Abstract
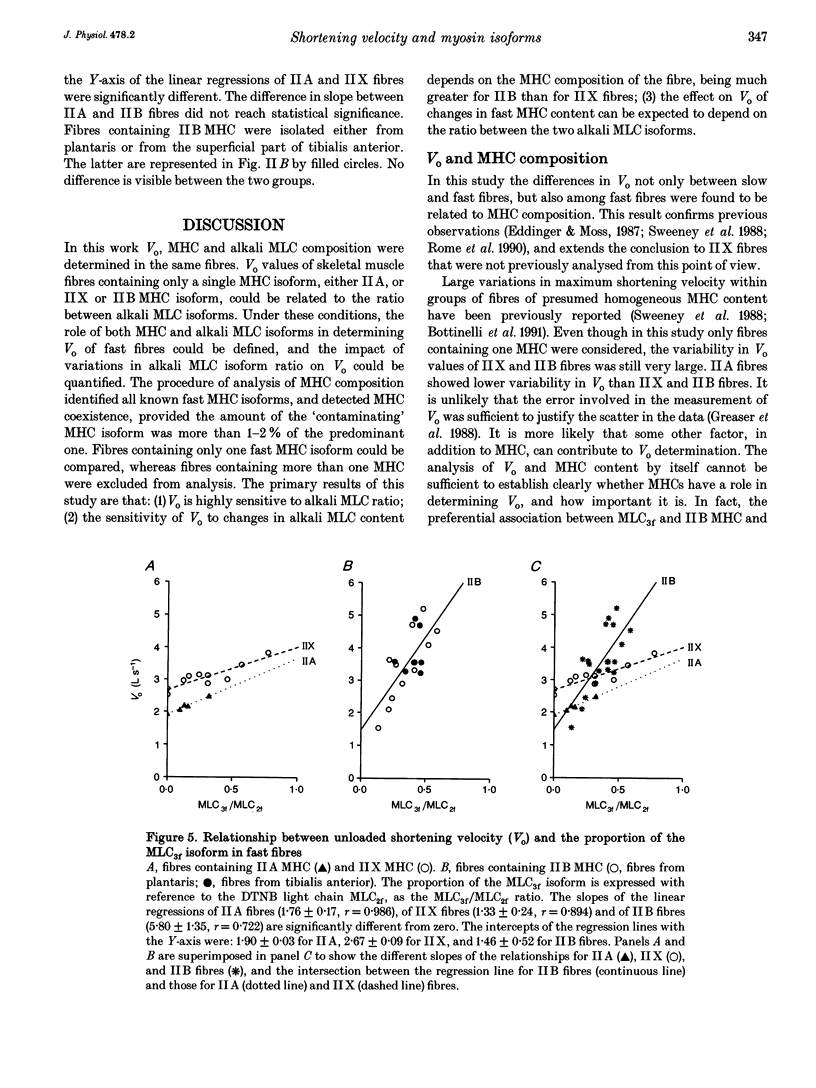
1. This study aims to assess the role of myosin heavy chain (MHC) and alkali myosin light chain (MLC) isoforms in determining maximum velocity of shortening in fast skeletal muscle fibres. 2. The maximum velocity of shortening as determined by the slack test (Vo) was tested for its relationship with MHC composition and with alkali MLC isoform ratio of fast fibres of known MHC composition. 3. MHC isoform composition was determined using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and monoclonal antibodies against MHCs, and combining the results obtained using the two methods. Three groups of fast fibres containing only one MHC isoform were identified: IIA, IIX and IIB fibres containing respectively IIA MHC, IIX MHC and IIB MHC. Fibres containing more than one MHC isoform were discarded. 4. The mean Vo value of IIA fibres was 2.33 +/- 0.29 muscle lengths per second (L s-1; mean +/- S.D.), this was significantly lower than that for IIX fibres (3.07 +/- 0.70 L s-1) which in turn had a mean Vo value significantly lower than that for IIB fibres (3.69 +/- 1.01 L s-1). 5. The relative proportion of alkali MLC isoforms (MLC3f, MLC1f) was determined by means of electrophoretic separation and densitometric quantification and was expressed as MLC3f/MLC2f with reference to the dithio-nitrobenzoic acid (DTNB) light chain (MLC2f). The mean value of the MLC3f/MLC2f ratio was significantly lower in IIA than in IIX and IIB fibres. 6. Vo was found to be proportional to the relative content of MLC3f in IIA, IIX and IIB fibres.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottinelli R., Schiaffino S., Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991 Jun;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär A., Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett. 1988 Aug 1;235(1-2):153–155. doi: 10.1016/0014-5793(88)81253-5. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L., Sartore S., Pierobon-Bormioli S., Schiaffino S. Fast-white and fast-red isomyosins in guinea pig muscles. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1662–1670. doi: 10.1016/0006-291x(80)91365-0. [DOI] [PubMed] [Google Scholar]
- Danieli Betto D., Zerbato E., Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem Biophys Res Commun. 1986 Jul 31;138(2):981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Danieli-Betto D., Betto R., Midrio M. Calcium sensitivity and myofibrillar protein isoforms of rat skinned skeletal muscle fibres. Pflugers Arch. 1990 Nov;417(3):303–308. doi: 10.1007/BF00370996. [DOI] [PubMed] [Google Scholar]
- DeNardi C., Ausoni S., Moretti P., Gorza L., Velleca M., Buckingham M., Schiaffino S. Type 2X-myosin heavy chain is coded by a muscle fiber type-specific and developmentally regulated gene. J Cell Biol. 1993 Nov;123(4):823–835. doi: 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddinger T. J., Moss R. L. Mechanical properties of skinned single fibers of identified types from rat diaphragm. Am J Physiol. 1987 Aug;253(2 Pt 1):C210–C218. doi: 10.1152/ajpcell.1987.253.2.C210. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L., Moss R. L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993 Dec;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- Lutz H., Weber H., Billeter R., Jenny E. Fast and slow myosin within single skeletal muscle fibres of adult rabbits. Nature. 1979 Sep 13;281(5727):142–144. doi: 10.1038/281142a0. [DOI] [PubMed] [Google Scholar]
- Mabuchi K., Szvetko D., Pintér K., Sréter F. A. Type IIB to IIA fiber transformation in intermittently stimulated rabbit muscles. Am J Physiol. 1982 May;242(5):C373–C381. doi: 10.1152/ajpcell.1982.242.5.C373. [DOI] [PubMed] [Google Scholar]
- Pastra-Landis S. C., Huiatt T., Lowey S. Assembly and kinetic properties of myosin light chain isozymes from fast skeletal muscle. J Mol Biol. 1983 Oct 25;170(2):403–422. doi: 10.1016/s0022-2836(83)80155-7. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Moss R. L., Giulian G. G., Greaser M. L. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985 Aug 5;260(16):9077–9080. [PubMed] [Google Scholar]
- Rome L. C., Sosnicki A. A., Goble D. O. Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol. 1990 Dec;431:173–185. doi: 10.1113/jphysiol.1990.sp018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochem J. 1987 May 1;243(3):687–693. doi: 10.1042/bj2430687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staron R. S., Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit tibialis anterior muscle. Biochem J. 1987 May 1;243(3):695–699. doi: 10.1042/bj2430695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J., Mabuchi K., Sréter F. A., Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. J Biol Chem. 1988 Jun 25;263(18):9034–9039. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Termin A., Staron R. S., Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single-fiber study. Eur J Biochem. 1989 Dec 22;186(3):749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Trayer I. P. Differential binding of rabbit fast muscle myosin light chain isoenzymes to regulated actin. FEBS Lett. 1985 Jan 28;180(2):170–173. doi: 10.1016/0014-5793(85)81065-6. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Trayer H. R., Levine B. A. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur J Biochem. 1987 Apr 1;164(1):259–266. doi: 10.1111/j.1432-1033.1987.tb11019.x. [DOI] [PubMed] [Google Scholar]
- Wada M., Pette D. Relationships between alkali light-chain complement and myosin heavy-chain isoforms in single fast-twitch fibers of rat and rabbit. Eur J Biochem. 1993 May 15;214(1):157–161. doi: 10.1111/j.1432-1033.1993.tb17908.x. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Hydrolysis of ATP and reversible binding to F-actin by myosin heavy chains free of all light chains. Nature. 1981 Aug 6;292(5823):560–562. doi: 10.1038/292560a0. [DOI] [PubMed] [Google Scholar]