recruitment, the majority are seen in the research clinic for their baseline visit, but a minority are seen at home. For the following biennial visits, participants continue to have the option of being seen at home related to poor mobility or transportation difficulties.[46] Regardless of the study visit location, the ACT study protocol is equivalent.
The Prospective Eye ACT Study Cohort
The Eye ACT study recruits and enrolls participants from the parent ACT study. Eye ACT is unique in that there are no exclusion criteria specific to the study. Instead, Eye ACT reaches out to the entire ACT cohort for potential recruitment. At the initiation of the study, only established ACT participants undergoing biennial visits at the research clinic were recruited for Eye ACT. More recently, ACT participants seen at home for biennial visits and agree to Eye ACT home study visits have been included. (Figure 1) Leveraging the parent ACT’s currently ongoing ambitious aims to increase overall cohort size with emphasis on diversity, Eye ACT also plans to increase cohort size to over 2,000 active participants and begin recruiting new ACT enrollees.
Figure 1.
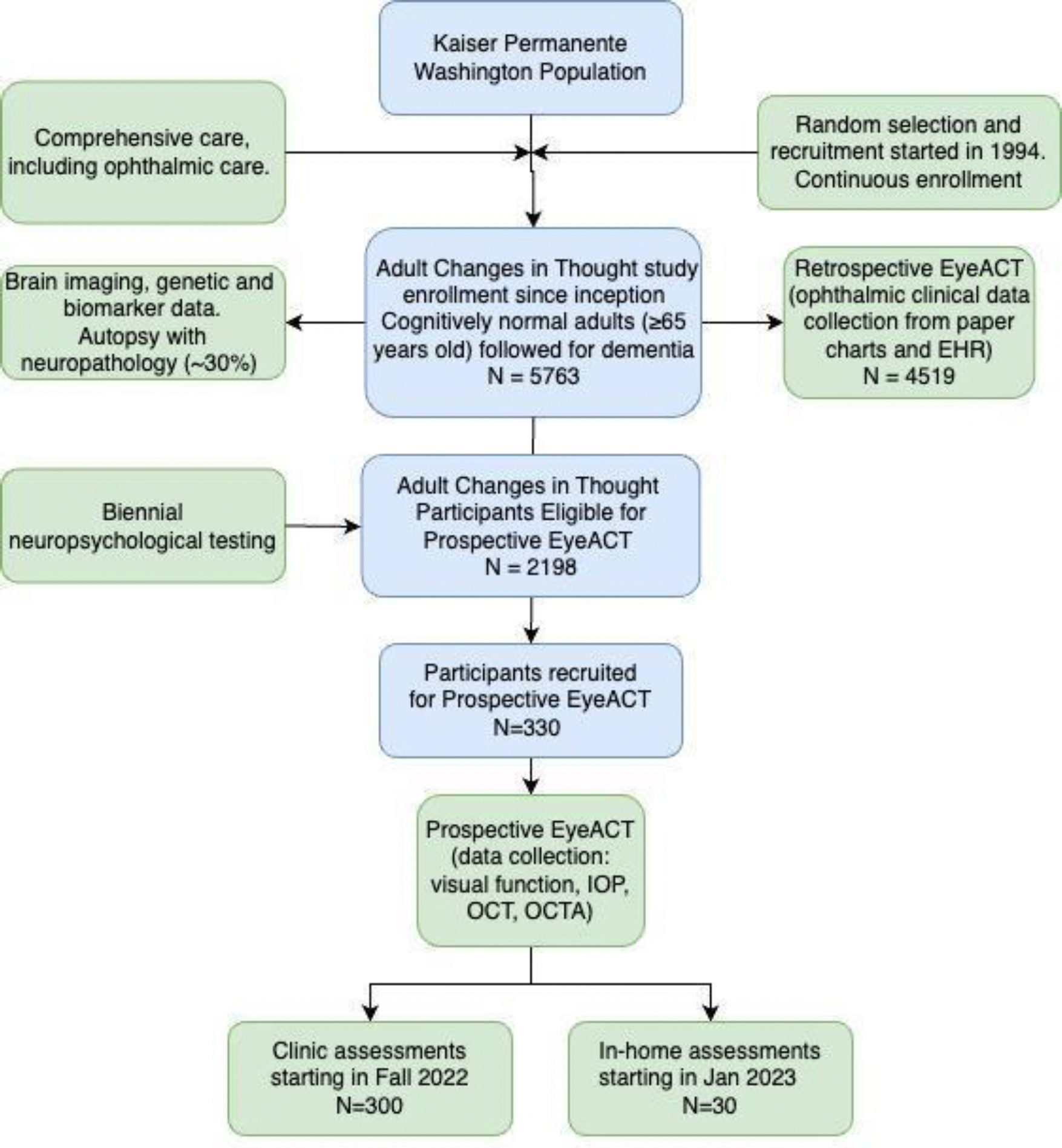
Flow chart of participants. Relationship between the parent ACT study and Eye ACT study cohorts (retrospective and prospective).
Prospective Eye ACT Study Data Collection Protocol
Following their regular ACT biennial visit and after providing informed consent, Eye ACT participants undergo a detailed eye exam, including: 1) visual function measures including visual acuity (VA) in Early Treatment Diabetic Retinopathy Study [ETDRS] letters[47,48] and contrast sensitivity (CS) using bulls-eye radial gratings[49] with a portable device (M&S Technology, Niles, IL), 2) intraocular pressure (IOP) with iCare TA01i tonometer (iCare, Raleigh, NC), 3) fundus photography/optical coherence tomography (OCT)/OCT angiography (OCTA) with Maestro2 (Topcon Medical Systems, Inc., Oakland, NJ), and 4) a second OCT/OCTA with PLEX® Elite 9000 OCTA (Carl Zeiss Meditec, Dublin, CA) for clinic visits only. With the exception of one imaging device (which is not portable), the same study protocol is followed regardless of whether participants are seen in the clinic or at home.
Retrospective Eye ACT Medical Record Data Collection Protocol
Eye ACT retrospective medical record data collection protocol includes extraction of eye-related data from all ACT study participants including from paper charts dating to the 1940s, diagnosis and procedure codes since 1993, electronic pharmacy records since 1978, and laboratory data since 1988 (Supplementary Methods).
Manual abstraction of paper medical records (i.e., “charts”) dating from 1950 to 2005 is performed by a group of trained and certified abstractors who undergo quarterly evaluations to ensure no deviation in quality. Chart abstraction is prioritized by: 1) participants who are deceased and underwent autopsy and 2) living participants who are autopsy consented. Our specialty-trained team reviews visual acuity, IOP, and extensive key clinical features, extracting up to 338 data fields per encounter, including 44 age-related macular degeneration (AMD)-related, 66 diabetic retinopathy (DR)-related, and 86 glaucoma-related fields.
In contrast, the extraction of the eye clinical data from EHR is obtained on all participants, regardless of their autopsy consent status, using an in-house natural language processing (NLP) algorithm to collect the same data fields as from paper charts. In addition, we obtain International Classification of Disease (ICD) and Current Procedural Terminology (CPT) codes of eye diseases and procedures from the EHR, and eye medication history from electronic pharmacy records. (Supplementary Table 1) Visual impairment was defined as any clinical encounter with Snellen VA worse than 20/40. A complete list of retrospectively collected ophthalmic variables is in Supplementary Table 2.
Statistical analysis
We compared the demographic and clinical characteristics of (1) all Eye ACT participants to currently living and dementia-free ACT participants who were not enrolled in Eye ACT as of 03/2023, and (2) Eye ACT participants who were seen in the clinic versus at home. We used Wilcoxon’s rank sum statistic for continuous variables and Fisher’s exact test for categorical variables to assess differences between groups. All tests were conducted at a significance level of 0.05.
RESULTS
Total enrollment of the parent ACT study was 5763 participants as of 2020. Of these, 2198 ACT participants were alive and at risk of developing AD/ADRD as of 12/2021. A total of 330 of these have been recruited to the Eye ACT study as of 03/2023, the most recent ACT data freeze date, and included in the analytic set, with 300 seen in the research clinic and 30 seen at home. A total of 109 individuals declined participation in Eye ACT (4% of research clinic and 75% of home participants).
Comparison of the parent ACT study participants and Eye ACT study participants
Compared to the active parent ACT study participants (i.e. at risk of developing dementia) not in Eye ACT, Eye ACT study participants were slightly younger at ACT enrollment (mean age 70.3 vs. 71.2, p=0.014), had slightly more education (mean [standard deviation] years of education 17.7 [2.4] vs. 16.2 [3.0], p<0.001), and a smaller proportion were female (50% vs. 60%, p<0.001). (Table 1) This cohort was also enrolled and followed in ACT for a shorter duration (median [range] number of ACT visits since ACT enrollment visit: 3 [1–13] vs. 4 [1–14], p<0.001). (Table 1) Furthermore, Eye ACT participants had lower self-reported rates of cardiovascular disease (10% vs. 16%, p=0.008), cerebrovascular disease (6% vs. 11%, p=0.01), and hypertension (45% vs. 53%, p=0.004) compared to parent ACT participants.
Table 1.
Demographic and clinical information of the ACT total enrollment cohort, ACT cohort at risk of dementia, and Eye ACT cohort as of 03/2023.
| ACT total enrollment N=5763 | Currently at risk of dementia in ACT (excluding Eye ACT participants) N=1868 | Prospective Eye ACT cohort to date N=330 | p-valuea | |
|---|---|---|---|---|
|
| ||||
| Total follow-up since ACT baseline, in person-years | 44,886.90 | 13,303.00 | 1574.9 | - |
|
| ||||
| Mean age at ACT enrollment (SD) | 74.1 (6.4) | 71.2 (4.7) | 70.3 (4.1) | 0.014 |
|
| ||||
| Median number of ACT visits since ACT baseline (range) | 4 (1–14) | 4 (1–14) | 3 (1–13) | < 0.001 |
|
| ||||
| Female | 58% | 60% | 50% | < 0.001 |
|
| ||||
| Raceb | 0.005 | |||
| White | 89.4% | 87.2% | 92.4% | |
| Black | 3.6% | 3.4% | 0.3% | |
| Asian | 3.5% | 4.4% | 2.4% | |
| American Indian, or Alaskan Native | 0.2% | 0.1% | - | |
| Native Hawaiian or Pacific Islander | 0.1% | 0.1% | - | |
| Mixed race or Other | 3.3% | 4.8% | 4.9% | |
|
| ||||
| Hispanic ethnicity | 1% | 2% | 2% | 0.84 |
|
| ||||
| Mean years of education (SD)c | 15.0 (3.2) | 16.2 (3.0) | 17.7 (2.4) | < 0.001 |
|
| ||||
| Any APOE ε4d | 26% | 26% | 27% | 0.68 |
|
| ||||
| Cardiovascular diseasee | 28% | 16% | 10% | 0.008 |
|
| ||||
| Cerebrovascular diseasee | 21% | 11% | 6% | 0.01 |
|
| ||||
| History of a diabetes prescriptionf | 16% | 13% | 10% | 0.07 |
|
| ||||
| Hypertensione | 60% | 53% | 45% | 0.004 |
|
| ||||
| Smokinge | 0.94 | |||
| Never | 48% | 54% | 54% | |
| Past | 48% | 43% | 44% | |
| Current | 3% | 3% | 2% | |
|
| ||||
| Median number of clinical eye encounters [IQR]f | 22 [10–39] | 25 [13–43] | 29 [15–41] | 0.19 |
|
| ||||
| Median number of VA measures [IQR]f | 11 [1–24] | 14 [6–25] | 16 [9–25] | 0.01 |
|
| ||||
| Median number of IOP measures [IQR]f | 10 [1–21] | 13 [6–22] | 13 [8–22] | 0.02 |
|
| ||||
| Presence of visual impairment in at least one eyef | 37% | 22% | 12% | <0.001 |
|
| ||||
| Presence of AMDf | 32% | 25% | 18% | 0.02 |
|
| ||||
| Presence of DRf | 8% | 6% | 4% | 0.18 |
|
| ||||
| Presence of glaucomaf | 19% | 14% | 14% | 1.00 |
|
| ||||
| Ever diagnosed with cataractsf | 86% | 90% | 93% | 0.09 |
|
| ||||
| History of cataract surgeryf | 48% | 54% | 50% | 0.15 |
ACT, Adult Changes in Thought; AMD, age-related macular degeneration; APOE ε4, Apolipoprotein E gene ε4 allele; DR, diabetic retinopathy; IOP, intraocular pressure; IQR, interquartile range; SD, standard deviation; VA, visual acuity.
P-values compare people in Eye ACT to the rest of those currently at risk of AD/ADRD. They are from Wilcoxon’s rank sum statistic for continuous variables and Fisher’s exact test for categorical variables.
ACT participants self-identified their race.
Truncated at 21 years.
Available for 4686 (81%) in ACT, 1698 (91%) Currently at risk of dementia and not in Eye ACT, and 224 (68%) in Eye ACT.
Self-report, most recent ACT study biennial visit.
Most recent data from electronic health records.
Parent ACT study participants had a slightly higher rate of subjective distance (12% vs 9%) and near (12% vs 10%) vision problems using self-reported questionnaire data than the Eye ACT cohort, but neither were statistically significant (p-values=0.25 and 0.51, respectively). On review of retrospective eye data, Eye ACT participants experienced lower rates of visual impairment (12% vs. 22%, p<0.001) and AMD (18 vs. 25%, p=0.02) than non-Eye ACT study participants.
Comparison of Eye ACT participants who were evaluated in research clinic and at home
Eye ACT participants evaluated in their homes were older at their first Eye ACT study visit (mean age 77.2 vs. 74.9, p=0.015) and more likely to be female (60% vs. 49%, p=0.026) than those evaluated at the research clinic (Table 2). On the ACT survey questionnaire, non-statistically significant differences in subjective distance (10% vs 9%) and near (17% vs 10%) vision problems were reported between home and clinic visit cohorts (p-values=0.74 and 0.22, respectively).
Table 2.
Characteristics and baseline Eye ACT study visit data from the eye with worse visual acuity in the first 300 prospective Eye ACT study clinic visit participants and 30 home visit participants.
| Clinic visit (N=300) | Home visit (N=30) | p-valueb | |||
|---|---|---|---|---|---|
|
| |||||
| Na | Mean (SD) or % | N1 | Mean (SD) or % | ||
|
| |||||
| Number of ACT visits as of 03/2023 | 300 | 3.3 (2.1) | 30 | 4.5 (2.2) | 0.003 |
|
| |||||
| Age at Eye ACT baseline | 300 | 74.9 (4.9) | 30 | 77.2 (5.7) | 0.015 |
|
| |||||
| Female sex | 300 | 49% | 30 | 60% | 0.026 |
|
| |||||
| Race c | 300 | 30 | 0.73 | ||
| White | 92.30% | 93.30% | |||
| Black | 0.30% | 0% | |||
| Asian | 2.70% | 0% | |||
| Mixed race or Other | 4.70% | 6.70% | |||
|
| |||||
| Education at ACT baseline (years) | 300 | 17.8 (2.4) | 30 | 17.0 (2.6) | 0.15 |
|
| |||||
| CASI-IRT scored | 297 | 0.00 (1.0) | 30 | 0.03 (1.1) | 0.97 |
|
| |||||
| Visual Acuity (ETDRS)e,f | 298 | 78.9 (8.9) | 29 | 71.1(15.6) | < 0.001 |
|
| |||||
| Contrast Sensitivity, 6CPD logf,g | 297 | -1.73 (0.40) | 29 | -1.46 (0.61) | 0.003 |
|
| |||||
| Contrast Sensitivity, 3CPD logf,g | 297 | -2.06 (0.37) | 29 | -1.85 (0.61) | 0.002 |
|
| |||||
| Intraocular pressure, mmHgf | 264 | 14.2 (4.0) | 29 | 13.6 (3.5) | 0.46 |
|
| |||||
| Total macular thickness, mmf | 247 | 266.8 (17.1) | 29 | 266.4 (26.5) | 0.37 |
|
| |||||
| Total foveal thickness, mmf | 247 | 214.4 (41.1) | 29 | 227.6 (60.9) | 0.27 |
|
| |||||
| Macular RNFL thickness, mmf | 247 | 33.9 (6.8) | 29 | 31.5 (7.7) | 0.14 |
|
| |||||
| Macular GC-IPL thickness, μmf | 247 | 63.8 (7.2) | 29 | 64.8 (10.2) | 0.89 |
ACT, Adult Changes in Thought; CASI-IRT, Cognitive Abilities Screening Instrument-Item Response Theory; ETDRS, Early Treatment Diabetic Retinopathy Study; CPD, cycles per degree; GC-IPL, ganglion cell/inner plexiform layer; RNFL, retinal nerve fiber layer.
Reasons for missing data included incomplete data collection, patient related factors, or insufficient data quality for analysis.
Wilcoxon rank sum for continuous variables, Fisher’s exact for categorical.
ACT participants self-identified their race.
IRT-based CASI score, standardized to a mean of 0 (SD 1) in this Eye ACT sample at the nearest ACT biennial visit before the initial Eye ACT visit.
Early Treatment Diabetic Retinopathy Study visual acuity scores. Higher ETDRS values indicate better visual acuity.
Data from the worst visual acuity eye.
More negative log value indicates better contrast sensitivity.
Unlike the subjective survey results, participants seen at home had significantly worse visual function, demonstrating worse VA (mean 71.1 vs. 78.9 ETDRS letters, p<0.001) and CS (−1.9 vs. −2.1 mean log units at 3-cycles-per-degree, p=0.002) compared to their counterparts in the eye with worse visual acuity. However, CASI-IRT scores (0.03 vs. 0.00, p=0.97) and OCT retinal layer thickness measurements were similar between the two groups (Table 2, Supplementary Methods). At home participants also showed worse visual function and thinner total macular thickness in the better seeing eye. (Supplementary Table 3) OCTA measures from participants seen in the clinic are shown in Supplementary Table 4. There were no statistically significant differences in clinical health variables between the clinic and home visit groups (Supplementary Table 5).
Data acquired since the most recent ACT data freeze
As of 4/1/24, a total of 610 participants have been recruited and have similar demographic characteristics as the inaugural cohort of 330 in the analytic set. Mean age at ACT enrollement is 70.0 (SD 3.8), mean age at Eye ACT enrollment is 78.8 (5.0), 49% are women, and 92% are self-reported White.
DISCUSSION
Eye ACT is a prospective, longitudinal, community-based study of older adults that provides an unparalleled opportunity to investigate the relationship between the eye and brain, with unique home visit capacity and unprecedented past eye health data from both paper chart and EHR data. In this initial report, we compare demographic and clinical characteristics of Eye ACT study participants to the parent ACT study to better understand potential sources of selection bias in future analyses of associations between ophthalmic biomarkers and AD/ADRD risk. Compared to the rest of the parent ACT participants, current Eye ACT study participants were younger at ACT enrollment, more recently enrolled in the ACT study, and overall healthier. However, these differences were small. Among the Eye ACT cohort, participants seen at home had significantly worse visual acuity and contrast sensitivity compared to participants seen in the research clinic, while cognitive scores and the majority of the optical coherence tomography measurements were similar.
The Eye ACT cohort should provide opportunities to address several conflicting findings in the existing literature on retinal imaging biomarkers of AD/ADRD. In many studies, retinal thinning of the RNFL and GC-IPL, and retinal vascular changes including decreased foveal avascular zone, increased vessel tortuosity, and lower vessel density of deep capillary plexus were reported to be associated with AD/ADRD.[3,11,24,25,31] However, many other studies found no significant associations.[18–20,27] Conflicting results may be due to cross-sectional designs,[24–27] small sample sizes, lack of longitudinal studies,[50] disparate protocols for vision measures and retinal imaging, and variability of dementia diagnosis characterization.[24–29] The Eye ACT study can address some of these gaps with the incorporation of prospective data collection on retinal biomarkers in a large, cognitively well-characterized cohort and by including vision function data, retinal imaging, and clinical ophthalmic data. Eye ACT is also unique in incorporating eye disease history and visual function in parallel with eye imaging data collection. Previous studies have found associations between eye diseases and decreased cognition,[51,52] vision impairment and dementia,[52,53] and correlations between cataract surgery and lower risk of AD/ADRD.[54,55]
When designing a prospective, observational study with voluntary participation, it is important to understand potential selection biases that would undermine accuracy of future association studies.[46,56–58] We found several factors that were considerably different between Eye ACT participants and other active ACT study participants. Eye ACT participants were overall healthier, displaying decreased frequency of cardiovascular disease, cerebrovascular disease, hypertension, visual impairment, and AMD. All of these comorbidities have been shown to be associated with risks of AD/ADRD.[51,52,54,59–61] Eye ACT participants were more likely to be male, and had slightly higher education levels compared to the parent ACT cohort. Although Eye ACT participants were generally healthier, a slightly higher percentage of them had cataracts compared to ACT participants. Even though this difference was nonsignificant, ascertainment bias is also a possibility since Eye ACT participants were more likely to seek eye care based on the higher numebr of eye encounters and therefore may be diagnosed with cataracts earlier. As Eye ACT recruitment from the parent ACT study expands to include more participants seen at home, these differences will likely decrease, and we will account for any remaining sources of selection bias in future studies. We routinely perform inverse probability weighting (IPW) to account for selection bias among participants with brain autopsy consent.[62] Survival bias may also be an issue, which we will address by weighting longitudinal models by the probability of death or dropout[54] or by treating death and dropout as competing risks.
Prior work by Crane et al. involving the parent ACT study revealed the critical importance of enabling study visits at home given the added difficulty in traveling to clinic settings for those who are frail, physically ill, or cognitively impaired.[46] Patients who cannot travel to research clinics are currently underrepresented in dementia research and may have differing dementia risk profiles. In that prior work, the authors found a 23%, 39% and 75% reduction in sample size, follow-up time, and number of incident AD/ADRD cases, respectively, in the cohort restricted to clinic-only participants compared to those seen either at home or in clinic. More importantly, analyses using data from clinic-only participants alone resulted in overestimation of the association of several factors—age, cerebrovascular disease, APOE, and several neuropathological findings—with AD/ADRD risk compared to the results for participants being seen at home or in clinic.[46] This highlights the issue of selection bias and consequently inaccurate conclusions in studies that only include research clinic data collection. Validation of clinic-based study results using community-based studies with home visit capacity is critical in dementia research.
Sensory impairment is one of the primary risk factors of interest for dementia development. Visual impairment may contribute to social isolation and decreased cognitive stimulation, which may exacerbate dementia risk.[54,63,64] Eye ACT participants evaluated at home were older and had significantly worse visual acuity and contrast sensitivity than those seen in clinic, potentially making them at higher dementia risk compared to those seen in clinic. While we did not find significant differences in CASI scores between participants evaluated in the research clinic versus at home in this pilot sample, the differences in risk factors between these groups highlight the need for the longitudinal follow-up.
Interestingly, while functional eye measurements (i.e. visual acuity and contrast sensitivity) were significantly different between clinic and at home groups, no obvious difference was noted in imaging measurements, except for a difference in total macular thickness in the better seeing eye between cohorts. Several studies have previously shown that functional ophthalmic measures are associated with cognitive impairment and dementia risks,[65–67] thus our findings may suggest that functional deficits could occur earlier than detectable imaging changes in AD/ADRD pathology development. Patient-dependent factors, such as cooperation and concentration level during data collection. can induce noise in measuring functional deficits, making later analysis and ability to draw meaningful insight from the data more difficult.[68–70] However, the anticipated Eye ACT longitudinal data, which will follow both functional and structural imaging deficits, will provide more insights.
Several limitations exist with our study. Currently, the ACT study is >90% non-Hispanic white. However, ongoing initiatives of the parent ACT study include increasing sample size from 2000 to 3000 with targeted recruitment of racial and ethnic minority groups. Thus, Eye ACT is also increasing its recruitment goal to enroll a more diverse population, which will increase the generalizability of the study findings. The pilot data only include cross-sectional evaluations, but future biennial visits will allow longitudinal data analyses. Recruitment is ongoing with over 600 participants to date towards a goal of ≥ 80% of all eligible ACT participants. We only analyzed data from the most recent ACT data freeze date (03/2023) since more recent data acquired after the data freeze are not as complete due extensive quality control that happens at the time of the data freeze, including conversion of the CASI scores into IRT. Missing data are an important consideration. OCTA was not available at home visits initially but is now being added to home visits. To our best knowledge, this will be the first OCTA capture of older adults at home, allowing assessment of whether retinal vascular health significantly differs between those seen at home vs in clinic. Image quality can vary depending on participant factors such as small pupil size, tremor, and fatigue during study visits. We plan to identify non-random missing variables and adjust for bias using statistical methods such as IPW.[62]
In summary, this study has identified potential sources of bias and is designed to address them, as well as other gaps in previous research. Home visit participants had significantly decreased visual function compared to research clinic participants. Given potentially high rates of dementia among home visit participants in the ACT study, continuing to recruit at-home participants is critical to remove selection bias in future ophthalmic biomarker and dementia risk association studies. With its novel combination of functional and imaging eye measurements, retrospective eye health data, study setting, and anticipated longitudinal follow-up, Eye ACT provides unique strengths to assess potential retinal biomarkers of dementia.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the participants of the Adult Changes in Thought (ACT) study for the data they have provided and the many ACT investigators and staff who steward that data. You can learn more about ACT at: https://actagingstudy.org/.
FUNDING
This research was funded by the National Institute on Aging (R01AG060942, U19AG066567). Data collection for this work was additionally supported, in part, by prior funding from the National Institute on Aging (U01AG006781). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the National Institute on Aging or the National Institutes of Health. Additional funding included NIH grant OT2OD032644, the Latham Vision Research Innovation Award (Seattle, WA), the Klorfine Family Endowed Chair, the Karalis Johnson Retina Center, and an unrestricted grant from Research to Prevent Blindness. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
CONFLICT OF INTEREST
Dr. A. Lee reports support from the US Food and Drug Administration, grants from Amazon, Carl Zeiss Meditec, iCareWorld, Meta, Microsoft, Novartis, NVIDIA, Regeneron, Santen Pharmaceutical, and Topcon, personal fees from Alcon, Boehringer Ingelheim, Genentech/Roche, Gyroscope, Janssen, Johnson & Johnson, and Verana Health outside of the submitted work, and nonfinancial support from Microsoft outside of the submitted work. This article does not reflect the opinions of the Food and Drug Administration. All other authors have no conflict of interest to report. Dr. Cecilia Lee is an Associate Editor of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review.
DATA AVAILABILITY STATEMENT
Data from this analysis cannot be made publicly available for ethical and legal reasons. In order to replicate our findings, a researcher may need access to personal health identifiers (PHI) Version 2/24/2023 2 including dates of birth and death, dates of diagnoses, and ages over 89. These are required variables for the analysis, and we cannot publicly release this information without IRB approval and a Data Use Agreement with interested researchers. However, the datasets used and/or analyzed in the current study are available upon reasonable request and execution of appropriate human subjects review and data sharing agreements by following the process described on the Adult Changes in Thought (ACT) website: actagingresearch.org.
REFERENCES
- [1].Hinton DR, Sadun AA, Blanks JC, Miller CA (1986) Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315, 485–487. [DOI] [PubMed] [Google Scholar]
- [2].Iseri PK, Altinaş O, Tokay T, Yüksel N (2006) Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 26, 18–24. [DOI] [PubMed] [Google Scholar]
- [3].Garcia-Martin E, Bambo MP, Marques ML, Satue M, Otin S, Larrosa JM, Polo V, Pablo LE (2016) Ganglion cell layer measurements correlate with disease severity in patients with Alzheimer’s disease. Acta Ophthalmol 94, e454–9. [DOI] [PubMed] [Google Scholar]
- [4].Bambo MP, Garcia-Martin E, Pinilla J, Herrero R, Satue M, Otin S, Fuertes I, Marques ML, Pablo LE (2014) Detection of retinal nerve fiber layer degeneration in patients with Alzheimer’s disease using optical coherence tomography: searching new biomarkers. Acta Ophthalmol 92, e581–2. [DOI] [PubMed] [Google Scholar]
- [5].Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M (2013) Retinal nerve fiber layer thickness in patients with Alzheimer disease. J Neuroophthalmol 33, 58–61. [DOI] [PubMed] [Google Scholar]
- [6].Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F, Beutelspacher SC (2014) Detection of Retinal Nerve Fiber Layer Defects in Alzheimer’s Disease Using SD-OCT. Front Psychiatry 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Almeida ALM, Pires LA, Figueiredo EA, Costa-Cunha LVF, Zacharias LC, Preti RC, Monteiro MLR, Cunha LP (2019) Correlation between cognitive impairment and retinal neural loss assessed by swept-source optical coherence tomography in patients with mild cognitive impairment. Alzheimers Dement 11, 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ko F, Muthy ZA, Gallacher J, Sudlow C, Rees G, Yang Q, Keane PA, Petzold A, Khaw PT, Reisman C, Strouthidis NG, Foster PJ, Patel PJ, UK Biobank Eye & Vision Consortium (2018) Association of Retinal Nerve Fiber Layer Thinning With Current and Future Cognitive Decline: A Study Using Optical Coherence Tomography. JAMA Neurol 75, 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Salobrar-Garcia E, Hoyas I, Leal M, de Hoz R, Rojas B, Ramirez AI, Salazar JJ, Yubero R, Gil P, Triviño A, Ramirez JM (2015) Analysis of Retinal Peripapillary Segmentation in Early Alzheimer’s Disease Patients. Biomed Res Int 2015, 636548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chua SYL, Lascaratos G, Atan D, Zhang B, Reisman C, Khaw PT, Smith SM, Matthews PM, Petzold A, Strouthidis NG, Foster PJ, Khawaja AP, Patel PJ, UK Biobank Eye, Vision Consortium (2021) Relationships between retinal layer thickness and brain volumes in the UK Biobank cohort. Eur J Neurol 28, 1490–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].López-de-Eguileta A, López-García S, Lage C, Pozueta A, García-Martínez M, Kazimierczak M, Bravo M, Irure J, López-Hoyos M, Muñoz-Cacho P, Rodríguez-Perez N, Tordesillas-Gutiérrez D, Goikoetxea A, Nebot C, Rodríguez-Rodríguez E, Casado A, Sánchez-Juan P (2022) The retinal ganglion cell layer reflects neurodegenerative changes in cognitively unimpaired individuals. Alzheimers Res Ther 14, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gharbiya M, Trebbastoni A, Parisi F, Manganiello S, Cruciani F, D’Antonio F, De Vico U, Imbriano L, Campanelli A, De Lena C (2014) Choroidal thinning as a new finding in Alzheimer’s disease: evidence from enhanced depth imaging spectral domain optical coherence tomography. J Alzheimers Dis 40, 907–917. [DOI] [PubMed] [Google Scholar]
- [13].Bulut M, Yaman A, Erol MK, Kurtuluş F, Toslak D, Doğan B, Turgut Çoban D, Kaya Başar E (2016) Choroidal Thickness in Patients with Mild Cognitive Impairment and Alzheimer’s Type Dementia. J Ophthalmol 2016, 7291257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cunha JP, Proença R, Dias-Santos A, Melancia D, Almeida R, Águas H, Santos BO, Alves M, Ferreira J, Papoila AL, Louro C, Castanheira-Dinis A (2017) Choroidal thinning: Alzheimer’s disease and aging. Alzheimers Dement 8, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL (2007) Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 48, 2285–2289. [DOI] [PubMed] [Google Scholar]
- [16].Bayhan HA, Aslan Bayhan S, Celikbilek A, Tanık N, Gürdal C (2015) Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Experiment Ophthalmol 43, 145–151. [DOI] [PubMed] [Google Scholar]
- [17].Rifai OM, McGrory S, Robbins CB, Grewal DS, Liu A, Fekrat S, MacGillivray TJ (2021) The application of optical coherence tomography angiography in Alzheimer’s disease: A systematic review. Alzheimers Dement 13, e12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheung CY-L, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig J-AS, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY (2014) Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement 10, 135–142. [DOI] [PubMed] [Google Scholar]
- [19].den Haan J, van de Kreeke JA, van Berckel BN, Barkhof F, Teunissen CE, Scheltens P, Verbraak FD, Bouwman FH (2019) Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement 11, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Heringa SM, Bouvy WH, van den Berg E, Moll AC, Kappelle LJ, Biessels GJ (2013) Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab 33, 983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoon SP, Grewal DS, Thompson AC, Polascik BW, Dunn C, Burke JR, Fekrat S (2019) Retinal Microvascular and Neurodegenerative Changes in Alzheimer’s Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmology Retina 3, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, Silvestri G, Maxwell AP, McKay GJ (2015) Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement 1, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC, Macaulay SL, Szoeke C, Ellis KA, Ames D, Masters CL, Rainey-Smith S, Martins RN, AIBL Research Group (2013) Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry 3, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yuan A, Lee CS (2022) Retinal Biomarkers for Alzheimer Disease: The Facts and the Future. Asia Pac J Ophthalmol (Phila) 11, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, Wong TY, Chen C, Ikram MK, Whitson HE, Lad EM, Mok VCT, Cheung CY (2019) Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 126, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang J, Wang H, Li W, Cao X, Li C (2016) Amyloid Plaques in Retina for Diagnosis in Alzheimer’s Patients: a Meta-Analysis. Frontiers in Aging Neuroscience 8,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jin Q, Lei Y, Wang R, Wu H, Ji K, Ling L (2021) A Systematic Review and Meta-Analysis of Retinal Microvascular Features in Alzheimer’s Disease. Front Aging Neurosci 13, 683824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ge Y-J, Xu W, Ou Y-N, Qu Y, Ma Y-H, Huang Y-Y, Shen X-N, Chen S-D, Tan L, Zhao Q-H, Yu J-T (2021) Retinal biomarkers in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Ageing Research Reviews 69, 101361. [DOI] [PubMed] [Google Scholar]
- [29].O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP (2018) Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol 136, 1242–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O’Bryhim BE, Lin JB, Van Stavern GP, Apte RS (2021) OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology 128, 1489–1491. [DOI] [PubMed] [Google Scholar]
- [31].López-de-Eguileta A, Lage C, López-García S, Pozueta A, García-Martínez M, Kazimierczak M, Bravo M, de Arcocha-Torres M, Banzo I, Jimenez-Bonilla J, Cerveró A, Rodríguez-Rodríguez E, Sánchez-Juan P, Casado A (2019) Ganglion cell layer thinning in prodromal Alzheimer’s disease defined by amyloid PET. Alzheimers Dement 5, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Santos CY, Johnson LN, Sinoff SE, Festa EK, Heindel WC, Snyder PJ (2018) Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement 10, 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Byun MS, Park SW, Lee JH, Yi D, Jeon SY, Choi HJ, Joung H, Ghim UH, Park UC, Kim YK, Shin SA, Yu HG, Lee DY, KBASE Research Group (2021) Association of Retinal Changes With Alzheimer Disease Neuroimaging Biomarkers in Cognitively Normal Individuals. JAMA Ophthalmol 139, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van de Kreeke JA, Nguyen HT, Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, Yaqub M, van Berckel B, Lammertsma AA, Boomsma DI, Tan HS, Visser PJ, Verbraak FD (2021) Longitudinal retinal layer changes in preclinical Alzheimer’s disease. Acta Ophthalmol 99, 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mutlu U, Colijn JM, Ikram MA, Bonnemaijer PWM, Licher S, Wolters FJ, Tiemeier H, Koudstaal PJ, Klaver CCW, Ikram MK (2018) Association of Retinal Neurodegeneration on Optical Coherence Tomography With Dementia: A Population-Based Study. JAMA Neurol 75, 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].den Haan J, van de Kreeke JA, Konijnenberg E, ten Kate M, den Braber A, Barkhof F, van Berckel BN, Teunissen CE, Scheltens P, Visser PJ, Verbraak FD, Bouwman FH (2019) Retinal thickness as a potential biomarker in patients with amyloid-proven early- and late-onset Alzheimer’s disease. Alzheimers Dement 11, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shin JY, Choi EY, Kim M, Lee HK, Byeon SH (2021) Changes in retinal microvasculature and retinal layer thickness in association with apolipoprotein E genotype in Alzheimer’s disease. Sci Rep 11, 1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59, 1737–1746. [DOI] [PubMed] [Google Scholar]
- [39].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].ACT study home. https://actagingresearch.org/ [Google Scholar]
- [41].Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144, 73–81. [DOI] [PubMed] [Google Scholar]
- [42].Montine TJ, Sonnen JA, Montine KS, Crane PK, Larson EB (2012) Adult Changes in Thought Study: Dementia is an Individually Varying Convergent Syndrome with Prevalent Clinically Silent Diseases that may be Modified by Some Commonly Used Therapeutics. Current Alzheimer Research 9, 718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB (2013) Glucose levels and risk of dementia. N Engl J Med 369, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gibbons LE, Power MC, Walker RL, Kumar RG, Murphy A, Latimer CS, Nolan AL, Melief EJ, Beller A, Bogdani M, Keene CD, Larson EB, Crane PK, Dams-O’Connor K (2023) Association of Traumatic Brain Injury with Late Life Neuropathological Outcomes in a Community-Based Cohort. J Alzheimers Dis 93, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D (1994) The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 6, 45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- [46].Crane PK, Gibbons LE, McCurry SM, McCormick W, Bowen JD, Sonnen J, Keene CD, Grabowski T, Montine TJ, Larson EB (2016) Importance of home study visit capacity in dementia studies. Alzheimers Dement 12, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47] <j/>(1991).Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 98, 741–756. [DOI] [PubMed] [Google Scholar]
- [48].Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I (1982) New visual acuity charts for clinical research. Am J Ophthalmol 94, 91–96. [PubMed] [Google Scholar]
- [49].Harris PA, German Z, Roberts L (2019) Normal Values for Mesopic and Photopic Contrast Sensitivity Function with and without Glare using a Sinusoidal Bull’s-Eye Target. Optometry & Visual Performance 7, 138–148. [Google Scholar]
- [50].Costanzo E, Lengyel I, Parravano M, Biagini I, Veldsman M, Badhwar A, Betts M, Cherubini A, Llewellyn DJ, Lourida I, MacGillivray T, Rittman T, Tamburin S, Tai XY, Virgili G (2023) Ocular Biomarkers for Alzheimer Disease Dementia: An Umbrella Review of Systematic Reviews and Meta-analyses. JAMA Ophthalmol 141, 84–91. [DOI] [PubMed] [Google Scholar]
- [51].Lee CS, Larson EB, Gibbons LE, Lee AY, McCurry SM, Bowen JD, McCormick WC, Crane PK (2019) Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement 15, 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kuźma E, Littlejohns TJ, Khawaja AP, Llewellyn DJ, Ukoumunne OC, Thiem U (2021) Visual Impairment, Eye Diseases, and Dementia Risk: A Systematic Review and Meta-Analysis. J Alzheimers Dis 83, 1073–1087. [DOI] [PubMed] [Google Scholar]
- [53].Ehrlich JR, Goldstein J, Swenor BK, Whitson H, Langa KM, Veliz P (2022) Addition of Vision Impairment to a Life-Course Model of Potentially Modifiable Dementia Risk Factors in the US. JAMA Neurol 79, 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee CS, Gibbons LE, Lee AY, Yanagihara RT, Blazes MS, Lee ML, McCurry SM, Bowen JD, McCormick WC, Crane PK, Larson EB (2021) Association Between Cataract Extraction and Development of Dementia. JAMA Intern Med 182, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ma L-Z, Zhang Y-R, Li Y-Z, Ou Y-N, Yang L, Chen S-D, Dong Q, Feng J-F, Cheng W, Tan L, Yu J-T (2022) Cataract, cataract surgery, and risk of incident dementia: a prospective cohort study of 300,823 participants. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- [56].Wrobel AJ, Shapiro NE (1999) Conducting research with urban elders: issues of recruitment, data collection, and home visits. Alzheimer Dis Assoc Disord 13 Suppl 1, S34–8. [PubMed] [Google Scholar]
- [57].Anglemyer A, Horvath HT, Bero L (2014) Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014, MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lu CY (2009) Observational studies: a review of study designs, challenges and strategies to reduce confounding. Int J Clin Pract 63, 691–697. [DOI] [PubMed] [Google Scholar]
- [59].Leszek J, Mikhaylenko EV, Belousov DM, Koutsouraki E, Szczechowiak K, Kobusiak-Prokopowicz M, Mysiak A, Diniz BS, Somasundaram SG, Kirkland CE, Aliev G (2021) The Links between Cardiovascular Diseases and Alzheimer’s Disease. Curr Neuropharmacol 19, 152–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Love S, Miners JS (2016) Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol 131, 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Santos CY, Snyder PJ, Wu W-C, Zhang M, Echeverria A, Alber J (2017) Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement 7, 69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cole SR, Hernán MA (2008) Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Whitson HE, Cronin-Golomb A, Cruickshanks KJ, Gilmore GC, Owsley C, Peelle JE, Recanzone G, Sharma A, Swenor B, Yaffe K, Lin FR (2018) American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: Sensory Impairment and Cognitive Decline in Older Adults. Journal of the American Geriatrics Society 66, 2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ward ME, Gelfand JM, Lui L-Y, Ou Y, Green AJ, Stone K, Pedula KL, Cummings SR, Yaffe K (2018) Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment: Reduced Contrast Sensitivity. Ann Neurol 83, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Elyashiv SM, Shabtai EL, Belkin M (2014) Correlation between visual acuity and cognitive functions. Br J Ophthalmol 98, 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee ATC, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW (2020) Higher Dementia Incidence in Older Adults with Poor Visual Acuity. J Gerontol A Biol Sci Med Sci 75, 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bennett CR, Bex PJ, Bauer CM, Merabet LB (2019) The Assessment of Visual Function and Functional Vision. Semin Pediatr Neurol 31, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mitchell RA (1993) Contrast sensitivity in elderly subjects with a diagnosed ocular disease. Optom Vis Sci 70, 102–106. [DOI] [PubMed] [Google Scholar]
- [70].Wood JM, Lovie-Kitchin JE (1992) Evaluation of the efficacy of contrast sensitivity measures for the detection of early primary open-angle glaucoma. Optom Vis Sci 69, 175–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this analysis cannot be made publicly available for ethical and legal reasons. In order to replicate our findings, a researcher may need access to personal health identifiers (PHI) Version 2/24/2023 2 including dates of birth and death, dates of diagnoses, and ages over 89. These are required variables for the analysis, and we cannot publicly release this information without IRB approval and a Data Use Agreement with interested researchers. However, the datasets used and/or analyzed in the current study are available upon reasonable request and execution of appropriate human subjects review and data sharing agreements by following the process described on the Adult Changes in Thought (ACT) website: actagingresearch.org.


