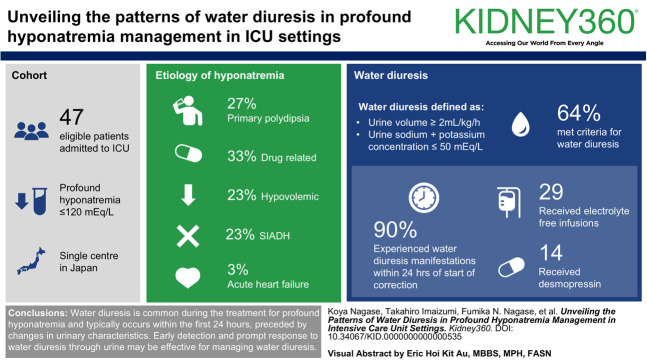
Abstract
Key Points
Water diuresis presents a conundrum during the treatment of profound hyponatremia, but its clinical characteristics remain unclear.
This study revealed that water diuresis mostly manifests within 24 hours of treatment, preceded by changes in urinary characteristics.
Careful urine monitoring in the early stages of hyponatremia treatment could facilitate the early detection of water diuresis.
Background
Hyponatremia treatment guidelines recommend avoiding excessive increases in serum sodium concentration (s[Na]) to prevent osmotic demyelination syndrome. Although an unexpected rise in s[Na] has been attributed to water diuresis during the treatment of hyponatremia, clinical courses of water diuresis are unclear. We conducted this study to investigate the clinical characteristics of water diuresis during profound hyponatremia management.
Methods
In this retrospective observational study, we examined patients with profound hyponatremia (s[Na] ≤120 mEq/L) admitted to the intensive care unit of a Japanese hospital. The manifestation of water diuresis was defined as a urine volume (UV) ≥2 ml/kg per hour and a urinary sodium plus potassium concentration (u[Na+K]) ≤50 mEq/L. We analyzed changes in UV and u[Na+K] over time for patients experiencing water diuresis. This analysis employed a mixed-effects model with spline terms for time, and the results are graphically presented.
Results
Among 47 eligible patients, 30 (64%) met the criteria for water diuresis. The etiologies of hyponatremia were drug-related hyponatremia (n=10; 33%), primary polydipsia (n=8; 27%), hypovolemic hyponatremia (n=7; 23%), syndrome of inappropriate antidiuresis (n=7; 23%), and acute heart failure (n=1; 3%). Among patients with water diuresis, 27 (90%) experienced the manifestation of water diuresis within 24 hours after the start of correction. The increased UV and decreased u[Na+K] levels began several hours before the peak manifestation of water diuresis. Within 6 hours after the manifestation of water diuresis, 29 patients (97%) received electrolyte-free infusions and 14 (47%) received desmopressin. One patient (3%) with water diuresis experienced overcorrection.
Conclusions
Water diuresis is common during the treatment for profound hyponatremia and typically occurs within the first 24 hours, preceded by changes in urinary characteristics. Early detection and prompt response to water diuresis through urine monitoring during the early periods of hyponatremia treatment may be effective for managing water diuresis.
Keywords: electrolytes, hyponatremia
Visual Abstract
Introduction
Hyponatremia is the most prevalent electrolyte imbalance, and in profound cases, it can cause serious neurologic symptoms owing to cerebral edema, necessitating prompt treatment.1–3 However, overly aggressive corrections are not recommended as they are associated with osmotic demyelination syndrome.4–6 This serious iatrogenic complication can lead to severe and irreversible symptoms, including dysarthria, dysphagia, tetraplegia, seizures, locked-in syndrome, coma, and death.7,8 To reduce the risk of these critical neurologic outcomes, guidelines have recommend correction limits of 10–12 mEq/L within the first 24 hours and 18 mEq/L within the first 48 hours of treatment.9,10
Overcorrection beyond these limits has been investigated in large clinical studies and is associated with younger age, lower body mass index (BMI), lower baseline serum sodium concentration (s[Na]), and severe comorbidities6–11; however, overcorrection remains prevalent.6,12–14 While the conundrum of overcorrection persists, some experts have discussed how unexpected s[Na] elevations may result from abrupt water diuresis during the treatment of hyponatremia.15–17 Generally, hyponatremia is attributed to disturbances in water homeostasis, primarily due to reduced effective arterial blood volume or elevated vasopressin levels, which reduce free water excretion and cause water retention.17 Triggers for water retention, such as hypovolemia, drugs, adrenal insufficiency, or syndrome of inappropriate antidiuresis (SIAD), are often reversible, resulting in water diuresis when resolved.9,10,15 Water diuresis decreases the total body water (TBW) and increases s[Na] as s[Na] is determined by exchangeable sodium (Nae), exchangeable potassium (Ke), and TBW18,19 using Equation 1.
| (Equation 1) |
To address s[Na] elevation due to reduced TBW, electrolyte-free infusions and desmopressin acetate (DDAVP) have been used empirically.9,10 In previous studies, three approaches have been proposed for the use of DDAVP: (1) when correction is initiated (proactive strategy), (2) when water diuresis occurs or overcorrection is anticipated (reactive strategy), and (3) when overcorrection is reached (rescue strategy).20,21 However, no consensus exist on the optimal treatment for water diuresis.
Although water diuresis is the primary factor leading to the overcorrection of hyponatremia, only few studies have focused on it, and the actual status still needs to be explored.22,23 Hence, in this study, we aimed to investigate the clinical course of patients with profound hyponatremia admitted to the intensive care unit (ICU) to clarify the clinical characteristics of water diuresis during the treatment of profound hyponatremia and thereby contribute to the effective management of water diuresis.
Methods
Inclusion Criteria
This retrospective observational study was conducted at Chubu Rosai Hospital, a tertiary hospital in Nagoya, Japan. Patients with profound hyponatremia (s[Na] ≤120 mEq/L) admitted to the ICU between January 2014 and December 2022 were included in this study. Those without urinary volume or biochemical data were excluded. In addition, patients whose natural history of urinary output was unknown owing to the proactive administration of DDAVP before the manifestation of water diuresis were also excluded. The research protocol was developed in accordance with the Declaration of Helsinki and was approved by the Chubu Rosai Hospital Clinical Research Ethics Committee (reference number 202309-03, approval date: October 30, 2023). The Committee waived the need for informed consent as the study data were anonymized, and the patients could not be identified individually.
Data Collection and Definition
Patient data, including demographic characteristics, comorbidities, daily medications, causes of hyponatremia, blood and urine test findings, urine volume (UV), treatment methods for hyponatremia, and outcomes, were collected by reviewing electronic health records. The severity of comorbidities was quantified using the Charlson Comorbidity Index (CCI).24 Symptoms of hyponatremia were classified as moderate (nausea without vomiting, headache, drowsiness, confusion, general weakness, and fatigue) or severe (vomiting, seizures, and coma [Glasgow Coma Scale score ≤8 points]) according to previous studies and guidelines.10,14,25 The manifestation of water diuresis was defined as a time point occurring when there is polyuria with a UV of ≥2 ml/kg per hour and the most recent urinary sodium plus potassium concentration (u[Na+K]) is ≤50 mEq/L. s[Na] data measured immediately before (defined as s[Na]bef) and immediately after (s[Na]aft) the manifestation of water diuresis and 6 hours after the s[Na]aft measurement (s[Na]aft+6h) were collected in this study (Figure 1). Data on UV and u[Na+K] were also collected at three specific times: immediately before the manifestation of water diuresis (UVbef and u[Na+K]bef), at the time of the manifestation of water diuresis (UVwd and u[Na+K]wd), and at the time of the s[Na]aft+6h measurement (UVaft+6h and u[Na+K]aft+6h). Using Equation 2, we also calculated electrolyte-free water clearance (EFWC) at three points: immediately before the manifestation of water diuresis (EFWCbef calculated with s[Na]bef, UVbef, and u[Na+K]bef), during and immediately after the manifestation of water diuresis (EFWCaft calculated with s[Na]aft, UVwd, and u[Na+K]wd), and at the time of the s[Na]aft+6h measurement (EFWCaft+6h calculated with s[Na]aft+6h, UVaft+6h, and u[Na+K]aft+6h).19,26
| (Equation 2) |
Figure 1.
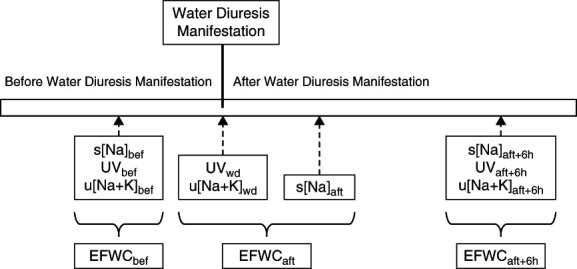
Time points and variables recorded before and after the manifestation of water diuresis. Aft, after; bef, before; EFWC, electrolyte-free water clearance; s[Na], serum sodium concentration; u[Na+K], urinary sodium plus potassium concentration; UV, urine volume; wd, during water diuresis.
We defined acute hyponatremia as cases having a normal s[Na] within 48 hours before the diagnosis of hyponatremia. The appropriate correction of hyponatremia was defined as a change in s[Na] of 4–10 mEq/L within the first 24 hours of treatment and of ≤18 mEq/L within the first 48 hours of treatment.9,10 Overcorrection was defined as a change in s[Na] >10 mEq/L within the first 24 hours of treatment or >18 mEq/L within the first 48 hours of treatment. Undercorrection was defined as a change in s[Na] <4 mEq/L within the first 24 hours of treatment. When s[Na] data were unavailable at exactly 24 hours of treatment, the extrapolated values were estimated using Equation 3.
| (Equation 3) |
Naa indicates the s[Na] measurement immediately before 24 hours, Nab indicates the s[Na] measurement immediately after 24 hours, and Ta and Tb are the times when Naa and Nab were obtained, respectively.13 The same method was used to extrapolate the s[Na] at 48 hours and s[Na]aft+6h.
Predictive Correction by Infusate and Fluid Loss Formula
The correction of hyponatremia was conducted using the infusate and fluid loss formula (derived from Equation 1) in some patients (Equation 4).25,27
| (Equation 4) |
Here, s[Na]1 represents the current s[Na], and s[Na]2 represents the projected s[Na]. All data sources related to Na, K, and water input/output (including infusion, urine, eating/drinking, drainage [Na, K, and water], electrolyte supplementation [Na and K], and insensible excretion [water]) were collected to optimize the accuracy of s[Na]2 predictions (Supplemental Figure 1). After diagnosing hyponatremia, spot urine samples were analyzed to estimate the amounts of Na, K, and water excreted through the urine, insensible excretion, and other routes. Subsequently, potential changes in s[Na] owing to the oral or intravenous administration of Na, K, and water were assessed. Based on these calculations, a treatment strategy to achieve the desired correction rate was derived. Water and sodium losses owed to sweating were typically negligible and not considered in the calculations.28,29
Statistical Analysis
Categorical data are presented as numbers and percentages, and continuous data are presented as medians and interquartile ranges (IQRs). The Wilcoxon signed-rank sum test was used to compare s[Na] (s[Na]bef versus s[Na]aft), UV (UVbef versus UVwd), u[Na+K] (u[Na+K]bef versus u[Na+K]wd), and EFWC (EFWCbef versus EFWCaft) before and after the manifestation of water diuresis. Assuming nonlinear trajectories, we employed a mixed-effects model with an unstructured variance-covariance matrix, patient-level random effects, and spline time terms to examine changes in s[Na], UV, and u[Na+K] over time in patients experiencing water diuresis. Additionally, we analyzed restricted cubic spline models for UV and u[Na+K] separately for patients administered DDAVP and those who were not administered DDAVP within 6 hours after water diuresis manifestation. Regarding s[Na], seven knots were placed at equal intervals every 6 hours from −18 to +18 hours relative to the timing of s[Na]aft measurement. Similarly, for UV and u[Na+K], five knots were placed at intervals from −12 to +12 hours relative to the manifestation of water diuresis. Linear mixed-effects models adjusted for the covariates of age, sex, BMI, and CCI score were used to compare the correction rates from s[Na]bef to s[Na]aft and from s[Na]aft to s[Na]aft+6h in patients with water diuresis. All statistical analyses were conducted using Stata 18.0 BE software (Stata Corp., College Station, TX). A P value < 0.05 was considered statistically significant.
Results
Patient Characteristics
Sixty patients in the ICU were diagnosed with profound hyponatremia (s[Na] ≤120 mEq/L) during the study period. After excluding two patients for whom urinary data were unavailable and 11 who were proactively administered DDAVP before the manifestation of water diuresis, 47 patients were included in the study. The proportion of acute hyponatremia was 0%, and all patients were classified as having chronic hyponatremia. Forty patients (85%) experienced polyuria with UV ≥2 ml/kg per hour, and 30 (64%) met the criteria for water diuresis with a u[Na+K] ≤50 mEq/L (Figure 2). Patients in the water diuresis group (16 females and 14 males) had a median age of 71 years (IQR, 54–83 years) and a baseline s[Na] of 111 mEq/L (IQR, 108–114 mEq/L) (Table 1). The etiologies of hyponatremia in the water diuresis group were drug-related hyponatremia (n=10; 33%), primary polydipsia (n=8; 27%), hypovolemic hyponatremia (n=7; 23%), SIAD (n=7; 23%), acute heart failure (n=1; 3%), and unknown (n=2; 7%). All patients with primary polydipsia and ≥50% of patients with hypovolemic hyponatremia, drug-related hyponatremia, or SIAD developed water diuresis (Supplemental Table 1). Among patients who experienced water diuresis, the symptoms of hyponatremia included nausea (n=8; 27%), drowsiness (n=8; 27%), vomiting (n=6; 20%), general weakness (n=4; 13%), unsteady gait (n=4; 13%), headache (n=1; 3%), coma (n=6; 20%), and seizure (n=2; 7%).
Figure 2.
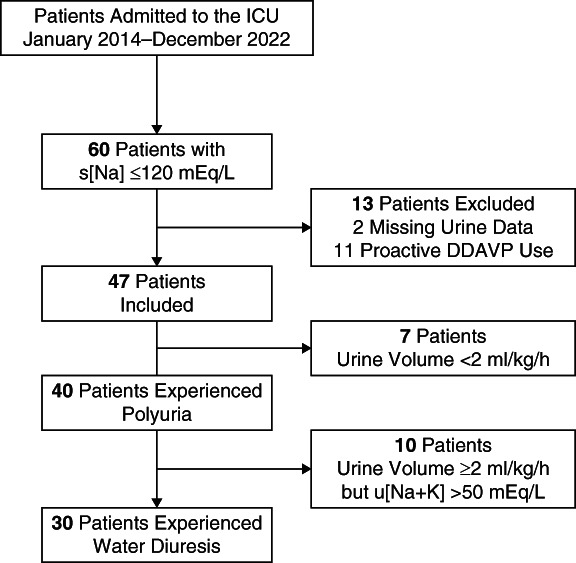
Patient selection process. DDAVP, desmopressin acetate; ICU, intensive care unit.
Table 1.
Baseline data of patients with water diuresis
| Patient No. | Baseline Data | Water Diuresis Manifestation Time from Correction Start (h) | Infusate and Fluid Loss Formula Use | Correction Methods until Water Diuresis Manifestation | Before Water Diuresis Manifestation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Sex | Body Weight (kg) | s[Na] (mEq/L) | Hyponatremia Cause | Hyponatremia Symptom | s[Na]bef (mEq/L) | UVbef (ml/h) | u[Na+K]bef (mEq/L) | EFWCbef (ml/h) | ||||
| 1 | 65 | Female | 40.0 | 117 | Unidentified | Coma | 6 | − | HTS (RBI), NS | 119 | 90 | 51.7 | 51 |
| 2 | 70 | Female | 29.6 | 112 | SIAD (vaccine stress) | Nausea, headache | 9 | + | HTS (RBI) | 118 | 55 | 106.3 | 5 |
| 3 | 82 | Male | 59.9 | 107 | SIAD (surgery stress) | Coma | 13 | − | HTS (SCI) | 109 | 33 | 91.3 | 5 |
| 4 | 43 | Male | 65.0 | 104 | Oral DDAVP | Nausea | 3 | − | D5W | 105 | N/A | 59.4 | N/A |
| 5 | 92 | Female | 52.6 | 108 | SIAD (stomacache) | Nausea, general weakness | 5 | + | NS | 109 | 270 | 110 | −2 |
| 6 | 54 | Male | 61.4 | 108 | Primary polydipsia | Drowsiness, general weakness | 3 | + | D5W | 108 | 60 | 11.2 | 54 |
| 7 | 61 | Male | 60.7 | 111 | Primary polydipsia | Seizure, coma | 4 | + | HTS (RBI), NS, D5W | 115 | 380 | 104 | 36 |
| 8 | 87 | Female | 43.1 | 114 | Hypovolemic | Nausea | 12 | + | NS | 115 | 10 | 14.3 | 9 |
| 9 | 55 | Female | 39.0 | 120 | Primary polydipsia+SNRI | Seizure, coma | 12 | + | HTS (RBI, SCI) | 131 | 117 | 57.7 | 65 |
| 10 | 93 | Female | 43.7 | 111 | SIAD (rib fracture) | Unsteady gait | 6 | + | NS | 112 | 650 | 86.8 | 146 |
| 11 | 51 | Male | 40.3 | 112 | Unidentified | Drowsiness, general weakness | 2 | − | HTS (SCI) | 116 | N/A | 46.5 | N/A |
| 12 | 53 | Male | 70.6 | 116 | Primary polydipsia+thiazide | Coma, vomiting | 2 | − | D5W | 116 | N/A | 49.7 | N/A |
| 13 | 51 | Male | 60.0 | 111 | Acute heart failure | Drowsiness | 6 | + | HTS (SCI), LD | 110 | 182 | 82.7 | 45 |
| 14 | 71 | Female | 56.3 | 110 | Primary polydipsia+thiazide | Drowsiness | 1 | + | HTS (RBI, SCI) | 112 | N/A | N/A | N/A |
| 15 | 74 | Male | 55.0 | 112 | SIAD (herpes zoster pain) | Vomiting | 22 | + | NS | 115 | 10 | 31.7 | 7 |
| 16 | 49 | Female | 42.2 | 113 | Primary polydipsia | Vomiting | 1 | + | NS | 113 | N/A | N/A | N/A |
| 17 | 66 | Male | 44.5 | 110 | Hypovolemic+SNRI | Drowsiness, unsteady gait | 3 | + | HTS (SCI) | 112 | 40 | 48.2 | 23 |
| 18 | 49 | Female | 49.5 | 115 | Carbamazepine | Drowsiness | 13 | − | NS, D5W | 118 | 93 | 111.4 | 5 |
| 19 | 84 | Female | 68.8 | 117 | Oral DDAVP | Unsteady gait | 10 | − | HTS (SCI), D5W | 121 | 100 | 54.7 | 55 |
| 20 | 73 | Male | 35.5 | 116 | Thiazide | Drowsiness | 19 | + | NS, D5W | 121 | 63 | 73.8 | 25 |
| 21 | 70 | Female | 44.0 | 112 | Hypovolemic | Coma | 34 | − | NS | 122 | 77 | 19.1 | 65 |
| 22 | 47 | Male | 44.4 | 99 | Primary polydipsia | Drowsiness | 2 | − | NS | 99 | 130 | 52.3 | 61 |
| 23 | 71 | Male | 47.4 | 110 | Hypovolemic | General weakness, fatigue | 4 | + | NS, LD | 112 | 400 | 79.6 | 116 |
| 24 | 97 | Female | 37.7 | 107 | SIAD (stomach ache) | Vomiting | 6 | + | HTS (RBI, SCI), NS | 115 | 20 | 118.8 | −1 |
| 25 | 74 | Female | 29.8 | 109 | Hypovolemic | Vomiting | 4 | + | HTS (RBI), D5W | 116 | 250 | 80.4 | 77 |
| 26 | 80 | Female | 62.2 | 114 | Primary polydipsia | Nausea, unsteady gait | 3 | + | NS | 115 | N/A | 61.2 | N/A |
| 27 | 79 | Male | 53.6 | 103 | Thiazide | Nausea | 40 | + | HTS (RBI, SCI), NS | 116 | 77 | 60.5 | 37 |
| 28 | 84 | Female | 40.3 | 107 | Hypovolemic+SNRI | Nausea | 30 | + | HTS (RBI), NS, D5W | 117 | 53 | 56.9 | 27 |
| 29 | 84 | Male | 49.4 | 110 | SIAD (arthralgia) | Nausea | 15 | + | HTS (RBI), NS, D5W | 116 | 75 | 38 | 50 |
| 30 | 78 | Female | 44.7 | 108 | Hypovolemic | Vomiting | 2 | + | HTS (RBI), D5W | 112 | 230 | 60.3 | 106 |
| Median | 71 | 46.1 | 111 | 6 | 115 | 84 | 59.9 | 41 | |||||
| IQR | 54–83 | 40.3–59.9 | 108–114 | 3–13 | 112–117 | 54–218 | 48.6–85.8 | 8–64 | |||||
D5W, dextrose 5% in water; DDAVP, desmopressin acetate; EFWC, electrolyte-free water clearance; HTS, hypertonic saline; IQR, interquartile range; LD, loop diuretics; N/A, not applicable; NS, normal saline; RBI, rapid bolus infusion; SCI, slow continuous infusion; SIAD, syndrome of inappropriate antidiuresis; s[Na], serum sodium concentration; SNRI, serotonin noradrenaline reuptake inhibitor; u[Na+K], urinary sodium plus potassium; UV, urine volume.
Blood and Urine Variables before and after Water Diuresis
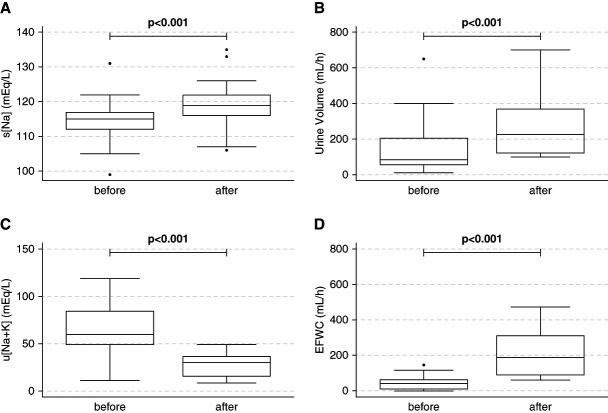
The median number of measurements in the first 24 hours was eight for s[Na] (IQR, 6–11 measurements), ten for UV (IQR, 8–12 measurements), and six for u[Na+K] (IQR, 4–7 measurements) among patients in the water diuresis group (Supplemental Table 2). The median time from correction initiation to the manifestation of water diuresis was 6 hours (IQR, 3–13 hours). Water diuresis occurred in 18 patients (60%) within 6 hours, in 22 (73%) within 12 hours, and in 27 (90%) within 24 hours after correction initiation (Table 1). Before the manifestation of water diuresis, the correction methods included hypertonic saline rapid bolus infusion in 11 patients (37%), hypertonic saline slow continuous infusion in nine (30%), normal saline (NS) in 17 (57%), dextrose 5% in water (D5W) in 11 (37%), and loop diuretics in two (7%). The treatment strategies were determined through calculations based on the infusate and fluid loss formula in 21 patients (70%) who developed water diuresis. Before the manifestation of water diuresis, the median s[Na]bef was 115 mEq/L (IQR, 112–117 mEq/L), UVbef was 84 ml/h (IQR, 54–218 ml/h), u[Na+K]bef was 59.9 mEq/L (IQR, 48.6–85.8 mEq/L), and EFWCbef was 41 ml/h (IQR, 8–64 ml/h) (Figure 3 and Table 1). After the manifestation of water diuresis, the median s[Na]aft was 119 mEq/L (IQR, 116–122 mEq/L), UVwd was 230 ml/h (IQR, 119–372 ml/h), u[Na+K]wd was 30.2 mEq/L (IQR, 15.4–36.8 mEq/L), and EFWCaft was 187 ml/h (IQR, 88–317 ml/h) (Figure 3 and Table 2). After the manifestation of water diuresis, s[Na], UV, and EFWC values were significantly higher (both P < 0.001) and the u[Na+K] value was significantly lower (P < 0.001) than the values before the manifestation of water diuresis (Figure 3). The median s[Na]aft+6h was 118 mEq/L (IQR, 114–122 mEq/L), UVaft+6h was 70 ml/h (IQR, 44–103 ml/h), u[Na+K]aft+6h was 43.8 mEq/L (IQR, 31.9–89.5 mEq/L), and EFWCaft+6h was 30 ml/h (IQR, 10–67 ml/h) (Table 2).
Figure 3.
Comparison of s[Na], UV, u[Na+K], and EFWC before and after water diuresis. Box plots illustrating the data distributions of s[Na] (A), UV (B), u[Na+K] (C), and EFWC (D) before and after water diuresis are shown. Each plot displays the median, IQRs (25th and 75th percentiles), and minimum and maximum observations for each variable. Outliers are depicted as individual dots. The Wilcoxon signed-rank sum test confirmed significant changes in all four metrics after the manifestation of water diuresis: higher s[Na], UV, and EFWC and lower u[Na+K] (all P < 0.001). IQR, interquartile range.
Table 2.
Variables and correction outcomes of patients with water diuresis
| Baseline Date | After Water Diuresis Manifestation | Correction Methods for the Next 6 h from s[Na]aft Measurement | 6 h after s[Na]aft Measurement | s[Na] Change | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. | Age (yr) | Sex | s[Na]aft Measurement Time from Water Diuresis Manifestation (h) | s[Na]aft (mEq/L) | UVwd (ml/h) | u[Na+K]wd (mEq/L) | EFWCaft (ml/h) | s[Na]aft+6h (mEq/L) | UVaft+6h (ml/h) | u[Na+K]aft+6h (mEq/L) | EFWCaft+6h (ml/h) | 24 h | 48 h | |
| 1 | 65 | Female | 1 | 123 | 180 | 14.7 | 158 | DDAVP, D5W | 122 | 12 | 53.9 | 7 | 9 | 15 |
| 2 | 70 | Female | 2 | 121 | 113 | 34.9 | 80 | DDAVP, D5W | 117 | 29 | 101.2 | 4 | 6 | 10 |
| 3 | 82 | Male | 6 | 119 | 243 | 8.5 | 226 | D5W | 116 | 50 | 31.4 | 36 | 9 | 15 |
| 4 | 43 | Male | 2 | 106 | 220 | 13.7 | 192 | D5W | 110 | 135 | 14.2 | 118 | 9 | 15 |
| 5 | 92 | Female | 1 | 112 | 640 | 46.5 | 374 | DDAVP, D5W | 114 | 110 | 99.9 | 14 | 4 | 5 |
| 6 | 54 | Male | 1 | 112 | 520 | 10.1 | 473 | D5W | 113 | 300 | 7.7 | 280 | 6 | 10 |
| 7 | 61 | Male | 6 | 122 | 450 | 31.8 | 333 | D5W | 121 | 44 | 93.2 | 10 | 7 | 13 |
| 8 | 87 | Female | 4 | 119 | 220 | 13.9 | 194 | D5W | 123 | 83 | 137.7 | −10 | 10 | 12 |
| 9 | 55 | Female | 1 | 133 | 217 | 36.6 | 157 | DDAVP, D5W | 127 | 80 | 139.6 | −8 | 10 | 16 |
| 10 | 93 | Female | 5 | 124 | 370 | 19.6 | 312 | DDAVP, D5W | 115 | 73 | 88.2 | 17 | 0 | 3 |
| 11 | 51 | Male | 1 | 119 | 100 | 22.2 | 81 | D5W | 121 | 93 | 71.2 | 38 | 10 | 14 |
| 12 | 53 | Male | 12 | 135 | 300 | 20.5 | 254 | D5W | 134 | 155 | 39 | 110 | 17 | 19 |
| 13 | 51 | Male | 4 | 116 | 379 | 15.1 | 330 | D5W | 116 | 117 | 9.8 | 107 | 10 | 8 |
| 14 | 71 | Female | 1 | 117 | 400 | 15.5 | 347 | DDAVP, D5W | 114 | 160 | 36.8 | 108 | 10 | 12 |
| 15 | 74 | Male | 1 | 116 | 230 | 39.1 | 152 | NS | 120 | 90 | 34.3 | 64 | 6 | 9 |
| 16 | 49 | Female | 1 | 123 | 150 | 10.1 | 138 | DDAVP, D5W | 122 | 38 | 13 | 34 | 8 | 17 |
| 17 | 66 | Male | 1 | 116 | 120 | 30.9 | 88 | D5W | 114 | 14 | 34.9 | 10 | 5 | 9 |
| 18 | 49 | Female | 2 | 121 | 117 | 24.3 | 94 | D5W | 121 | 47 | 45.3 | 29 | 6 | 15 |
| 19 | 84 | Female | 2 | 126 | 250 | 29.5 | 191 | DDAVP, D5W | 123 | 43 | 113.6 | 3 | 8 | 9 |
| 20 | 73 | Male | 2 | 122 | 115 | 32 | 85 | D5W | 123 | 63 | 32 | 47 | 6 | 9 |
| 21 | 70 | Female | 6 | 124 | 100 | 16.7 | 87 | D5W | 126 | 50 | 33.8 | 37 | 5 | 14 |
| 22 | 47 | Male | 1 | 107 | 660 | 42.3 | 399 | DDAVP, D5W | 105 | 399 | 42.3 | 238 | 5 | 8 |
| 23 | 71 | Male | 1 | 113 | 700 | 46.6 | 411 | DDAVP, D5W | 114 | 40 | 29.4 | 30 | 8 | 10 |
| 24 | 97 | Female | 2 | 116 | 250 | 30.9 | 183 | DDAVP, D5W | 113 | 77 | 170.3 | −39 | 6 | 12 |
| 25 | 74 | Female | 1 | 117 | 320 | 31.7 | 233 | DDAVP, D5W | 118 | 70 | 65.7 | 31 | 9 | 18 |
| 26 | 80 | Female | 2 | 118 | 240 | 21.9 | 195 | DDAVP, D5W | 119 | 50 | 50.7 | 29 | 5 | 12 |
| 27 | 79 | Male | 2 | 119 | 133 | 49.3 | 78 | D5W | 115 | 70 | 80.3 | 21 | 6 | 13 |
| 28 | 84 | Female | 2 | 120 | 100 | 32.2 | 73 | D5W | 123 | 60 | 66.6 | 28 | 10 | 18 |
| 29 | 84 | Male | 2 | 117 | 100 | 46.7 | 60 | D5W | 118 | 100 | 30.8 | 74 | 8 | 10 |
| 30 | 78 | Female | 2 | 107 | 230 | 37.2 | 150 | DDAVP, D5W | 112 | 41 | 38.9 | 27 | 8 | 13 |
| Median | 71 | 3 | 119 | 230 | 30.2 | 187 | 118 | 70 | 43.8 | 30 | 8 | 12 | ||
| IQR | 54–83 | 2–4 | 116–122 | 119–372 | 15.4–36.8 | 88–317 | 114–122 | 44–103 | 31.9–89.5 | 10–67 | 6–9 | 9–15 | ||
D5W, dextrose 5% in water; DDAVP, desmopressin acetate; EFWC, electrolyte-free water clearance; IQR, interquartile range; NS, normal saline; s[Na], serum sodium concentration; u[Na+K], urinary sodium plus potassium concentration; UV, urine volume.
Changes in s[Na], UV, and Urine Sodium plus Potassium Concentration over Time
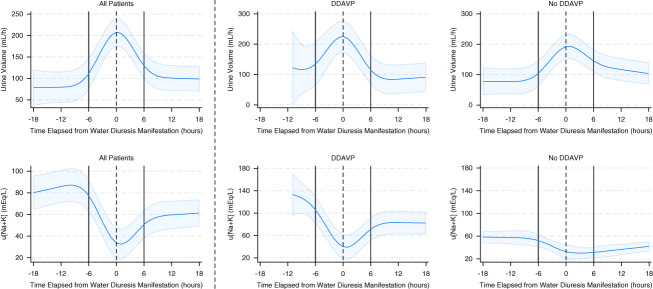
The inflection points of s[Na] were identified at −6, 0, and +6 hours from the timing of the s[Na]aft measurement (Figure 4). After adjusting for age, sex, BMI, and CCI score, the slope of s[Na] significantly decreased from 0.80±0.19 mEq/L per hour before the s[Na]aft to −0.09±0.40 mEq/L per hour after the s[Na]aft measurement (P < 0.001) (Figure 5). The number of patients who received hypertonic and NS solutions decreased over time, whereas the number of patients who received D5W and DDAVP increased over time. Within 6 hours after the manifestation of water diuresis, 29 patients (97%) received D5W and 14 (47%) received DDAVP. Analysis of changes in urinary variables over time indicated that increase in UV and decrease in u[Na+K] began several hours before the peak manifestation of water diuresis (Figure 6). Following this peak, the UV rapidly decreased, and the u[Na+K] increased in patients who were administered DDAVP compared with those who were not administered DDAVP.
Figure 4.
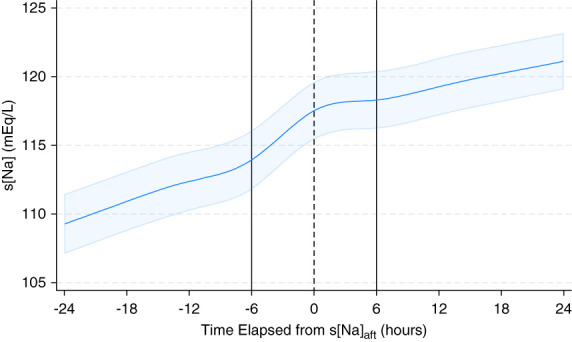
Changes in s[Na] over time after the s[Na]aft measurement. A restricted cubic spline model was used to depict changes in s[Na] levels over time in patients who experienced water diuresis. The solid curve represents the predicted trajectory of s[Na], while the surrounding shaded area indicates its 95% confidence interval. s[Na]aft, serum sodium concentration immediately after manifestation of water diuresis.
Figure 5.
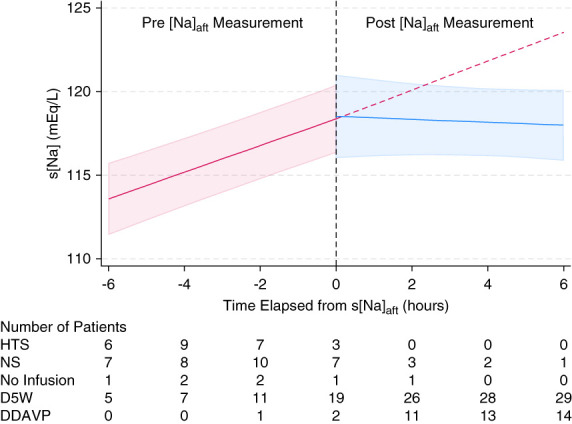
Changes in s[Na] over time and correction method used. Linear mixed-effects models were used to depict the changes in the s[Na] levels over time in patients who experienced water diuresis. This figure shows the predicted trajectory of s[Na] (solid line) and the 95% confidence interval (shaded area). The dashed red line illustrates a hypothetical trajectory based on that before the s[Na]aft measurements. The table below the graph shows the number of patients who underwent each correction method at each time point. HTS, hypertonic saline; NS, normal saline.
Figure 6.
Changes in UV and u[Na+K] over time based on the administration of DDAVP in patients who experienced water diuresis. A restricted cubic spline model was used to depict the changes in UV and u[Na+K] in all patients, patients administered DDAVP, and patients who were not administered DDAVP. The solid curve represents the predicted trajectory of these measurements, and the shaded area denotes the 95% confidence interval.
Treatment Outcomes of Patients with Water Diuresis
The median change in s[Na] within the first 24 hours of treatment was 8 mEq/L (IQR, 6–9 mEq/L) and that within the first 48 hours was 12 mEq/L (IQR, 9–15 mEq/L) among patients who developed water diuresis (Table 2). Appropriate correction was achieved in 28 patients (93%), and undercorrection and overcorrection were each observed in one patient (3%) (Supplemental Table 2). No osmotic demyelination syndrome was noted among patients with water diuresis.
Discussion
The investigation of the clinical course of patients with profound hyponatremia admitted to the ICU identified several critical clinical characteristics of water diuresis. First, water diuresis occurred in 64% of patients with profound hyponatremia, triggering a steep elevation in s[Na] following increased UV and decreased u[Na+K]. Second, water diuresis manifested within the initial 6 hours of treatment in 60% of patients and within 24 hours in 90% of patients. Third, the increase in UV and decrease in u[Na+K] began several hours before the peak manifestation of water diuresis. Finally, the early detection of water diuresis and prompt administration of electrolyte-free solutions and DDAVP may contribute to appropriate correction.
In this study, among patients with water diuresis, s[Na], UV, and u[Na+K] were measured every 3, 2.4, and 4 hours, respectively, within the first 24 hours. Despite close monitoring, significant changes in s[Na] were observed before and after the manifestation of water diuresis and accompanied by increased UV and decreased u[Na+K]. These changes indicate that water diuresis leads to the loss of electrolyte-free water, resulting in decreased TBW and increased s[Na]. The increased EFWC also supports this result. In addition, the increased s[Na] may be augmented by hypertonic or NS administration, the recommended initial treatment for hyponatremia.9,10 In this study, physicians promptly discontinued saline infusion when water diuresis occurred. D5W was administered in 37% of the cases, even before the onset of water diuresis. This suggests that electrolyte-free water infusion can be used preventively to slow the increase in s[Na] levels, even if it did not meet the definition of water diuresis in this study. If the hypertonic or NS administration had not been discontinued, the s[Na] would have increased beyond the correction limit (Figure 5). Furthermore, because water diuresis was prevalent in this study, the initial treatment of hyponatremia with hypertonic and NS should often be discontinued at the appropriate time, anticipating the onset of water diuresis.
While anticipating the onset of water diuresis is challenging, the results of this study contribute to the appropriate vigilance for water diuresis when treating profound hyponatremia. First, water diuresis developed within the first 6 hours in over half of the patients and within 24 hours in all but three patients after the initiation of hyponatremia correction. On admission, most patients with primary polydipsia (physiological electrolyte-free water excretion) already had water diuresis. In addition, most patients with immediately removable causes of hyponatremia (such as hypovolemia, drugs, pain, or physical stress) developed water diuresis within 24 hours. These findings indicate the need for clinicians to remain vigilant during the early phases of correction. Second, the cubic spline model for UV and u[Na+K] revealed that urinary characteristics of water diuresis change before the peak manifestation of water diuresis, highlighting the importance of careful monitoring of urine variables to detect the onset of increased UV and decreased u[Na+K] for the early detection of water diuresis.
In addition to early detection of water diuresis, appropriate interventions are crucial for effectively managing hyponatremia. Although no standardized treatment exists for water diuresis, electrolyte-free infusions and DDAVP are empirically administered in patients with imminent overcorrection.9,10 DDAVP reduces water loss from the urine by promoting water reabsorption in the collecting ducts, decreasing the need for electrolyte-free infusions to lower s[Na] levels.30 In this study, nearly all patients were administered electrolyte-free infusions and half of them were administered DDAVP promptly to counteract water diuresis. The cubic spline model indicated that DDAVP administration effectively reduced UV and increased u[Na+K], even in patients with severe water diuresis. Therefore, administering DDAVP to patients with severe water diuresis is reasonable to reduce the reliance on high-dose electrolyte-free infusions.
In addition, the infusate and fluid loss formula was used to determine the optimal therapy in most patients considering Na, K, and water input/outputs such as urine, eating/drinking, and extrarenal losses. While the commonly used infusate formula provides a convenient prediction of the change in s[Na] owed to infusion,31,32 it tends to significantly underestimate s[Na] changes in patients experiencing water diuresis. This is because it does not consider the effect of the electrolyte-free water loss owing to water diuresis.12,33 By contrast, the infusate and fluid loss formula allows for the adjustment of treatment according to variations in the UV and u[Na+K] when water diuresis occurs, potentially minimizing the risk of overcorrection.12,25
This study had some limitations. First, it was a retrospective, single-center analysis, warranting caution when extrapolating the findings to diverse settings. Specifically, the impact of water diuresis on s[Na] may be more pronounced in settings where monitoring is less frequent, delaying the recognition of water diuresis. Second, the small sample size of this study limited the statistical power to identify significant outcomes. In particular, overcorrection was present in only one patient with water diuresis, making it difficult to perform additional analyses, such as computing a cutoff or predicting overcorrection based on an apparent change in u[Na+K]. Third, the diagnosis of hyponatremia and ICU admission did not occur concurrently, and UV data during the initial hours of admission were unavailable for some patients. We may have missed the manifestations of water diuresis during that period. Fourth, the time points for measuring blood and urine parameters were not standardized, so the measurement intervals varied from case to case. Finally, although this study defined water diuresis as a UV of ≥2 ml/kg per hour and u[Na+K] of ≤50 mEq/L, there is no universal standard. In this study, we incorporated UV and u[Na+K] in the definition of water diuresis because they influence changes in s[Na] caused by urine.16,34 Regarding UV, in this study, UV per body weight was used to account for differences in the effect of body size on s[Na].16 Although some experts define a UV of ≥100 ml/h as water diuresis,10,17 all manifestations of water diuresis in this study exceeded this threshold. For u[Na+K], the threshold of a u[Na+K] of ≤50 mEq/L was selected because s[Na] tends to increase when u[Na+K] is less than half the s[Na].12,22 This study also showed that relaxing the u[Na+K] threshold to 75 or 100 mEq/L may result in a smaller rate of increase in s[Na] from s[Na]bef to s[Na]aft (0.74±0.22 mEq/L per hour for a u[Na+K] of ≤75 mEq/L and 0.64±0.23 mEq/L per hour for a u[Na+K] of ≤100 mEq/L, respectively). There is no consensus on these thresholds, which may change with future findings.
In conclusion, this study highlights the characteristics of patients with water diuresis during the treatment of profound hyponatremia in the ICU. Water diuresis is common in patients with profound hyponatremia, leading to a rapid elevation in s[Na] levels following increased UV and decreased u[Na+K]. Water diuresis typically manifests within the first 24 hours of treatment and is preceded by the onset of changes in urinary characteristics. Early detection of water diuresis via monitoring urinary characteristics during the early periods of hyponatremia treatment and prompt administration of electrolyte-free infusions and desmopressin may contribute to the effective management of water diuresis. Future multicenter studies involving larger sample sizes are required to validate and further refine the findings of this study.
Supplementary Material
Acknowledgments
We are grateful to all members of the Division of Nephrology, Rheumatology, and the intensive care unit at Chubu Rosai Hospital for their invaluable contribution to the clinical practice of hyponatremia.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A625.
Funding
None.
Author Contributions
Conceptualization: Koya Nagase, Tsuyoshi Watanabe.
Data curation: Koya Nagase, Tsuyoshi Watanabe.
Formal analysis: Takahiro Imaizumi, Koya Nagase, Tsuyoshi Watanabe.
Investigation: Koya Nagase, Tsuyoshi Watanabe.
Methodology: Takahiro Imaizumi, Koya Nagase, Tsuyoshi Watanabe.
Project administration: Tsuyoshi Watanabe.
Supervision: Yoshiro Fujita, Takahiro Imaizumi, Yoshihiro Nakamura, Hideaki Shimizu, Naoho Takizawa, Waka Yokoyama-Kokuryo.
Visualization: Koya Nagase, Tsuyoshi Watanabe.
Writing – original draft: Koya Nagase.
Writing – review & editing: Yoshiro Fujita, Hiroki Ikai, Takahiro Imaizumi, Yuuki Ito, Keita Iwasaki, Yukari Murai, Fumika N. Nagase, Yoshihiro Nakamura, Hideaki Shimizu, Naoho Takizawa, Tsuyoshi Watanabe, Mari Yamamoto, Waka Yokoyama-Kokuryo.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A624.
Supplemental Table 1. Comparison of baseline characteristics of patients with and without water diuresis.
Supplemental Table 2. Correction method and outcomes of patients with and without water diuresis.
Supplemental Figure 1. Predictive correction by infusate and fluid loss formula.
References
- 1.Ayus JC, Arieff AI. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281(24):2299–2304. doi: 10.1001/jama.281.24.2299 [DOI] [PubMed] [Google Scholar]
- 2.Nzerue CM, Baffoe-Bonnie H, You W, Falana B, Dai S. Predictors of outcome in hospitalized patients with severe hyponatremia. J Natl Med Assoc. 2003;95(5):335–343. PMID: 12793790 [PMC free article] [PubMed] [Google Scholar]
- 3.Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70–76. doi: 10.1093/ndt/gfi082 [DOI] [PubMed] [Google Scholar]
- 4.Brunner JE, Redmond JM, Haggar AM, Kruger DF, Elias SB. Central pontine myelinolysis and pontine lesions after rapid correction of hyponatremia: a prospective magnetic resonance imaging study. Ann Neurol. 1990;27(1):61–66. doi: 10.1002/ana.410270110 [DOI] [PubMed] [Google Scholar]
- 5.Laureno R, Karp BI. Myelinolysis after correction of hyponatremia. Ann Intern Med. 1997;126(1):57–62. doi: 10.7326/0003-4819-126-1-199701010-00008 [DOI] [PubMed] [Google Scholar]
- 6.George JC, Zafar W, Bucaloiu ID, Chang AR. Risk factors and outcomes of rapid correction of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(7):984–992. doi: 10.2215/CJN.13061117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004;75(suppl 3):iii22–iii28. doi: 10.1136/jnnp.2004.045906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aegisdottir H, Cooray C, Wirdefeldt K, Piehl F, Sveinsson O. Incidence of osmotic demyelination syndrome in Sweden: a nationwide study. Acta Neurol Scand. 2019;140(5):342–349. doi: 10.1111/ane.13150 [DOI] [PubMed] [Google Scholar]
- 9.Verbalis JG Goldsmith SR Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 suppl 1):S1–S42. doi: 10.1016/j.amjmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Spasovski G Vanholder R Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur J Endocrinol. 2014;170(3):G1–G47. doi: 10.1530/EJE-13-1020 [DOI] [PubMed] [Google Scholar]
- 11.Woodfine JD, Sood MM, MacMillan TE, Cavalcanti RB, van Walraven C. Derivation and validation of a novel risk score to predict overcorrection of severe hyponatremia: the Severe Hyponatremia Overcorrection Risk (SHOR) Score. Clin J Am Soc Nephrol. 2019;14(7):975–982. doi: 10.2215/CJN.12251018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adrogué HJ, Madias NE. The challenge of hyponatremia. J Am Soc Nephrol. 2012;23(7):1140–1148. doi: 10.1681/ASN.2012020128 [DOI] [PubMed] [Google Scholar]
- 13.Geoghegan P Harrison AM Thongprayoon C, et al. Sodium correction practice and clinical outcomes in profound hyponatremia. Mayo Clin Proc. 2015;90(10):1348–1355. doi: 10.1016/j.mayocp.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 14.Baek SH Jo YH Ahn S, et al. Risk of Overcorrection in rapid intermittent bolus vs slow continuous infusion therapies of hypertonic saline for patients with symptomatic hyponatremia: the SALSA randomized clinical trial. JAMA Intern Med. 2021;181(1):81–92. doi: 10.1001/jamainternmed.2020.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterns RH, Hix JK, Silver S. Treating profound hyponatremia: a strategy for controlled correction. Am J Kidney Dis. 2010;56(4):774–779. doi: 10.1053/j.ajkd.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 16.Buchkremer F, Segerer S, Bock A. Monitoring urine flow to prevent overcorrection of hyponatremia: derivation of a safe upper limit based on the edelman equation. Am J Kidney Dis. 2019;73(1):143–145. doi: 10.1053/j.ajkd.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 17.Adrogué HJ, Tucker BM, Madias NE. Diagnosis and management of hyponatremia: a review. JAMA. 2022;328(3):280–291. doi: 10.1001/jama.2022.11176 [DOI] [PubMed] [Google Scholar]
- 18.Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37(9):1236–1256. doi: 10.1172/JCI103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose BD. New approach to disturbances in the plasma sodium concentration. Am J Med. 1986;81(6):1033–1040. doi: 10.1016/0002-9343(86)90401-8 [DOI] [PubMed] [Google Scholar]
- 20.Workeneh BT, Meena P, Christ-Crain M, Rondon-Berrios H. Hyponatremia demystified: integrating physiology to shape clinical practice. Adv Kidney Dis Health. 2023;30(2):85–101. doi: 10.1053/j.akdh.2022.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMillan TE, Tang T, Cavalcanti RB. Desmopressin to prevent rapid sodium correction in severe hyponatremia: a systematic review. Am J Med. 2015;128(12):1362.e15-24. doi: 10.1016/j.amjmed.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 22.Pham PC, Chen PV, Pham PT. Overcorrection of hyponatremia: where do we go wrong? Am J Kidney Dis. 2000;36(2):E12. doi: 10.1053/ajkd.2000.9013 [DOI] [PubMed] [Google Scholar]
- 23.Mohmand HK, Issa D, Ahmad Z, Cappuccio JD, Kouides RW, Sterns RH. Hypertonic saline for hyponatremia: risk of inadvertent overcorrection. Clin J Am Soc Nephrol. 2007;2(6):1110–1117. doi: 10.2215/CJN.00910207 [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 25.Nagase K Watanabe T Nomura A, et al. Predictive correction of serum sodium concentration with formulas derived from the Edelman equation in patients with severe hyponatremia. Sci Rep. 2023;13(1):1783. doi: 10.1038/s41598-023-28380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen MK, Kurtz I. Derivation of a new formula for calculating urinary electrolyte-free water clearance based on the Edelman equation. Am J Physiol Renal Physiol. 2005;288(1):F1–F7. doi: 10.1152/ajprenal.00259.2004 [DOI] [PubMed] [Google Scholar]
- 27.Barsoum NR, Levine BS. Current prescriptions for the correction of hyponatraemia and hypernatraemia: are they too simple? Nephrol Dial Transplant. 2002;17(7):1176–1180. doi: 10.1093/ndt/17.7.1176 [DOI] [PubMed] [Google Scholar]
- 28.Turner MJ, Avolio AP. Does replacing sodium excreted in sweat attenuate the health benefits of physical activity? Int J Sport Nutr Exerc Metab. 2016;26(4):377–389. doi: 10.1123/ijsnem.2015-0233 [DOI] [PubMed] [Google Scholar]
- 29.Hall JE, Hall ME, Guyton AC. Textbook of Medical Physiology, 14th ed. Elsevier; 2021. [Google Scholar]
- 30.Rafat C Schortgen F Gaudry S, et al. Use of desmopressin acetate in severe hyponatremia in the intensive care unit. Clin J Am Soc Nephrol. 2014;9(2):229–237. doi: 10.2215/CJN.00950113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adrogué HJ, Madias NE. Aiding fluid prescription for the dysnatremias. Intensive Care Med. 1997;23(3):309–316. doi: 10.1007/s001340050333 [DOI] [PubMed] [Google Scholar]
- 32.Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581–1589. doi: 10.1056/NEJM200005253422107 [DOI] [PubMed] [Google Scholar]
- 33.Liamis G, Kalogirou M, Saugos V, Elisaf M. Therapeutic approach in patients with dysnatraemias. Nephrol Dial Transplant. 2006;21(6):1564–1569. doi: 10.1093/ndt/gfk090 [DOI] [PubMed] [Google Scholar]
- 34.Portales-Castillo I, Sterns RH, Bress J, Proano RA. Where do the salt and water go? A case of profound hyponatremia. Am J Kidney Dis. 2018;72(6):885–889. doi: 10.1053/j.ajkd.2018.07.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information.