Abstract
Background:
Ear keloids are pathologic scar hyperplasia in the ear region. The most therapeutic approach was surgical shave excision with radiation therapy. However, radiation therapy is easily delivered to healthy surrounding tissues. In the last years, injections with botulinum toxin type A (BTX-A) have been proven to improve surgical scars effectively in clinical trials. This study aimed to evaluate the effect of immediate injections of BTX-A after surgical excision for ear keloids.
Methods:
From January 2020 to January 2023, 33 consecutive patients with ear keloids were enrolled. All patients underwent scar excision and revision at the same time when they needed BTX-A. It was injected into surgical wound closure immediately after surgery. The results of this study were evaluated at follow-up from 7 to 18 months using the Vancouver Scar Scale (VSS) and the Visual Analogue Scale (VAS).
Results:
From January 2020 to January 2023, 33 patients received concomitant therapy of immediate injections of BTX-A after surgery for ear keloids. The patients were evaluated at follow-ups lasting 7 to 18 months. Only one case recurred within the follow-up period, and no adverse effects were reported.
Conclusion:
This study demonstrates that significant cosmetic outcomes in ear keloid treatment were achieved after early postsurgical BTX-A injections. The patients reported high satisfaction and few complications.
Key words: BTX-A injections, concomitant therapy, ear keloids
The ear keloids are hypertrophic scars that commonly arise in the ear region that mostly affect patients' psychosocial.1 Although different therapies have been reported to prevent or treat ear keloids, successful treatment remains difficult, and few treatment modalities have been proven ideal. First-line treatment of keloids including steroid creams and silicone creams have been shown to have little, if any, benefit.2,3 The surgical shave excision with radiation therapy may delivered to healthy surrounding tissues.4 It is, therefore, clear that a simple, safe, and effective treatment for ear keloids is greatly needed.
In recent years, injections with botulinum toxin type A (BTX-A) were proposed for the treatment of established scars in clinical investigation and could be an effective treatment.5,6 To this day, its potential mechanism theory still remains unclear.7 Currently, there are no studies evaluating immediate injections of BTX-A after surgical excision for ear keloids treatment. We conducted the study to investigate the safety and efficacy of intralesional BTX-A for the early postsurgical treatment of ear keloids using objective measurements. At the end of the follow-up period, most subjects showed significant reduction after treatment in comparison to that before treatment with more degree of clinical improvement. The primary objective of this study is to evaluate the efficacy of immediate injections of BTXA after surgical excision to make recommendations for the management of ear keloids.
SURGERY DESIGN AND PROCEDURE
Patient Sample Size
From January 2020 to January 2023, 33 patients received early postoperative injections of BTXA after surgical excision for ear keloids. Twenty-five females and 8 males were included, with a mean age of 32 years (ages 17–65 y). We performed an institutional board-approved retrospective review of our database for them. The follow-up period was 7 to 18 months.
Patients and Randomization
From January 2020 to January 2023, 33 consecutive patients who underwent postoperative injections of BTXA after surgical excision for ear keloids were enrolled. The original diagnoses were secondary ear keloids. We referred to a prospective trial that described a mean dose of 18 U for ear keloids.
Study Procedures
All procedures were performed under local anesthesia. Vials containing 100 U of BTX-A were mixed with 2 to 3 mL of 0.9% saline. Immediately after skin closure, patients were submitted for BTXA treatment. The injections were intradermal during all procedures, and the maximum drug injected was 20 U.
Evaluation of Clinical Assessments
At the 7- to 18-month follow-up appointment, plastic surgeons were asked to make the treatment effect using the VSS and the VAS (from 0 to 10, with 0 = worst and 10 = best) score. The surgeon recorded the recurrence within the follow-up period, and adverse events were also observed. Standardized digital photographs were taken at the same time.
RESULTS
Thirty-three enrolled subjects (25 female patients and 8 male patients) with 44 ear keloids completed this study. The mean age was 32.6 years (ages 17–65 y). All the incisions healed well and near-complete absence of side effects such as skin atrophy in the follow-up appointment. Only one case recurrence was observed in subjects. The case was again treated with BTX-A injection after surgical excision, still in the observation stage (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/SCS/G734). A significant reduction of subjective symptoms was achieved after the treatment. The treatment produced better, narrower, and flatter surgical scars (Figs. 1, 2, 3). The VSS scores and the VAS scores were statistically significant (Figs. 4, 5). This suggests that concomitant therapy can prevent the recurrence of ear keloid and optimize the therapeutic effect.
FIGURE 1.
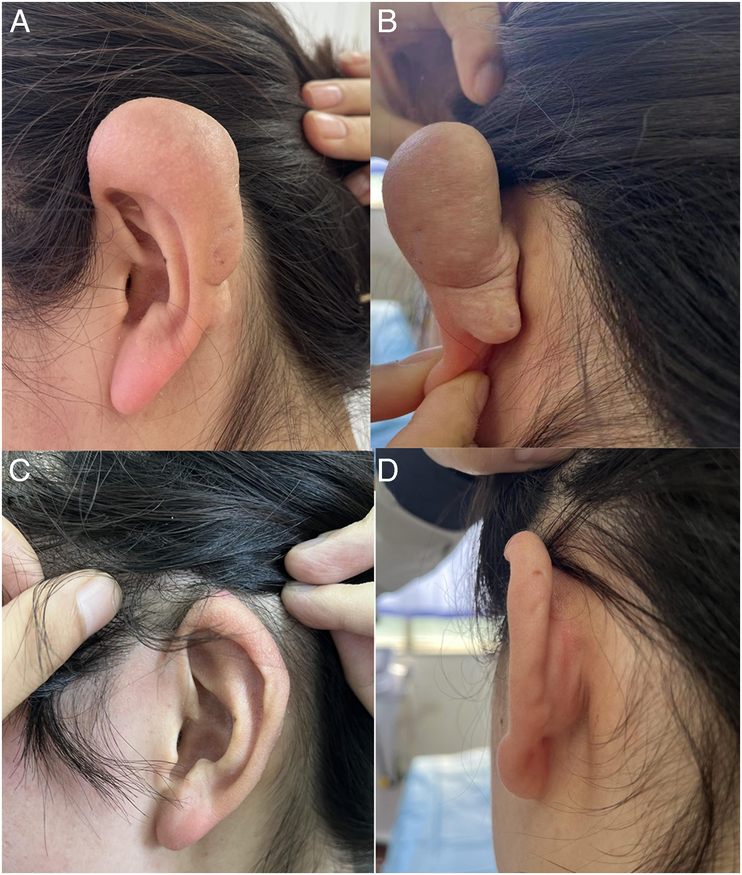
This patient had a keloid on the auricular. (A and B) Preoperative view. (C and D) View 10 months after the operation with BTXA injection.
FIGURE 2.
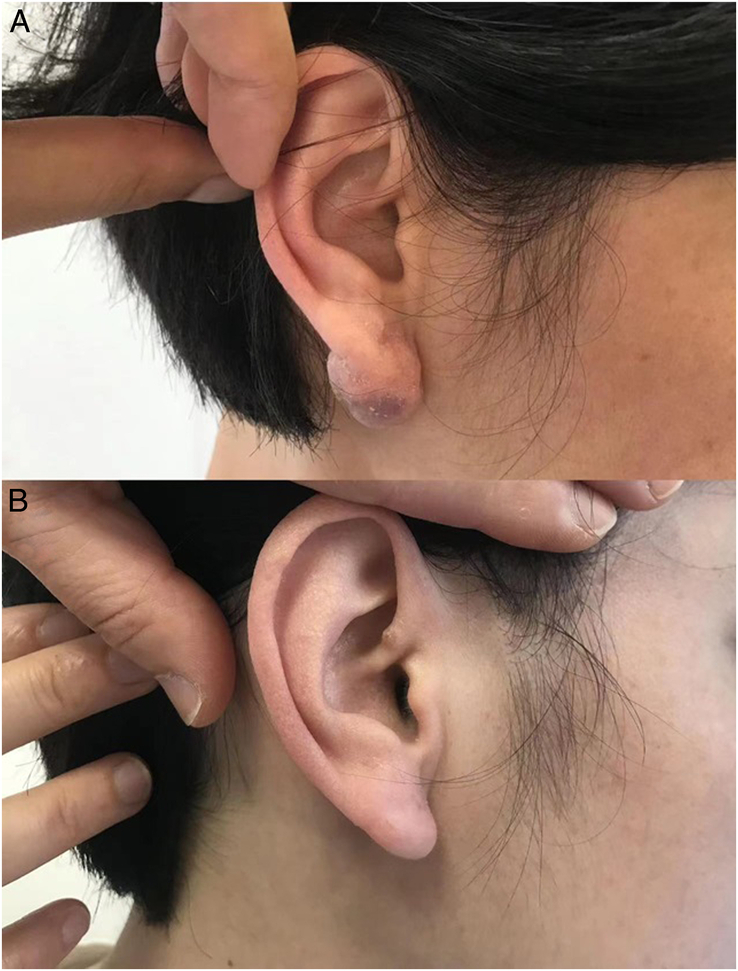
A 23-year-old woman had a keloid on her ear lobe (A) Preoperative view of a 23-year-old patient showing keloid on the ear lobe. (B) Postoperative view at 9 months after the treatment.
FIGURE 3.
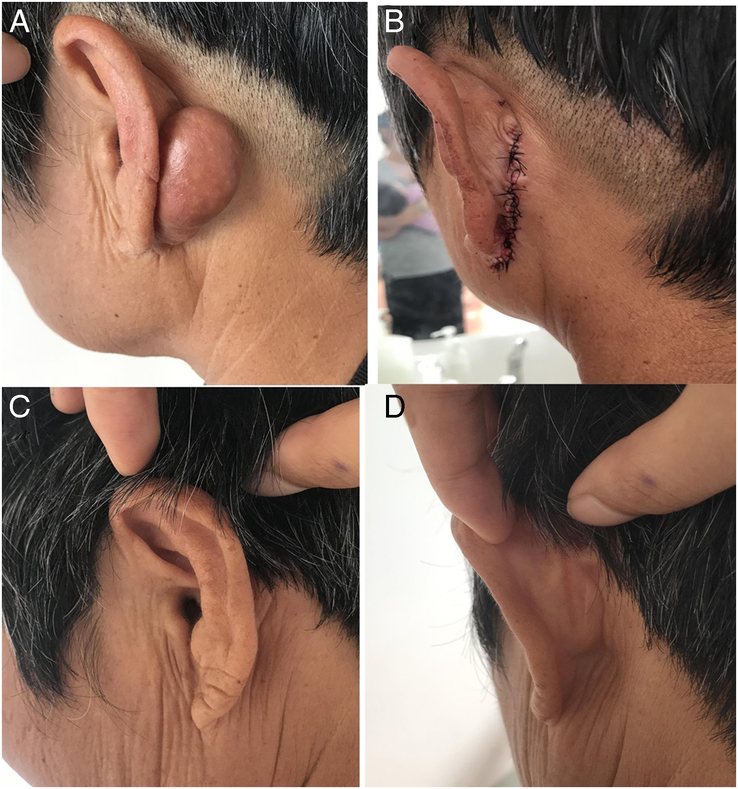
A 65-year-old woman had a keloid behind her ear. (A and B) Preoperative view of the patient. (C and D) Postoperative view at 18 months after the operation with BTXA injection.
FIGURE 4.
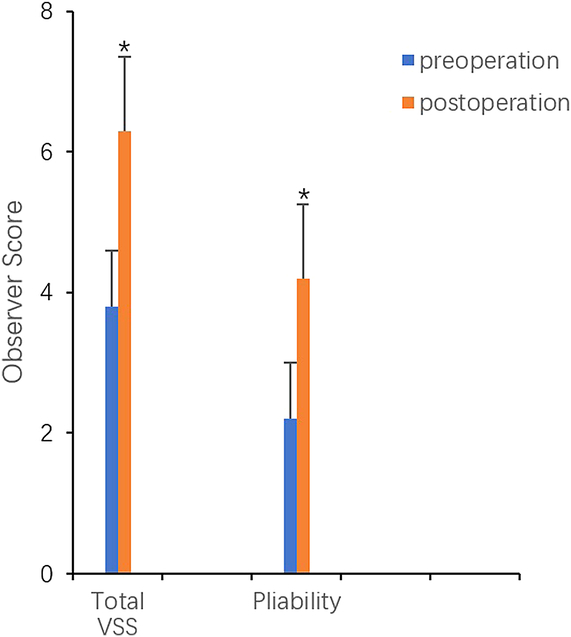
Improvement of Vancouver Scar Scale scores (*P < 0.05).
FIGURE 5.
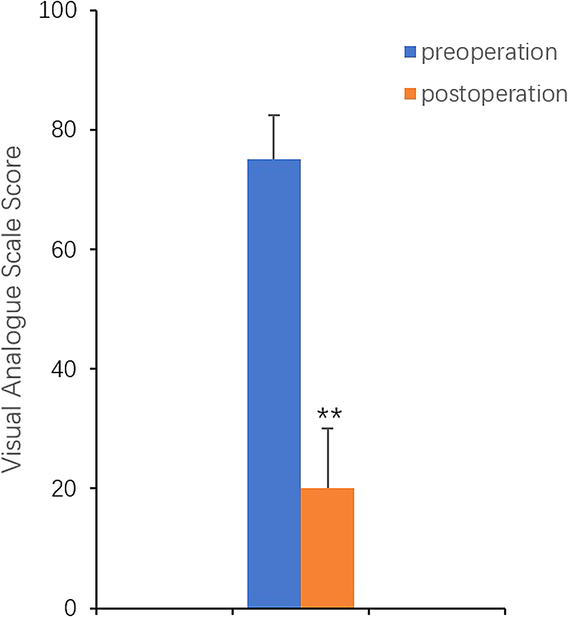
Improvement of visual analogue scale scores (**P < 0.01).
DISCUSSION
Ear keloids are cutaneous scarring that commonly arise in the ear region that mostly affects patients psychosocial and sometimes behave more like benign tumors.8,9 Besides the poor esthetic appearance, ear keloids can be associated with severe clinical symptoms such as itching and rigidity. Most therapeutic approaches have been used for ear keloids in recent years, from surgical to nonsurgical methods. Although surgery is one of the main options, which are prone to relapse and cannot be completely cured, so combination of surgical measures with injection or radiation for ear keloids is recommended.10,11 Unfortunately, the side effects quality of corticosteroids, radiation therapy, and 5-FU were such as skin atrophy and telangiectasia.
New esthetic uses of BTX-A have been discovered for decades, and it is still an active area of research. Recently, there has been an increasing use of BTX-A for the treatment of postsurgical scars, which has been proposed that BTX-A can inhibit the secretion of inflammatory factors and growth factors that promote scar formation.12–14
At present, the treatment of early postoperative injections of BTXA after surgical excision used for ear keloids has never been tested in clinical trials, as the use of BTX-A in the treatment of keloids has been an issue of dispute. The concomitant therapy for ear keloids was found to be effective in our subjects. All the patients in this study were safe and had no complications. Our study detected a significant clinical improvement in ear keloids assessed by VSS and VAS. The therapeutic approaches remain clinically satisfactory.
Our results come in agreement with several previous reports that documented the efficacy of BTX-A in the treatment of keloids.15,16 One study17 conducted an analysis of BTX-A in treating keloids, and they concluded that BTX-A is better than steroids. Aslo Hu et al18 found that the scars after BTX-A injection during revision surgery were highly satisfactory, which was in agreement with our results.
There have been different theories on the mechanism of BTX-A on keloids.19–21 Although the mechanism is still debatable, BTX-A has already been used in patients for the prevention and treatment of scars and keloids, and its clinical effectiveness is becoming more evident,22,23 and the results of our study documented the efficacy of BTX-A in the management of ear keloids.
Our technology is an effective therapy that is highly satisfying in preventing ear keloid recurrence, and it is, therefore, recommended as a successful treatment for ear keloids.
According to published reports and clinical investigations, our study was the first to pilot the efficacy of concomitant therapy of BTXA injections after surgical excision for ear keloids and detected significant clinical improvements.
In conclusion, the high patient satisfaction, higher security, and more rapid efficacy suggest that this therapy should be considered a preferential treatment for ear keloids.
Limitations of the Study
Although our study involved only 33 patients, studies with a large sample size are necessary to confirm the results. The period of follow-up still required longer observation to estimate the effect of this treatment modality on ear keloid management.
CONCLUSION
In summary, this study demonstrates the results on the effectiveness of concomitant surgical excision and BTXA injections for ear keloids could be a safe and effective method.
Supplementary Material
Footnotes
S.H.L. is the first author.
The authors report no conflicts of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by the author.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.jcraniofacialsurgery.com.
Contributor Information
Shu Hua Li, Email: caozhe7531@163.com.
Xiu Juan Shan, Email: 15863621025@163.com.
Zhen Hua Wang, Email: wfzcwzh@126.com.
Shu Jie Tao, Email: 610181718@qq.com.
REFERENCES
- 1.Jeong HS, Lee BH, Sung HM, et al. Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg 2015;136:171e–178e [DOI] [PubMed] [Google Scholar]
- 2.Zhang DZ, Liu XY, Xiao WL, et al. Botulinum toxin type A and the prevention of hypertrophic scars on the maxillofacial area and neck: a meta-analysis of randomized controlled trials. Plos One 2016;11:e0151627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu DQ, Li XJ, Weng XJ. Effect of BTXA on inhibiting hypertrophic scar formation in a rabbit ear model. Aesthetic Plast Surg 2017;41:721–728 [DOI] [PubMed] [Google Scholar]
- 4.Ru-Lin Huang, Chia-Kang Ho, Mathias Tremp, et al. Early postoperative application of botulinum toxin type A prevents hypertrophic scarring after epicanthoplasty: a split-face, double-blind. Randomized Trial Plast Reconstr Surg 2019;144:835–844 [DOI] [PubMed] [Google Scholar]
- 5.Perdanasari AT, Lazzeri D, Su W, et al. Recent developments in the use of intralesional injections keloid treatment. Arch Plast Surg 2014;41:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JJ, Bayat A. Genetic susceptibility to raised dermal scarring. Br J Dermatol 2009;161:8–18 [DOI] [PubMed] [Google Scholar]
- 7.Sohrabi C, Goutos I. The use of botulinum toxin in keloid scar management: a literature review. Scars Burn Heal 2020;26:2059513120926628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasyanju Carrero LM, Ma WW, Liu HF, et al. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: Updated review. J Cosmet Dermatol 2019;18:10–15 [DOI] [PubMed] [Google Scholar]
- 9.Jeong HS, Lee BH, Sung HM, et al. Effect of botulinum toxin type A on differentiation of fibroblasts derived from scar tissue. Plast Reconstr Surg 2015;136:171e–178e [DOI] [PubMed] [Google Scholar]
- 10.Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: a randomized controlled trial. JCosmetDermatol 2015;14:161–166 [DOI] [PubMed] [Google Scholar]
- 11.El-Hamd NYM, Ahmad ET, Ali MD, et al. Botulinum toxin and platelet rich plasma as innovative therapeutic modalities for keloids. Dermatol Ther 2021;34:e14900. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Lei T-C. Botulinum toxin A promotes the transdifferentiation of primary keloid myofibroblasts into adipocyte-like cells. Basic Clin Pharmacol Toxicol 2021;129:462–469 [DOI] [PubMed] [Google Scholar]
- 13.He Y, Deng Z, Alghamdi M, et al. From genetics to epigenetics: new insights into keloid scarring. Cell Prolif 2017;50:e12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauglitz GG, Bureik D, Dombrowski Y, et al. Botulinum toxin A for the treatment of keloids. Skin Pharmacol Physiol 2012;25:313–318 [DOI] [PubMed] [Google Scholar]
- 15.Austin E, Koo E, Jagdeo J. The cellular response of keloids and hypertrophic scars to botulinum toxin A: a comprehensive literature review. Dermatol Surg 2018;44:149–157 [DOI] [PubMed] [Google Scholar]
- 16.Ismail Sahar A, Mohammed Noorhan HK, Sotohy M, et al. Botulinum toxin type A versus 5-Fluorouracil in treatment of keloid. Arch Dermatol Res 2021;313 7:549–556 [DOI] [PubMed] [Google Scholar]
- 17.Carrero LMK, Ma W-Wi, Liu H-F, et al. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: updated review. J Cosmet Dermatol 2019;l18 1:10–15 [DOI] [PubMed] [Google Scholar]
- 18.Hu L, Zou Y, Chang S-J, et al. Effects of botulinum toxin on improving facial surgical scars: a prospective, split-scar, double-blind, randomized controlled trial. Plast Reconstr Surg 2018;141:646–650 [DOI] [PubMed] [Google Scholar]
- 19.Haubner F, Leyh M, Ohmann E, et al. Effects of botulinum toxin A on patient-specific keloid fibroblasts in vitro. Laryngoscope 2014;124:1344–1351 [DOI] [PubMed] [Google Scholar]
- 20.Fanous A, Bezdjian A, Caglar D, et al. Treatment of keloid scars with botulinum toxin type A versus triamcinolone in an athymic nude mouse model. Plast Reconstr Surg 2019;143:760–767 [DOI] [PubMed] [Google Scholar]
- 21.Wang XX, Chen X, Xiao ZB. Effects of botulinum toxin type A on expression of genes in keloid fibroblasts. Aesthet Surg J 2014;34:154–159. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Min HJ, Kim YW. The efficacy and safety of early postoperative botulinum toxin A injection for facial scars. Aesthetic Plast Surg 2018;42 2:530–537 [DOI] [PubMed] [Google Scholar]
- 23.Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg 2017;43(suppl 1):S3–S18 [DOI] [PubMed] [Google Scholar]


