Abstract
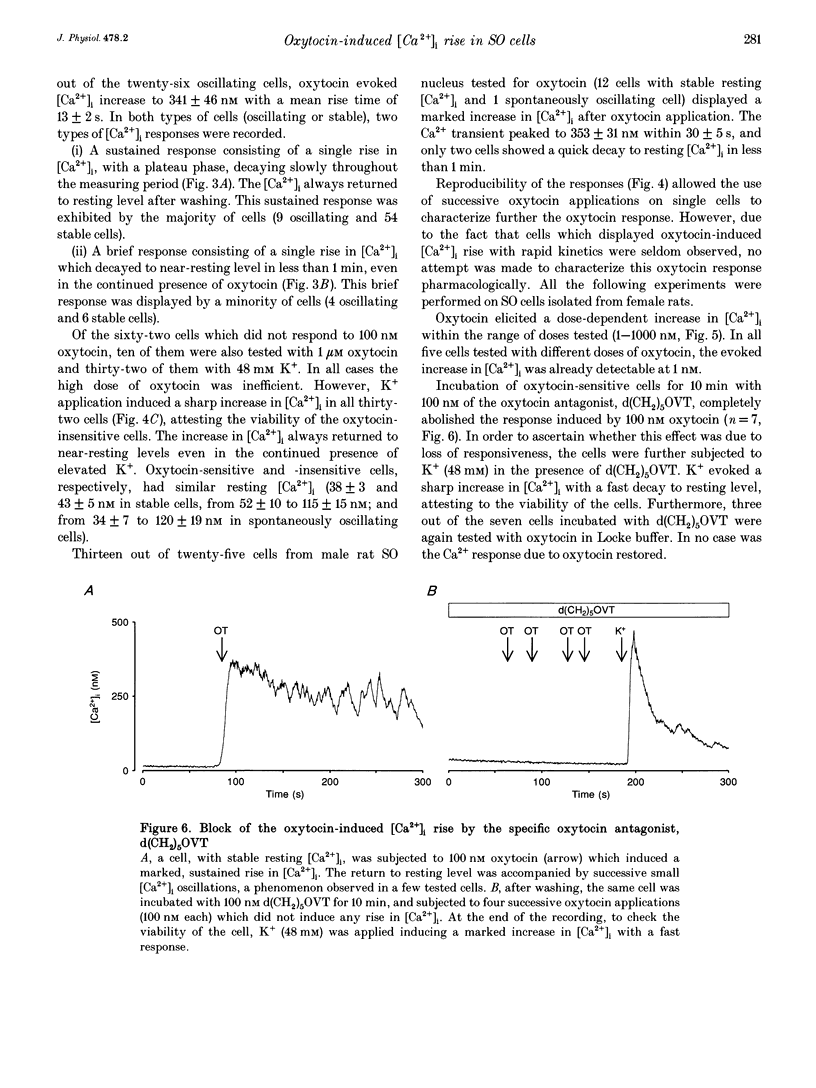
1. Intracellular Ca2+ concentration ([Ca2+]i) was monitored in single cells isolated from adult rat supraoptic (SO) nuclei. The great majority of cells (85%) were neurones and most were immunoreactive to oxytocin or to vasopressin (AVP). 2. The resting [Ca2+]i of the majority (80%) of the neurones remained stable while 20% of the neurones displayed spontaneous [Ca2+]i oscillations which disappeared in low-Ca2+ (100 nM) EGTA buffer. 3. Addition of 100 nM oxytocin increased the [Ca2+]i in both stable and oscillating cells. Two types of responses were observed: (i) a sustained response with [Ca2+]i being maintained at an elevated level and (ii) a brief response with [Ca2+]i quickly returning to a near-resting level. Responses were reproducible, dose dependent and blocked with a specific oxytocin antagonist. 4. Removal of extracellular Ca2+ did not block the oxytocin response. In EGTA buffer, application of thapsigargin (200 nM) onto oxytocin-sensitive cells induced an increase in [Ca2+]i and inhibited the oxytocin response. These effects were not induced by other intracellular Ca2+ mobilizers such as tBuBHQ (see Methods) or caffeine. 5. In conclusion, half of the SO cells respond to oxytocin with a rise in [Ca2+]i. The effect is mediated by oxytocin receptors and results from release of Ca2+ from thapsigargin-sensitive stores.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer K., Sanborn B. M. Changes in intracellular free calcium in isolated myometrial cells: role of extracellular and intracellular calcium and possible involvement of guanine nucleotide-sensitive proteins. Endocrinology. 1989 Jan;124(1):17–23. doi: 10.1210/endo-124-1-17. [DOI] [PubMed] [Google Scholar]
- Bankowski K., Manning M., Seto J., Haldar J., Sawyer W. H. Design and synthesis of potent in vivo antagonists of oxytocin. Int J Pept Protein Res. 1980 Nov;16(5):382–391. doi: 10.1111/j.1399-3011.1980.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Batra S. Effect of oxytocin on calcium influx and efflux in the rat myometrium. Eur J Pharmacol. 1986 Jan 14;120(1):57–61. doi: 10.1016/0014-2999(86)90639-4. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. The caffeine-sensitive Ca2+ store in bovine adrenal chromaffin cells; an examination of its role in triggering secretion and Ca2+ homeostasis. FEBS Lett. 1990 Jun 18;266(1-2):91–95. doi: 10.1016/0014-5793(90)81514-o. [DOI] [PubMed] [Google Scholar]
- Cheek T. R., Thastrup O. Internal Ca2+ mobilization and secretion in bovine adrenal chromaffin cells. Cell Calcium. 1989 May-Jun;10(4):213–221. doi: 10.1016/0143-4160(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Duddy S. K., Kass G. E., Orrenius S. Ca2(+)-mobilizing hormones stimulate Ca2+ efflux from hepatocytes. J Biol Chem. 1989 Dec 15;264(35):20863–20866. [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harvey J., Collingridge G. L. Thapsigargin blocks the induction of long-term potentiation in rat hippocampal slices. Neurosci Lett. 1992 May 25;139(2):197–200. doi: 10.1016/0304-3940(92)90551-h. [DOI] [PubMed] [Google Scholar]
- Inenaga K., Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 1986 Jan;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. Interactions between Ca2+ mobilizing mechanisms in cultured rat cerebellar granule cells. J Physiol. 1992 Oct;456:667–680. doi: 10.1113/jphysiol.1992.sp019360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A. J., Collingridge G. L., Schofield J. G. L-glutamate and acetylcholine mobilise Ca2+ from the same intracellular pool in cerebellar granule cells using transduction mechanisms with different Ca2+ sensitivities. Cell Calcium. 1992 May;13(5):293–301. doi: 10.1016/0143-4160(92)90064-y. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T., Kuriyama K., Nakashima T., Kiyohara T., Sugimori H. Oxytocin modulates oxytocin neurons in the paraventricular nuclei of female rats throughout pregnancy and parturition. Am J Obstet Gynecol. 1993 Mar;168(3 Pt 1):969–974. doi: 10.1016/s0002-9378(12)90854-6. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Kirischuk S. I. Spatial heterogeneity of caffeine- and inositol 1,4,5-trisphosphate-induced Ca2+ transients in isolated snail neurons. Neuroscience. 1993 Apr;53(4):943–947. doi: 10.1016/0306-4522(93)90479-y. [DOI] [PubMed] [Google Scholar]
- Kuriyama K., Nakashima T., Kawarabayashi T., Kiyohara T. Oxytocin inhibits nonphasically firing supraoptic and paraventricular neurons in the virgin female rat. Brain Res Bull. 1993;31(6):681–687. doi: 10.1016/0361-9230(93)90141-w. [DOI] [PubMed] [Google Scholar]
- Leng G. Lateral hypothalamic neurones: osmosensitivity and the influence of activating magnocellular neurosecretory neurones. J Physiol. 1982 May;326:35–48. doi: 10.1113/jphysiol.1982.sp014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link H., Dayanithi G., Föhr K. J., Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992 Apr;130(4):2183–2191. doi: 10.1210/endo.130.4.1312449. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Léránth C., Záborszky L., Marton J., Palkovits M. Quantitative studies on the supraoptic nucleus in the rat. I. Synaptic organization. Exp Brain Res. 1975 May 22;22(5):509–523. doi: 10.1007/BF00237351. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Ho Y. W., Hatton G. I. Axon collaterals of supraoptic neurones: anatomical and electrophysiological evidence for their existence in the lateral hypothalamus. Neuroscience. 1984 Jan;11(1):169–182. doi: 10.1016/0306-4522(84)90221-5. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Leng G. Complex action potential waveform recorded from supraoptic and paraventricular neurones of the rat: evidence for sodium and calcium spike components at different membrane sites. Exp Brain Res. 1984;56(1):135–143. doi: 10.1007/BF00237449. [DOI] [PubMed] [Google Scholar]
- Moos F., Poulain D. A., Rodriguez F., Guerné Y., Vincent J. D., Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76(3):593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989 Jan;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I., Russell J. A., Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993 Mar;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Oldershaw K. A., Taylor C. W. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone mobilizes inositol 1,4,5-trisphosphate-sensitive and -insensitive Ca2+ stores. FEBS Lett. 1990 Nov 12;274(1-2):214–216. doi: 10.1016/0014-5793(90)81366-v. [DOI] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Properties of supraoptic magnocellular neurones isolated from the adult rat. J Physiol. 1992 Sep;455:291–306. doi: 10.1113/jphysiol.1992.sp019302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins G. M., Bremel R. D. Oxytocin-stimulated myosin phosphorylation in mammary myoepithelial cells: roles of calcium ions and cyclic nucleotides. Endocrinology. 1984 May;114(5):1617–1626. doi: 10.1210/endo-114-5-1617. [DOI] [PubMed] [Google Scholar]
- Richard P., Moos F., Freund-Mercier M. J. Central effects of oxytocin. Physiol Rev. 1991 Apr;71(2):331–370. doi: 10.1152/physrev.1991.71.2.331. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Burgoyne R. D. A distinct 2,5-di-(tert-butyl)-1,4-benzohydroquinone-sensitive calcium store in bovine adrenal chromaffin cells. FEBS Lett. 1991 Sep 9;289(2):151–154. doi: 10.1016/0014-5793(91)81057-f. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Winiger B. P., Mollard P., Vacher P., Wuarin F., Zahnd G. R., Wollheim C. B., Dufy B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987 Oct 22;329(6141):719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- Siaud P., Denoroy L., Assenmacher I., Alonso G. Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol. 1989 Dec 15;290(3):323–335. doi: 10.1002/cne.902900302. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Hirning L. D., Miller R. J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol Pharmacol. 1988 Nov;34(5):664–673. [PubMed] [Google Scholar]
- Thayer S. A., Perney T. M., Miller R. J. Regulation of calcium homeostasis in sensory neurons by bradykinin. J Neurosci. 1988 Nov;8(11):4089–4097. doi: 10.1523/JNEUROSCI.08-11-04089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. A., Yacono P. W., Golan D. E., Tashjian A. H., Jr Mechanism of spontaneous intracellular calcium fluctuations in single GH4C1 rat pituitary cells. Biochem J. 1993 May 15;292(Pt 1):175–182. doi: 10.1042/bj2920175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H., Okuya S., Inenaga K., Kasai M., Uesugi S., Kannan H., Kaneko T. Oxytocin predominantly excites putative oxytocin neurons in the rat supraoptic nucleus in vitro. Brain Res. 1987 Jul 28;416(2):364–368. doi: 10.1016/0006-8993(87)90920-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura R., Kiyama H., Kimura T., Araki T., Maeno H., Tanizawa O., Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993 Sep;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]



