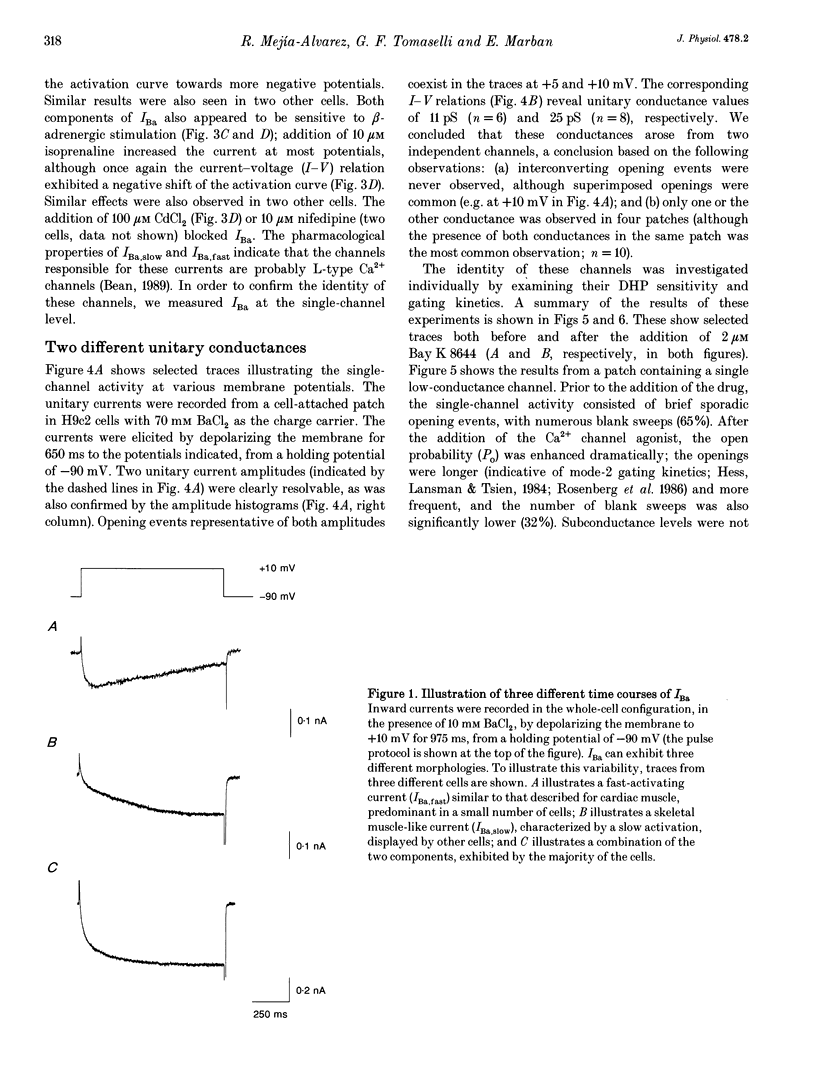
Abstract
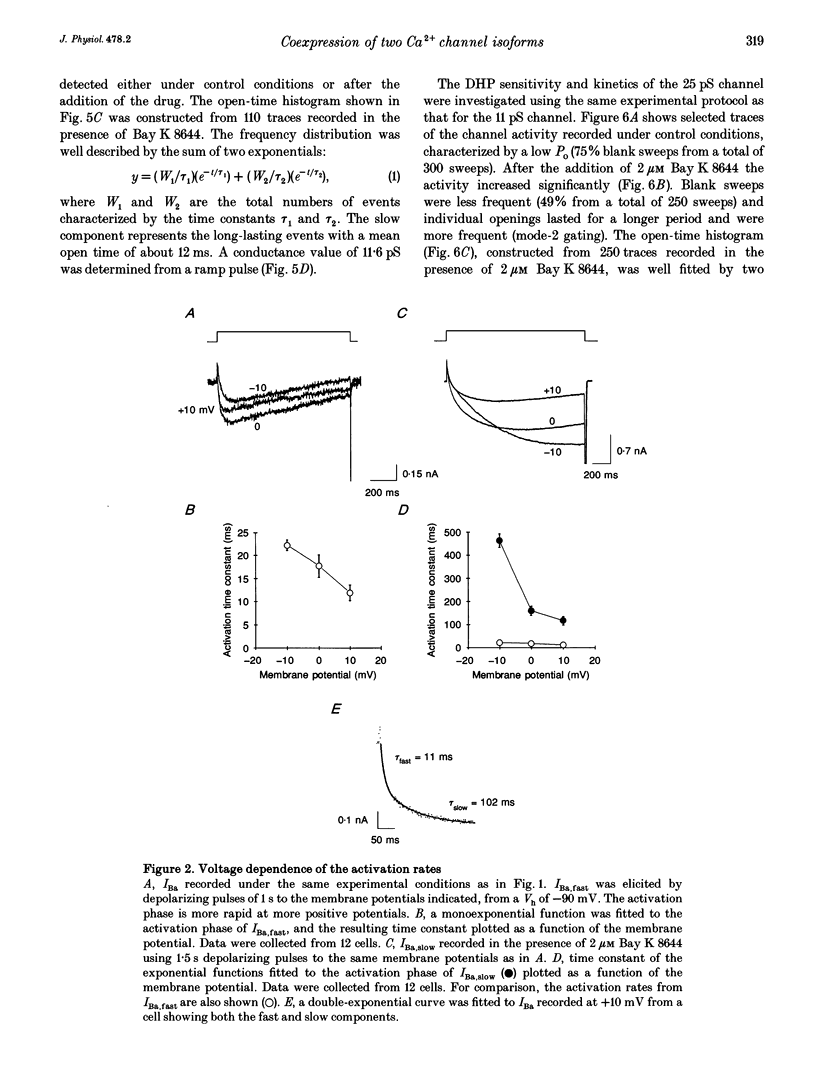
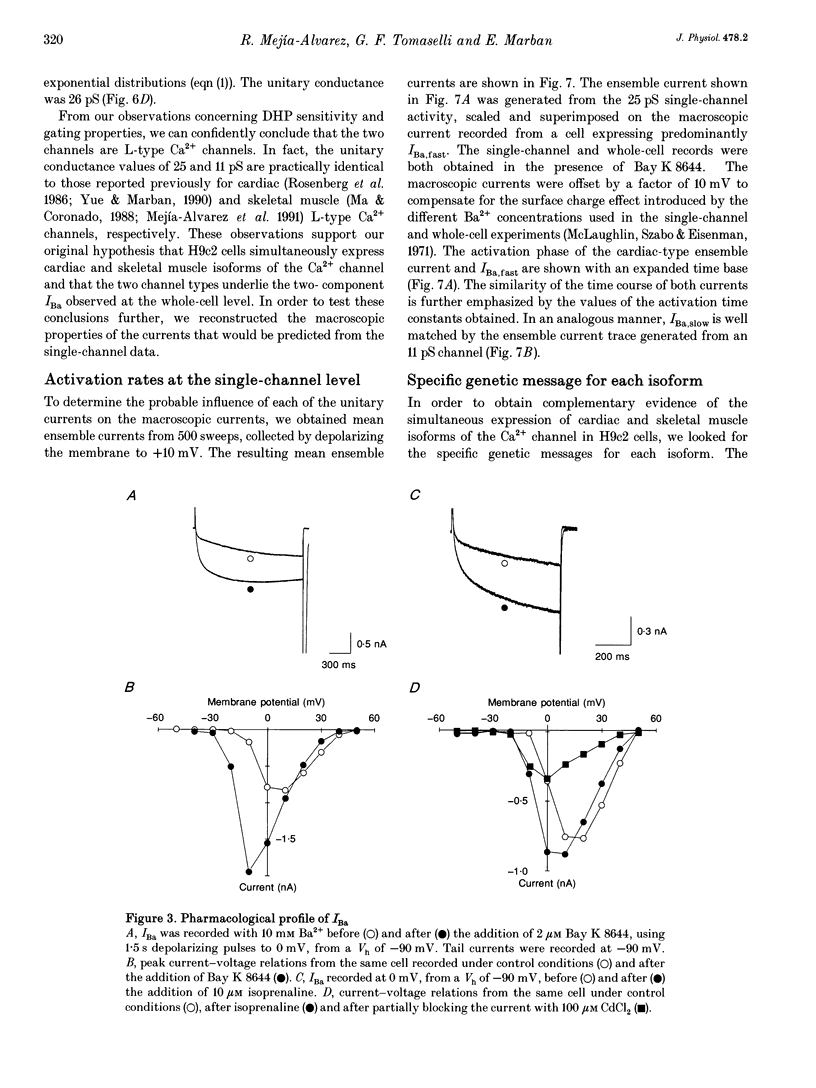
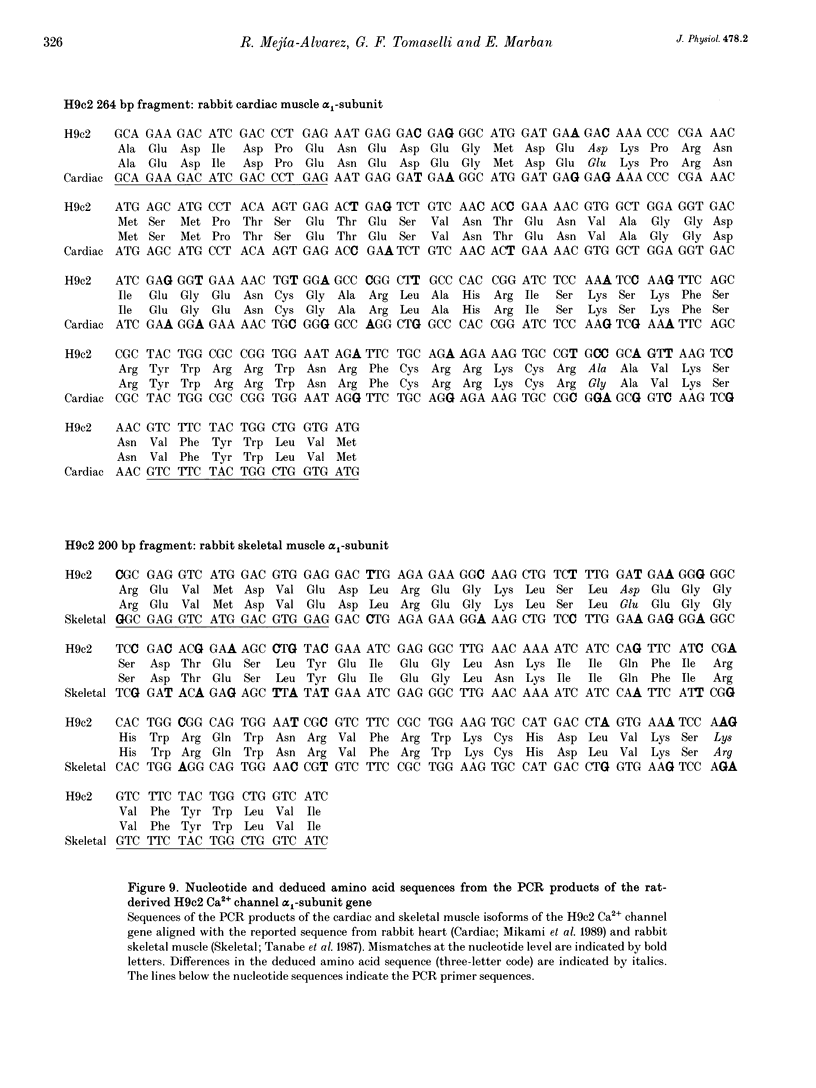
1. We have investigated the identity of the L-type Ca2+ channels present in the H9c2 myoblast line derived from embryonic rat ventricle. To this end, we characterized macroscopic and unitary Ba2+ currents through Ca2+ channels, and looked for specific genetic messages encoding different L-type Ca2+ channel isoforms. 2. The macroscopic Ba2+ current (recorded in 10 mM BaCl2) revealed two components with different time courses of activation. The fast component (IBa,fast) activates with a time constant of 23 +/- 12 ms (at +10 mV), while the slow component activates with a time constant of 125 +/- 12 ms (at +10 mV). 3. Single-channel recordings revealed the presence of two independent channels with conductance values of 11 and 25 pS (in 70 mM Ba2+). These values are identical to those reported previously for skeletal muscle and cardiac Ca2+ channels, respectively. 4. The mean ensemble current from the 11 pS channel reproduced the time course of the slow component observed at the macroscopic level, while the 25 pS ensemble time course paralleled that of the fast component. 5. Reverse transcriptase polymerase chain reaction (PCR) with alpha 1-isoform-specific primers revealed the presence of two distinct transcripts in H9c2 cells. The sequences of the PCR products showed a high degree of homology with the corresponding segments of the rabbit cardiac and skeletal muscle L-type Ca2+ channel isoforms. Adult rat skeletal and cardiac muscle expressed only one type of transcript. 6. H9c2 cells appear to be unique in that they simultaneously express both skeletal muscle and cardiac isoforms of the L-type Ca2+ channel alpha 1-subunit. Thus, the H9c2 cell line may prove to be useful when studying the regulation of subtype-specific Ca2+ channel gene expression.
Full text
PDF


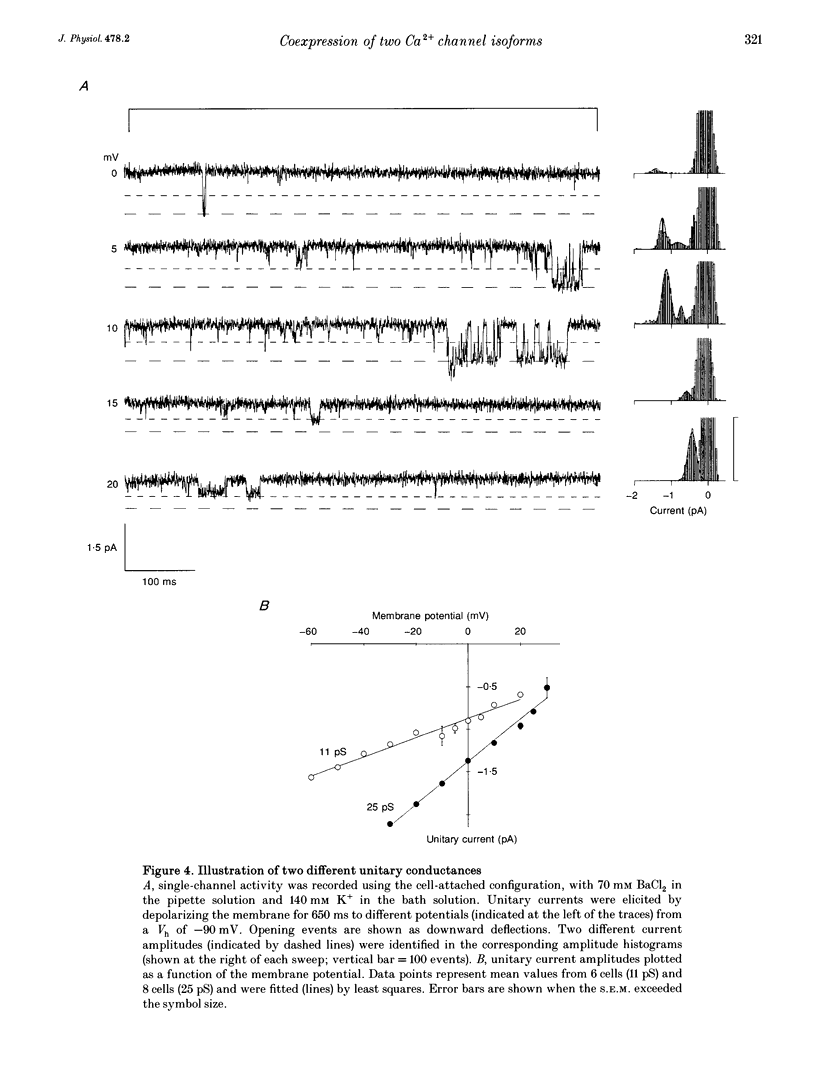
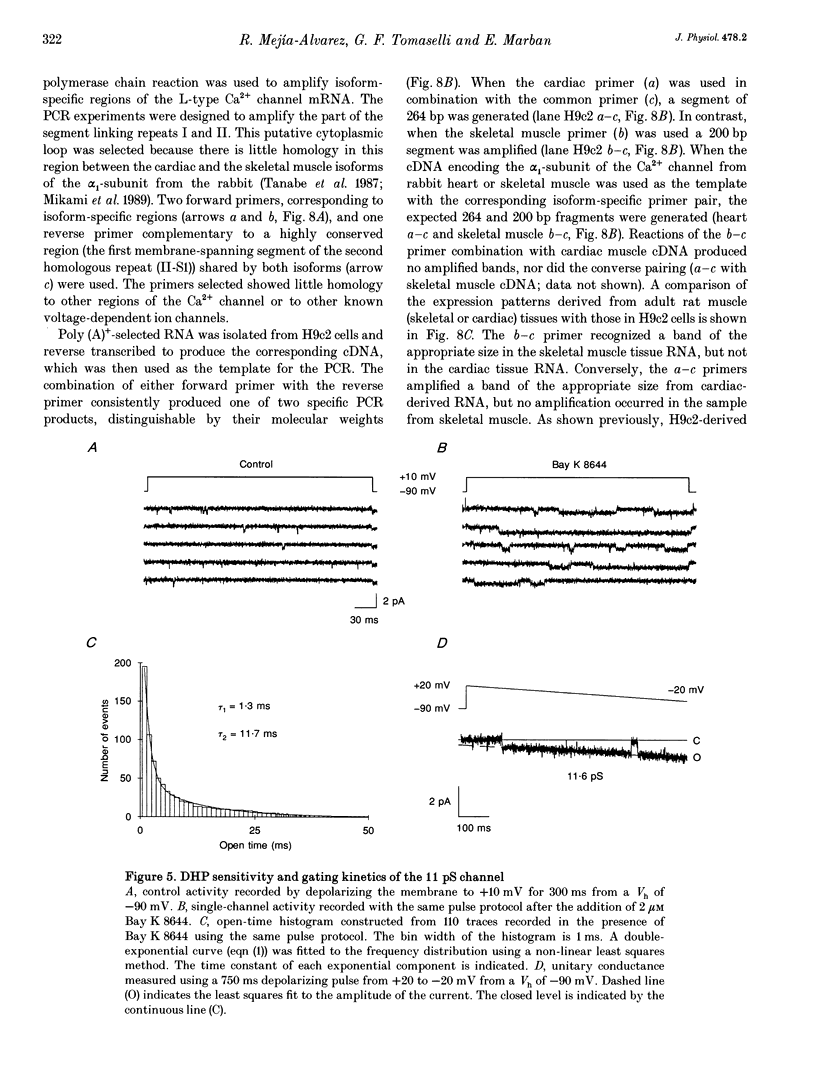
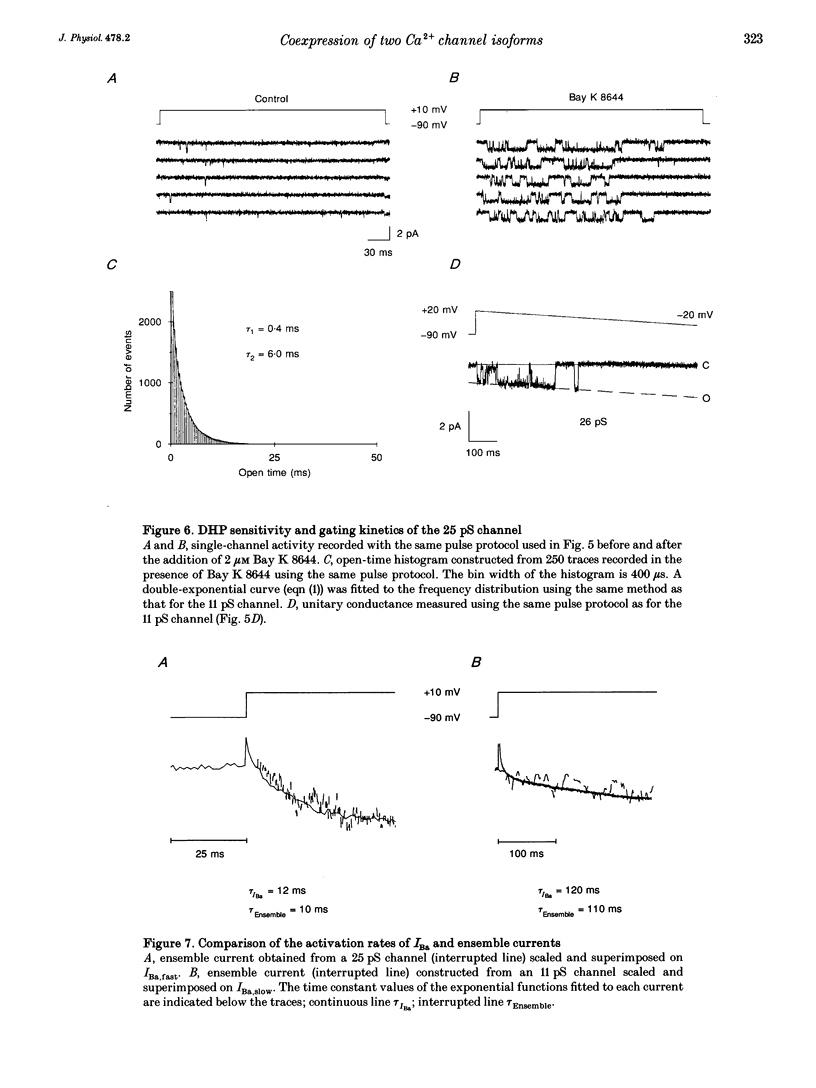




Images in this article
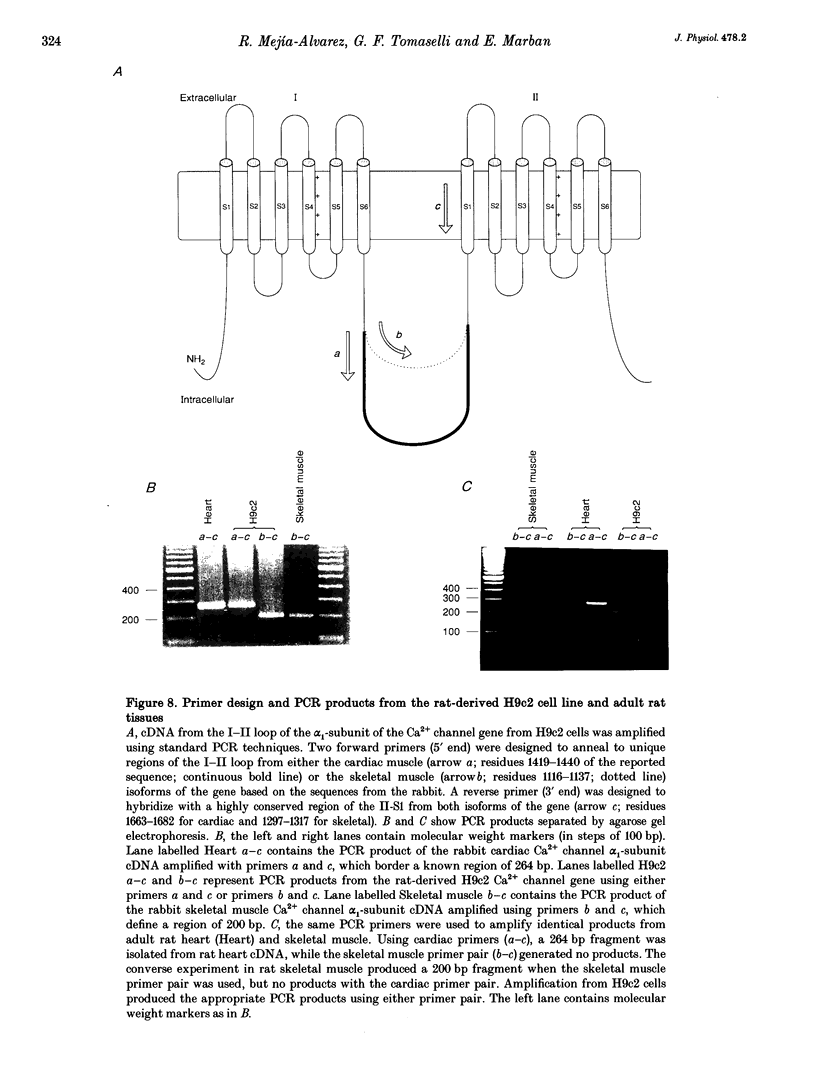
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. A novel calcium current in dysgenic skeletal muscle. J Gen Physiol. 1989 Sep;94(3):429–444. doi: 10.1085/jgp.94.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989 Oct;9(10):3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson P. L., Beam K. G. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol. 1983 Oct;82(4):449–468. doi: 10.1085/jgp.82.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. Modulation of calcium current gating in frog skeletal muscle by conditioning depolarization. J Physiol. 1992 Nov;457:639–653. doi: 10.1113/jphysiol.1992.sp019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Avila-Sakar A. J., Stefani E. Repetitive stimulation increases the activation rate of skeletal muscle Ca2+ currents. Pflugers Arch. 1990 Apr;416(1-2):210–212. doi: 10.1007/BF00370245. [DOI] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., Alvarez R. M., Fill M., Hawkes M. J., Brush K. L., Schilling W. P., Stefani E. [3H]PN200-110 and [3H]ryanodine binding and reconstitution of ion channel activity with skeletal muscle membranes. Anal Biochem. 1989 Nov 15;183(1):31–41. doi: 10.1016/0003-2697(89)90167-x. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Meyer R., Plant S., Krautwurst D., Rosenthal W., Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991 Dec;69(6):1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976 Mar 15;98(2):367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Brown A. M. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989 Jun;93(6):1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. High selectivity of calcium channels in single dialysed heart cells of the guinea-pig. J Physiol. 1984 Sep;354:253–272. doi: 10.1113/jphysiol.1984.sp015374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Coronado R. Heterogeneity of conductance states in calcium channels of skeletal muscle. Biophys J. 1988 Mar;53(3):387–395. doi: 10.1016/S0006-3495(88)83115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Mundiña-Weilenmann C., Hosey M. M., Ríos E. Dihydropyridine-sensitive skeletal muscle Ca channels in polarized planar bilayers. 1. Kinetics and voltage dependence of gating. Biophys J. 1991 Oct;60(4):890–901. doi: 10.1016/S0006-3495(91)82123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía-Alvarez R., Fill M., Stefani E. Voltage-dependent inactivation of T-tubular skeletal calcium channels in planar lipid bilayers. J Gen Physiol. 1991 Feb;97(2):393–412. doi: 10.1085/jgp.97.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Rampe D., Caffrey J. M., Schneider M. D., Brown A. M. Control of expression of the 1,4-dihydropyridine receptor in BC3H1 cells. Biochem Biophys Res Commun. 1988 Apr 29;152(2):769–775. doi: 10.1016/s0006-291x(88)80104-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Reeves J. P., Smilowitz H., Tsien R. W. Calcium channels in planar lipid bilayers: insights into mechanisms of ion permeation and gating. Science. 1986 Mar 28;231(4745):1564–1566. doi: 10.1126/science.2420007. [DOI] [PubMed] [Google Scholar]
- Shih H. T., Wathen M. S., Marshall H. B., Caffrey J. M., Schneider M. D. Dihydropyridine receptor gene expression is regulated by inhibitors of myogenesis and is relatively insensitive to denervation. J Clin Invest. 1990 Mar;85(3):781–789. doi: 10.1172/JCI114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido K. R., Marban E. L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circ Res. 1991 Dec;69(6):1487–1499. doi: 10.1161/01.res.69.6.1487. [DOI] [PubMed] [Google Scholar]
- Sánchez J. A., Stefani E. Kinetic properties of calcium channels of twitch muscle fibres of the frog. J Physiol. 1983 Apr;337:1–17. doi: 10.1113/jphysiol.1983.sp014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tomaselli G. F., Feldman A. M., Yellen G., Marban E. Human cardiac sodium channels expressed in Xenopus oocytes. Am J Physiol. 1990 Mar;258(3 Pt 2):H903–H906. doi: 10.1152/ajpheart.1990.258.3.H903. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E. Permeation in the dihydropyridine-sensitive calcium channel. Multi-ion occupancy but no anomalous mole-fraction effect between Ba2+ and Ca2+. J Gen Physiol. 1990 May;95(5):911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]