Abstract
Key Points
Sudden cardiac death is a major concern for hemodialysis patients. Mortality is higher on dialysis days and is associated with higher dialysate bicarbonate (DBIC).
Contrary to our hypothesis, there was no consistent association of higher DBIC with a higher risk of arrhythmia.
Further research is needed to assess the optimal DBIC and mechanisms by which it may improve outcomes for maintenance hemodialysis patients.
Background
Sudden death accounts for approximately 25% of deaths among maintenance hemodialysis patients, occurring more frequently on hemodialysis days. Higher dialysate bicarbonate (DBIC) may predispose to alkalemia and arrhythmogenesis.
Methods
We conducted a 12-month analysis of session-level data from 66 patients with implantable loop recorders. We fit logistic regression and negative binomial mixed-effects regression models to assess the association of DBIC with clinically significant arrhythmia (ventricular tachycardia ≥115 beats per minute [BPM] for at least 30 seconds, bradycardia ≤40 BPM for at least 6 seconds, or asystole for at least 3 seconds) and reviewer confirmed arrhythmia (RCA—implantable loop recorder-identified or patient-marked event for which a manual review of the stored electrocardiogram tracing confirmed the presence of atrial fibrillation, supraventricular tachycardia, sinus tachycardia with rate >130 BPM, ventricular tachycardia, asystole, or bradycardia). Models adjusted for age, sex, race, hemodialysis vintage, vascular access, and prehemodialysis serum bicarbonate and additionally for serum and dialysate potassium levels.
Results
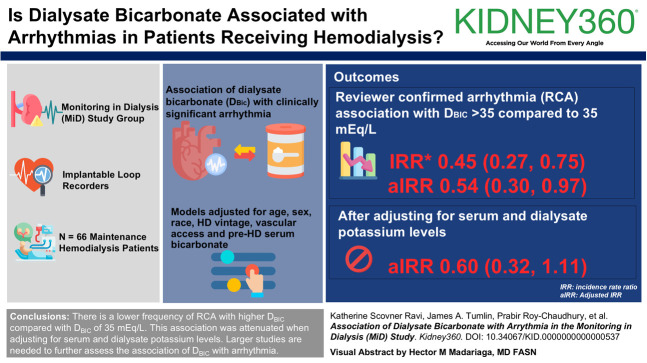
The mean age was 56±12 years, 70% were male, 53% were Black, and 35% were Asian. Fewer RCA episodes were associated with DBIC >35 than 35 mEq/L (incidence rate ratio 0.45 [0.27 to 0.75] and adjusted incident rate ratio 0.54 [0.30 to 0.97]), but the association was not significant when adjusting for serum and dialysate potassium levels (adjusted incident rate ratio, 0.60 [0.32 to 1.11]). Otherwise, no associations between DBIC and arrhythmia were identified.
Conclusions
We observed a lower frequency of RCA with higher DBIC, compared with DBIC of 35 mEql/L, contrary to our original hypothesis, but this association was attenuated in fully adjusted models. Validation of these findings in larger studies is required, with a further need for interventional studies to explore the optimal DBIC concentration.
Keywords: acidosis, cardiovascular, cardiovascular events, dialysis
Visual Abstract
Introduction
More than 550,000 patients are dependent on maintenance hemodialysis in the United States alone.1 Their mortality rate is nearly 20% per year, with more than 40% dying from cardiovascular (CV) disease.2,3 Sudden cardiac death (SCD) accounts for around a quarter of deaths in the maintenance hemodialysis patient population,4 with cardiac arrhythmia likely to be a major etiological factor.5 CV mortality and hospitalization rates are higher on the days on which hemodialysis occurs,6 which suggests there may be something intrinsic to the hemodialysis procedure that contributes to adverse CV outcomes.
Patients on maintenance hemodialysis are reliant on the delivery of base in the dialysate, in an attempt to minimize metabolic acidosis and its complications. This is achieved mostly via the use of dialysate bicarbonate (DBIC) at each hemodialysis session, which is delivered thrice weekly, in a highly nonphysiologic fashion via the prescription of dialysate concentrations above the normal physiologic range of serum bicarbonate (SBIC).7,8 Practitioners prescribe DBIC for millions of hemodialysis sessions per year. In light of observational evidence that higher DBIC is associated with mortality and SCD,9 and perhaps under duress from many highly publicized lawsuits against the use of higher DBIC, some authorities are encouraging physicians to manipulate the DBIC prescription, based on prehemodialysis SBIC levels.10 However, the optimal DBIC prescription is unknown.
Previous reports on the Monitoring in Dialysis (MiD) study illustrate that SBIC increases from prehemodialysis to posthemodialysis,11 and in these data, the DBIC is one of the most important contributors to changes in SBIC that occur during and after each individual hemodialysis session. Furthermore, for any given DBIC, the prehemodialysis SBIC is a strong predictor of the posthemodialysis SBIC concentration.11 To assess the interplay between DBIC and SBIC in terms of arrhythmogenesis, this study assesses how DBIC relates to arrhythmias described in the MiD study while accounting for prehemodialysis SBIC levels.
Methods
Study Design and Population
This study is a secondary analysis of the MiD study.12 MiD was a prospective cohort study that enrolled 66 patients on maintenance hemodialysis from 10 centers (n=43 from the United States; n=23 from India). The study used implantable loop recorders to record continuous electrocardiographic readings over a 6-month period. Patients were enrolled from January 2013 to January 2014 in the United States and from March 2014 to December 2015 in India. The primary eligibility criteria were age 21 or older, thrice weekly in-center hemodialysis, or eGFR <15 ml/min per 1.73 m2 with expected hemodialysis initiation within 2 months, although no patients were enrolled before their hemodialysis initiation. Key exclusion criteria were unsuitability for implantation, expected survival <6 months, left-sided hemodialysis catheter interfering with implantation, thoracic surgery within the preceding 6 months, bacteremia within the preceding 60 days or nonbacteremic infection within the preceding 14 days, hemoglobin <10 g/dl on consecutive measurements within the preceding 2 months, end-stage liver failure, transplantation or modality transfer expected within 6 months, or existing pacemaker or implantable cardioverter defibrillator. The design and main results of the MiD study have been reported.12,13 Dialysate prescriptions were as deemed clinically indicated by the patient's nephrologist (Figure 1).
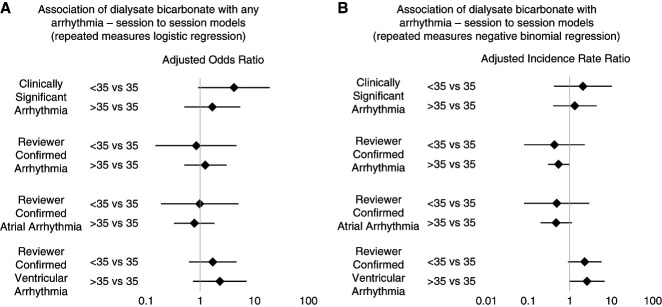
Figure 1.
Association of dialysate bicarbonate with arrhythmia—forest plots. (A) Association of dialysate bicarbonate with any arrhythmia—session to session models (repeated measures logistic regression). (B) Association of dialysate bicarbonate with arrhythmia—session to session models (repeated measures negative binomial regression). The diamonds represent the adjusted incident rate ratio, and the horizontal lines through the diamonds represent the 95% CIs. Models adjusted for age, sex, race, dialysis vintage, vascular access, and pre-hemodialysis serum bicarbonate. Note: Estimates are provided on the log scale. CI, confidence interval; OR, odds ratio.
Exposures and Outcomes
The primary exposure in this study was categorical DBIC (<35, 35, and >35 mEq/L). DBIC of 35 mEq/L was considered as the reference because it was the most common prescription used in the MiD cohort,14 and it is commonly used in clinical practice.15 Hemodialysis parameters, including DBIC, were recorded for all hemodialysis sessions, and updated parameters were used for the analysis of each repeated measure of hemodialysis session within individuals. The primary outcomes were arrhythmias from the beginning of the respective hemodialysis sessions to the start of the next. Arrhythmia events were defined as per the definitions used in the MiD study: (1) clinically significant arrhythmia (CSA)—those most likely to be associated with SCD, defined as episodes of ventricular tachycardia ≥115 beats per minute (BPM) for at least 30 seconds; episodes of bradycardia ≤40 BPM for at least 6 seconds; instances of asystole for at least 3 seconds; and any symptomatic event during which the stored electrocardiogram showed a CSA and (2) reviewer-confirmed arrhythmia (RCA)—any implantable loop recorder-identified or patient-marked event in which a manual review of the stored electrocardiogram tracing confirmed the presence of atrial fibrillation, supraventricular tachycardia, sinus tachycardia with rate >130 BPM, ventricular tachycardia, asystole, or bradycardia. Implantable loop recorders were interrogated at each hemodialysis session for 6 months. As previously described, the RCA end point defines events that represent clinically relevant electrical instability and chronotropic dysfunction which likely share common physiologies with CSA. RCA thus merits analysis in conjunction with CSA.14 Tracings were independently adjudicated by a core laboratory.13 The number of CSA, RCA, and all their subtype events were assessed across groups of DBIC. We included data where the number of events was sufficient to make a determination regarding associations.
Laboratory Analysis
Blood samples were obtained before hemodialysis twice weekly for the first 4 weeks and then once weekly through the remaining 5 months of the 6-month observation period. Blood samples were collected at study sites by trained personnel, centrifuged, refrigerated, and then shipped to a central laboratory in the United States or India according to the recruitment site for measurement, using standard techniques.12
Statistical Analysis
Continuous variables were examined graphically and recorded as means (±SD) for normally distributed data or medians (25th–75th percentile) for non-normally distributed data. Categorical variables were examined by frequency distribution and recorded as proportions. Associations between demographic, laboratory, and dialysis session data and DBIC were assessed using ANOVA for continuous variables or chi-squared or Fisher exact tests for categorical variables, as appropriate.
The associations between DBIC with the number of hemodialysis sessions complicated by arrhythmia from the beginning of the session until the initiation of the next hemodialysis session and with the number of arrhythmias occurring from the start of one hemodialysis session to the initiation of the next hemodialysis session were assessed using repeated measures logistic regression models and negative binomial mixed-effects regression models, respectively, with patient ID included as a random effect. DBIC was reviewed at each session and recorded if it was changed permitting accuracy of repeated measures. Initially, unadjusted models were fit; subsequently, adjusted models accounted for age, sex, race, hemodialysis vintage, and vascular access (covariates used in initial analyses)13 (model 1). Our fully adjusted model included the aforementioned covariates and added prehemodialysis SBIC as a time varying covariate with SBIC from the respective session; sessions for which SBIC was unavailable were not included in these results (model 2). The number of covariates was limited by the small sample size and number of outcomes. However, given the particular interest regarding the interactions with potassium, we included an exploratory model which included the aforementioned variables and added prehemodialysis serum potassium and dialysate potassium levels. All analyses were carried out using the statistical software package SAS version 9.4 (Cary, NC). Two-sided P values of <0.05 were considered statistically significant.
Ethics
The MiD study was approved by applicable institutional review boards or ethical review committees at each participating center. The investigation conforms with the principles outlined in the Declaration of Helsinki. Participants provided written informed consent before the beginning of the study.
Results
Baseline Characteristics
A total of 66 patients were included in the present analysis, contributing a total of 3655 sessions with recorded DBIC. The mean age at baseline was 56±12 years, 70% were male, 53% were Black, and 35% were Asian. A total of 64% of participants had a history of diabetes, 26% had heart failure, and 11% had atrial fibrillation at baseline (Table 1). Patients with higher DBIC were more likely to be Black, to have older hemodialysis vintage, and had higher prehemodialysis SBIC levels. Baseline characteristics and hemodialysis treatment characteristics are presented by country of origin in Supplemental Tables 1 and 2, respectively. Laboratory data and dialysis characteristics across all sessions are shown in Supplemental Tables 3 and 4, respectively.
Table 1.
Characteristics of the participants at baseline
| Baseline Characteristic | All Patients (N=66) | Baseline DBIC Recorded (n=62) | Baseline DBIC <35 (n=15) | Baseline DBIC=35 (n=27) | Baseline DBIC >35 (n=20) | P Valuea |
|---|---|---|---|---|---|---|
| Age, yr | 56±12 | 56±12 | 59±15 | 59±10 | 51±12 | 0.09 |
| Male, No. (%) | 46 (70) | 42 (68) | 11 (73) | 19 (70) | 12 (60) | 0.70 |
| Race, No. (%) | ||||||
| Asian | 23 (35) | 19 (31) | 8 (53) | 11 (41) | 0 (0) | <0.001 |
| Black | 35 (53) | 35 (57) | 7 (47) | 13 (48) | 15 (75) | |
| White | 7 (11) | 7 (11) | 0 (0) | 3 (11) | 4 (20) | |
| Other | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 1 (5) | |
| ESKD vintage, yr | 2.4 (1.2–5.3) | 2.5 (1.1–5.5) | 2.9 (1.3–4.8) | 2.2 (0.9–4.8) | 3.4 (1.2–11.9) | 0.02 |
| Vascular access, No. (%) | 0.58 | |||||
| AV fistula | 45 (68) | 41 (66) | 10 (67) | 20 (74) | 11 (55) | |
| AV graft | 17 (26) | 17 (27) | 5 (33) | 5 (19) | 7 (35) | |
| Catheter | 3 (5) | 3 (5) | 0 (0) | 2 (7) | 1 (5) | |
| Comorbid conditions, No. (%) | ||||||
| Diabetes mellitus | 42 (64) | 38 (61) | 9 (60) | 17 (63) | 12 (60) | 1.00 |
| Hyperlipidemia | 40 (61) | 39 (63) | 8 (53) | 17 (63) | 14 (70) | 0.63 |
| Hypertension | 56 (85) | 56 (86) | 12 (80) | 22 (82) | 19 (95) | 0.35 |
| Ischemic heart disease | 32 (49) | 28 (45) | 7 (47) | 12 (44) | 9 (45) | 1.00 |
| Congestive heart failure | 17 (26) | 17 (27) | 5 (33) | 4 (15) | 8 (40) | 0.15 |
| Arrhythmia | 21 (32) | 21 (34) | 4 (27) | 8 (30) | 9 (45) | 0.49 |
| Atrial fibrillation | 7 (11) | 7 (11) | 1 (7) | 2 (7) | 4 (20) | 0.40 |
| Systolic BP, mm Hg | 141±23 | 140±23 | 142±25 | 140±23 | 137±24 | 0.83 |
| Diastolic BP, mm Hg | 77±13 | 76±13 | 75±12 | 75±11 | 79±17 | 0.62 |
| Medication use, No. (%) | ||||||
| Aspirin | 24 (36) | 22 (36) | 5 (33) | 8 (30) | 9 (45) | 0.59 |
| Antilipidemic | 32 (49) | 31 (50) | 10 (67) | 12 (44) | 9 (45) | 0.37 |
| ACEI or ARB | 22 (33) | 21 (34) | 4 (27) | 6 (22) | 11 (55) | 0.06 |
| β blockers | 38 (58) | 36 (58) | 9 (60) | 15 (56) | 12 (60) | 1.00 |
| Predialysis serum laboratory values | ||||||
| BUN, mg/dl | 60±18 | 60±18 | 51±18 | 64±20 | 60±14 | 0.11 |
| Creatinine, mg/dl | 10.0±3.4 | 10.2±3.4 | 9.2±3.2 | 10.0±3.4 | 11.2±3.5 | 0.25 |
| Sodium, mEq/L | 137±5 | 137±4 | 137±5 | 137±3 | 138±5 | 0.71 |
| Potassium, mEq/L | 5.0±1.0 | 4.9±0.9 | 4.5±0.7 | 5.1±1.2 | 4.8±0.7 | 0.14 |
| Calcium, mg/dl | 8.7±0.8 | 8.7±0.9 | 8.8±0.9 | 8.7±0.9 | 8.8±0.9 | 0.86 |
| Bicarbonate, mEq/L | 22±4 | 22±4 | 22±4 | 21±4 | 24±3 | 0.04 |
| Magnesium, mg/dl | 2.4±0.5 | 2.4±0.5 | 2.3±0.7 | 2.5±0.5 | 2.3±0.3 | 0.47 |
| Phosphorus, mg/dl | 5.5±2.0 | 5.5±2.0 | 4.5±1.0 | 6.0±2.5 | 5.5±1.8 | 0.13 |
| Hemoglobin, g/dl | 11±1 | 11±1 | 10±2 | 11±1 | 11±1 | 0.16 |
| Serum albumin, g/dl | 3.9±0.3 | 4.0±0.3 | 3.9±0.5 | 3.9±0.3 | 4.0±0.2 | 0.28 |
Continuous variables are presented as means ± SD or median (25th–75th percentiles). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AV, arteriovenous; DBIC, dialysate bicarbonate.
P value from ANOVA for continuous variables or chi-squared or Fisher exact tests for categorical variables, as appropriate.
Hemodialysis Treatment Characteristics
The median duration of hemodialysis was 4.0 (3.5–4.0) hours, with a mean single-pool Kt/V of 1.5±0.4 and ultrafiltration rate of 12±6 ml/kg per hour across all participants (Table 2). Patients with higher DBIC had shorter hemodialysis durations, were heavier and further above their target dry weight prehemodialysis, and were more likely to have higher blood flows, high-flux dialyzers, and higher dialysate calcium concentrations. Of the sessions for which DBIC was <35 mEq/L, the baseline mean was 30.9±2.4 mEq/L, and the mean for all sessions was 30.4±4.1 mEq/L. Of the sessions for which DBIC was >35 mEq/L, the baseline mean was 38.8±1.7 mEq/L, and the mean for all sessions was 38.5±1.6 mEq/L. Regarding intraparticipant prescription variability, 3408/3655 (93.2%) hemodialysis sessions had DBIC that was the same as the participant's baseline.
Table 2.
Characteristics of the dialysis prescriptions at baseline
| Baseline Characteristic | All Patients (N=66) | Baseline Dialysate Recorded (n=62) | Baseline DBIC <35 (n=15) | Baseline DBIC=35 (n=27) | Baseline DBIC >35 (n=20) | P Valuea |
|---|---|---|---|---|---|---|
| Duration of hemodialysis, h | 4.0 (3.5–4.0) | 4.0 (3.5–4.0) | 4.0 (4.0–4.0) | 3.7 (3.5–4.0) | 3.6 (3.5–4.0) | 0.02 |
| spKt/V | 1.5±0.4 | 1.5±0.3 | 1.5±0.3 | 1.4±0.3 | 1.5±0.3 | 0.53 |
| Predialysis weight, kg | 86.7±28.8 | 88.5±28.8 | 72.4±16.8 | 85.3±19.1 | 104.7±38.2 | <0.01 |
| Kilogram over dry weight target before dialysis | 4.2 (2.7–5.2) | 4.3 (2.8–5.5) | 3.9 (2.3–4.8) | 4.3 (2.3–5.0) | 4.9 (3.5–6.9) | 0.01 |
| UFR, ml/kg per hour | 12±6 | 12±6 | 12±6 | 11±5 | 13±6 | 0.64 |
| Dialysate flow, ml/min | 600 (500–800) | 600 (500–800) | 500 (500–800) | 600 (500–800) | 600 (600–800) | 0.20 |
| Blood flow, ml/min | 387 (300–467) | 400 (300–475) | 314 (294–500) | 375 (300–400) | 471 (407–500) | <0.001 |
| High-flux dialyzer, No. (%) | 42 (64) | 41 (66) | 7 (47) | 15 (56) | 19 (95) | <0.01 |
| Membrane reuse, No. (%) | 18 (27) | 17 (27) | 1 (7) | 11 (41) | 5 (25) | 0.06 |
| Cellulose membrane, No. (%) | 5 (8) | 4 (7) | 1 (7) | 3 (11) | 0 (0) | 0.35 |
| Dialysate temperature, °C, No. (%) | 0.06 | |||||
| 35.5 | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 1 (5) | |
| 36.0 | 3 (5) | 3 (5) | 0 (0) | 1 (4) | 2 (10) | |
| 36.5 | 5 (8) | 5 (8) | 0 (0) | 5 (19) | 0 (0) | |
| 37.0 | 57 (86) | 53 (86) | 15 (100) | 21 (78) | 17 (85) | |
| Dialysate potassium, mEq/L, No. (%) | 0.77 | |||||
| 1.0 | 1 (2) | 1 (2) | 0 (0) | 0 (0) | 1 (5) | |
| 2.0 | 53 (80) | 49 (79) | 11 (73) | 22 (82) | 16 (80) | |
| 3.0 | 11 (17) | 11 (18) | 4 (27) | 4 (15) | 3 (15) | |
| 4.0 | 1 (2) | 1 (2) | 0 (0) | 1 (4) | 0 (0) | |
| Dialysate calcium, mEq/L, No. (%) | <0.001 | |||||
| 1.5 and 1.6 | 13 (20) | 11 (18) | 1 (7) | 10 (37) | 0 (0) | |
| 2.0 and 2.5 | 39 (59) | 39 (63) | 7 (47) | 16 (59) | 16 (80) | |
| 3.0 and 3.5 | 14 (21) | 12 (19) | 7 (47) | 1 (4) | 4 (20) | |
| Dialysate sodium, mEq/L, No. (%) | 0.36 | |||||
| 135 | 6 (10) | 5 (9) | 1 (9) | 4 (15) | 0 (0) | |
| 138 | 6 (10) | 5 (9) | 1 (9) | 3 (11) | 1 (5) | |
| 140 | 49 (80) | 48 (83) | 9 (82) | 20 (74) | 19 (95) |
Continuous variables are presented as means ± SD or median (25th–75th percentiles). DBIC, dialysate bicarbonate; spKt/V, single-pool Kt/V; UFR, ultrafiltration rate.
P value from ANOVA for continuous variables, or chi-squared or Fisher exact tests for categorical variables, as appropriate.
Note: Blood flow rate is the mean of 12 time points during the baseline dialysis session.
Laboratory Analysis
Blood samples were obtained twice weekly for the first 4 weeks after the insertion of internal loop recorders and then weekly for the subsequent 5 months.12 Regarding the day of the week blood was obtained, 1040/1229 (84.6%) blood samples were obtained on a Monday or a Tuesday, and 189/1229 (15.4%) were obtained on other days of the week.
Risk of Arrhythmia by DBIC
The association of variable DBIC with the various types of arrhythmia is reported in Tables 3 and 4. There were fewer episodes of RCA for DBIC >35 mEq/L compared with 35 mEq/L in unadjusted (incidence rate ratio [IRR], 0.45; 95% CI, 0.27 to 0.75) and fully adjusted (model 2, adjusted incident rate ratio [aIRR], 0.54; 95% CI, 0.30 to 0.97) models, respectively. Although the trend persisted, the association lost significance in the exploratory model. Otherwise, no association between DBIC and the frequency of other arrhythmia subtypes was observed (DBIC >35 mEq/L compared with 35 mEq/L, aIRR [95% CI] in fully adjusted model 2 for each arrhythmia subtype: CSA: 1.33 [0.40 to 4.44]; reviewer-confirmed atrial arrhythmia: 0.47 [0.20 to 1.13]; reviewer-confirmed ventricular arrhythmia 2.60 [0.97 to 6.93]). For DBIC <35 mEq/L compared with 35 mEq/L, aIRR (95% CI) in fully adjusted model 2 for each arrhythmia subtype: CSA: 2.09 (0.42 to 10.36); RCA: 0.43 (0.08 to 2.29); reviewer-confirmed atrial arrhythmia: 0.49 (0.08 to 2.93); reviewer-confirmed ventricular arrhythmia 2.30 (0.91 to 5.83).
Table 3.
Association of dialysate bicarbonate with any arrhythmia—session to session models (repeated measures logistic regression)
| Model | DBIC (mEq/L) |
n/N P Valuea |
|||
|---|---|---|---|---|---|
| <35 versus 35 OR (95% CI) n/N |
>35 versus 35 OR (95% CI) n/N |
<35 versus >35 OR (95% CI) n/N |
|||
| Arrhythmia subtype | |||||
| CSA | Unadjusted | 1.68 (0.49 to 5.72) 87/816 versus 56/1552 |
1.22 (0.41 to 3.58) 78/1287 versus 56/1552 |
1.38 (0.36 to 5.23) 87/816 versus 78/1287 |
221/3655 0.71 |
| Model 1 | 2.00 (0.55 to 7.22) 87/795 versus 56/1552 |
1.01 (0.32 to 3.16) 75/1181 versus 56/1552 |
1.99 (0.37 to 10.76) 87/795 versus 75/1181 |
218/3528 0.57 |
|
| Model 2 | 4.22 (0.92 to 19.33) 46/249 versus 29/549 |
1.68 (0.51 to 5.51) 41/388 versus 29/549 |
2.52 (0.37 to 17.13) 46/249 versus 41/388 |
116/1186 0.13 |
|
| Exploratory model | 3.28 (0.66 to 16.39) 44/240 versus 29/548 |
1.65 (0.49 to 5.60) 41/388 versus 29/548 |
1.98 (0.26 to 15.29) 44/240 versus 41/388 |
114/1176 0.24 |
|
| RCA | Unadjusted | 0.43 (0.09 to 2.10) 271/816 versus 533/1552 |
0.61 (0.25 to 1.51) 587/1287 versus 533/1552 |
0.71 (0.16 to 3.20) 271/816 versus 587/1287 |
1391/3655 0.44 |
| Model 1 | 0.41 (0.06 to 2.67) 265/795 versus 533/1552 |
0.58 (0.24 to 1.38) 541/1181 versus 533/1552 |
0.72 (0.10 to 4.95) 265/795 versus 541/1181 |
1339/3528 0.35 |
|
| Model 2 | 0.85 (0.15 to 4.67) 86/249 versus 173/549 |
1.25 (0.51 to 3.07) 193/388 versus 173/549 |
0.68 (0.10 to 4.54) 86/249 versus 193/388 |
452/1186 0.87 |
|
| Exploratory model | 0.91 (0.16 to 5.07) 84/240 versus 173/548 |
1.47 (0.58 to 3.71) 193/388 versus 173/548 |
0.62 (0.09 to 4.24) 84/240 versus 193/388 |
450/1176 0.71 |
|
| RCA subtypes | |||||
| Reviewer-confirmed atrial arrhythmia | Unadjusted | 0.45 (0.13 to 1.54) 238/816 versus 421/1552 |
0.77 (0.43 to 1.39) 454/1287 versus 421/1552 |
0.58 (0.18 to 1.83) 238/816 versus 454/1287 |
1113/3655 0.41 |
| Model 1 | 0.60 (0.11 to 3.25) 235/795 versus 421/1552 |
0.61 (0.35 to 1.09) 412/1181 versus 421/1552 |
0.98 (0.17 to 5.50) 235/795 versus 412/1181 |
1068/3528 0.23 |
|
| Model 2 | 0.98 (0.19 to 5.10) 73/249 versus 139/549 |
0.78 (0.33 to 1.84) 141/388 versus 139/549 |
1.25 (0.19 to 8.11) 73/249 versus 141/388 |
353/1186 0.85 |
|
| Exploratory model | 1.00 (0.19 to 5.29) 71/240 versus 139/548 |
0.86 (0.36 to 2.07) 141/388 versus 139/548 |
1.16 (0.18 to 7.55) 71/240 versus 141/388 |
351/1176 0.95 |
|
| Reviewer-confirmed ventricular arrhythmia | Unadjusted | 1.94 (0.49 to 7.75) 117/816 versus 108/1552 |
2.33 (0.54 to 10.01) 143/1287 versus 108/1552 |
0.83 (0.20 to 3.42) 117/816 versus 143/1287 |
368/3655 0.48 |
| Model 1 | 2.44 (0.75 to 7.93) 116/795 versus 108/1552 |
2.22 (0.60 to 8.21) 140/1181 versus 108/1552 |
1.10 (0.28 to 4.33) 116/795 versus 140/1181 |
364/3528 0.27 |
|
| Model 2 | 1.71 (0.62 to 4.69) 34/249 versus 38/549 |
2.31 (0.74 to 7.20) 53/388 versus 38/549 |
0.74 (0.23 to 2.36) 34/249 versus 53/388 |
125/1186 0.32 |
|
| Exploratory model | 1.45 (0.53 to 3.97) 34/240 versus 38/548 |
2.47 (0.75 to 8.13) 53/388 versus 38/548 |
0.59 (0.17 to 1.99) 34/240 versus 53/388 |
125/1176 0.33 |
|
Model 1 adjusted for age, sex, race, dialysis vintage, and vascular access. CSA, clinically significant arrhythmia; DBIC, dialysate bicarbonate; n/N, number of sessions with event/number of sessions; OR, odds ratio; RCA, reviewer confirmed arrhythmia.
Model 2 adjusted for age, sex, race, dialysis vintage, vascular access, and prehemodialysis serum bicarbonate.
Exploratory model adjusted for age, sex, race, dialysis vintage, vascular access, prehemodialysis serum bicarbonate, prehemodialysis serum potassium, and dialysate potassium levels.
Overall P value from repeated measures model F test.
Table 4.
Association of dialysate bicarbonate with arrhythmia—session to session models (repeated measures negative binomial regression)
| Model | DBIC (mEq/L) |
n/N P Valuea |
|||
|---|---|---|---|---|---|
| <35 versus 35 IRR (95% CI) n/N |
>35 versus 35 IRR (95% CI) n/N |
<35 versus >35 IRR (95% CI) n/N |
|||
| Arrhythmia subtype | |||||
| CSA | Unadjusted | 1.35 (0.33 to 5.53) 586/816 versus 170/1552 |
0.92 (0.27 to 3.15) 455/1287 versus 170/1552 |
1.47 (0.33 to 6.60) 586/816 versus 455/1287 |
1211/3655 0.87 |
| Model 1 | 1.39 (0.30 to 6.37) 586/795 versus 170/1552 |
0.72 (0.21 to 2.39) 452/1181 versus 170/1552 |
1.94 (0.29 to 12.83) 586/795 versus 452/1181 |
1208/3528 0.78 |
|
| Model 2 | 2.09 (0.42 to 10.36) 205/249 versus 83/549 |
1.33 (0.40 to 4.44) 217/388 versus 83/549 |
1.57 (0.22 to 11.09) 205/249 versus 217/388 |
505/1186 0.61 |
|
| Exploratory model | 1.59 (0.29 to 8.85) 203/240 versus 83/548 |
1.51 (0.46 to 4.95) 217/388 versus 83/548 |
1.05 (0.13 to 8.35) 203/240 versus 217/388 |
503/1176 0.69 |
|
| RCA | Unadjusted | 0.27 (0.07 to 1.02) 2127/816 versus 3617/1552 |
0.45 (0.27 to 0.75) 3974/1287 versus 3617/1552 |
0.60 (0.20 to 1.82) 2127/816 versus 3974/1287 |
9718/3655 <0.01 |
| Model 1 | 0.30 (0.03 to 2.66) 2120/795 versus 3617/1552 |
0.40 (0.26 to 0.61) 3623/1181 versus 3617/1552 |
0.75 (0.09 to 6.30) 2120/795 versus 3623/1181 |
9360/3528 <0.001 |
|
| Model 2 | 0.43 (0.08 to 2.29) 565/249 versus 1221/549 |
0.54 (0.30 to 0.97) 1250/388 versus 1221/549 |
0.79 (0.14 to 4.36) 565/249 versus 1250/388 |
3036/1186 0.09 |
|
| Exploratory model | 0.52 (0.08 to 3.21) 553/240 versus 1221/548 |
0.60 (0.32 to 1.11) 1250/388 versus 1221/548 |
0.86 (0.14 to 5.25) 553/240 versus 1250/388 |
3024/1176 0.25 |
|
| RCA subtypes | |||||
| Reviewer-confirmed atrial arrhythmia | Unadjusted | 0.30 (0.07 to 1.30) 1387/816 versus 2391/1552 |
0.53 (0.26 to 1.10) 2054/1287 versus 2391/1552 |
0.57 (0.15 to 2.12) 1387/816 versus 2054/1287 |
5832/3655 0.15 |
| Model 1 | 0.39 (0.05 to 3.05) 1384/795 versus 2391/1552 |
0.45 (0.24 to 0.87) 1772/1181 versus 2391/1552 |
0.86 (0.11 to 6.75) 1384/795 versus 1772/1181 |
5547/3528 0.05 |
|
| Model 2 | 0.49 (0.08 to 2.93) 342/249 versus 862/549 |
0.47 (0.20 to 1.13) 547/388 versus 862/549 |
1.03 (0.16 to 6.72) 342/249 versus 547/388 |
1751/1186 0.21 |
|
| Exploratory model | 0.45 (0.07 to 2.93) 330/240 versus 862/548 |
0.51 (0.21 to 1.23) 547/388 versus 862/548 |
0.87 (0.13 to 5.92) 330/240 versus 547/388 |
1739/1176 0.28 |
|
| Reviewer-confirmed ventricular arrhythmia | Unadjusted | 2.24 (0.58 to 8.69) 246/816 versus 161/1552 |
2.13 (0.68 to 6.66) 330/1287 versus 161/1552 |
1.05 (0.28 to 3.93) 246/816 versus 330/1287 |
737/3655 0.35 |
| Model 1 | 3.00 (0.93 to 9.70) 245/795 versus 161/1552 |
2.18 (0.80 to 5.94) 327/1181 versus 161/1552 |
1.38 (0.40 to 4.77) 245/795 versus 327/1181 |
733/3528 0.12 |
|
| Model 2 | 2.30 (0.91 to 5.83) 72/249 versus 58/549 |
2.60 (0.97 to 6.93) 114/388 versus 58/549 |
0.89 (0.32 to 2.47) 72/249 versus 114/388 |
244/1186 0.09 |
|
| Exploratory model | 2.03 (0.01 to 276.52) 72/240 versus 58/548 |
2.86 (0.20 to 41.06) 114/388 versus 58/548 |
0.71 (0.07 to 6.92) 72/240 versus 114/388 |
244/1176 <0.001 |
|
Model 1 adjusted for age, sex, race, dialysis vintage, and vascular access. CSA, clinically significant arrhythmia; DBIC, dialysate bicarbonate; IRR, incidence rate ratio; n/N, number of events/number of sessions; RCA, reviewer-confirmed arrhythmia.
Model 2 adjusted for age, sex, race, dialysis vintage, vascular access, and prehemodialysis serum bicarbonate.
Exploratory model adjusted for age, sex, race, dialysis vintage, vascular access, prehemodialysis serum bicarbonate, prehemodialysis serum potassium, and dialysate potassium levels.
Overall P value from repeated measures model F test.
An association of DBIC with the presence of arrhythmias was not observed using logistic regression models (Table 3). For DBIC >35 mEq/L compared with 35 mEq/L, aOR (95% CI) in fully adjusted model 2 for each arrhythmia subtype: CSA: 1.68 (0.51 to 5.51); RCA: 1.25 (0.51 to 3.07); reviewer-confirmed atrial arrhythmia: 0.78 (0.33 to 1.84); reviewer-confirmed ventricular arrhythmia 2.31 (0.74 to 7.20). For DBIC <35 mEq/L compared with 35 mEq/L, aOR (95% CI) in fully adjusted model 2 for each arrhythmia subtype: CSA: 4.22 (0.92 to 19.33); RCA: 0.85 (0.15 to 4.67); reviewer-confirmed atrial arrhythmia: 0.98 (0.19 to 5.10); reviewer-confirmed ventricular arrhythmia 1.71 (0.62 to 4.69).
Discussion
In this secondary analysis of the MiD study, we tested whether higher DBIC is associated with cardiac arrhythmia in patients with implantable loop recorders. We observed a lower frequency of RCA with higher DBIC (>35 mEq/L), compared with DBIC of 35 mEq/L. Within RCA, a majority of events were atrial arrhythmia. We found no association of DBIC with CSA or other arrhythmia subtypes.
Cardiac arrhythmias are common in patients receiving hemodialysis, with mean estimates of 2 (95% confidence interval, 1.8 to 2.2) episodes of cardiac arrhythmia (defined as sinus bradycardia, asystole, high degree atrioventricular block, sustained ventricular tachycardia, ventricular fibrillation, or atrial fibrillation) per patient year16 and are thought to predispose to SCD. The highest incidence of CV death, hospitalization, and SCD occur on the days that patients receive hemodialysis treatments.6,17 Arrhythmias appear to cluster around the hemodialysis procedure, with previous studies demonstrating a three-fold increased risk of SCD in the 12 hours preceding and 1.7-fold increased risk of SCD in the 12 hours after hemodialysis sessions.18 The MiD study previously demonstrated that CSA occurs most frequently just preceding hemodialysis sessions and after the first hemodialysis session of the week.13 Because patients are at an increased risk of SCD in the perihemodialysis period,18 it is tempting to hypothesize that extremes of electrolyte derangement before hemodialysis and/or rapid changes during hemodialysis may contribute to the development of malignant cardiac arrhythmia. Although higher DBIC was shown to be associated with mortality and a trend toward more SCD (OR per 4 mEq/L higher DBIC, 1.03 [95% confidence interval, 0.88 to 1.19]) in the Dialysis Outcomes and Practice Patterns Study,9 this analysis used administrative codes and was unable to granularly define the presence or timing of an underlying arrhythmia in relation to hemodialysis sessions. Using MiD study data, we are able to address some of these issues, as well as adjust for the prehemodialysis SBIC concentrations in our models.
In this study, we describe the association of DBIC with CSA, RCA, and RCA subtypes, reviewer-confirmed atrial arrhythmia and reviewer-confirmed ventricular arrhythmia. Overall, we did not observe any meaningful association of DBIC >35 or <35 (versus 35 mEq/L) with CSA in either the logistic or negative binomial regression models. Regarding RCA, the observed effect estimates were on the side of a lower risk of RCA with DBIC >35 versus 35 and for DBIC <35 versus 35, which was mostly driven by a lower risk of reviewer-confirmed atrial arrhythmia. Conversely, the effect estimates were on the side of higher risk of reviewer-confirmed ventricular arrhythmia for DBIC >35 versus 35 and <35 versus 35. However, these estimates should be interpreted with caution, given the relative paucity of events in some models, wide confidence intervals, and inability to perform further multivariable adjustment. Ultimately, larger studies and trials are warranted to further assess for the potential associations of DBIC with arrhythmia.
In other MiD analyses of RCA in the 8 hours subsequent to respective hemodialysis sessions, Tumlin et al. reported that DBIC of >35 mEq/L was associated with fewer RCA events, compared with DBIC of 35 mEq/L (incidence rate ratio, 0.51 [0.27 to 0.97]).14 Our analysis adds clarity to the duration of this association in that it assessed the association in the full intradialytic and subsequent interdialytic interval (allowing for potential delayed events), and it also adjusted for SBIC. Soomro et al. assessed the association between DBIC and clinically significant bradycardia or asystole in the final 12 hours of the interdialytic interval and from the end of one dialysis session to the initiation of the next.19 Similar to our data, their study did not show evidence of an association of DBIC with clinically significant bradycardia or asystole during these time frames, but did not adjust for SBIC. Our study confirms these findings, even after adjustment for SBIC.
There are several mechanisms by which changing pH rapidly with higher DBIC could lead to arrhythmia. Alkalemia can promote intracellular shifts in serum potassium20 and lead to a change in the electrical charge of proteins, which promotes protein-to-calcium complexes and results in lower ionized calcium concentrations.10 Both lower serum calcium and potassium levels are associated with longer QTc durations,21 which may partially explain previous observations of QTc prolongation during hemodialysis with higher DBIC.22 However, other mechanisms for the association of higher DBIC with mortality warrant consideration.9 Metabolic alkalosis during hemodialysis may result in vasodilation and hemodynamic instability.23–25 Furthermore, metabolic alkalosis during hemodialysis may lead to decreased cerebral blood flow and respiratory suppression.26,27 High DBIC may lead to posthemodialysis alkalosis which promotes precipitation of calcium phosphate in the vasculature potentially contributing to vascular disease.28–30 Furthermore, higher DBIC is associated with mortality due to infections,9 possibly as alkalosis inhibits dendritic cell antigen-presenting capacity.31 Our findings demonstrate that mechanisms beyond arrhythmia for explaining the association of DBIC with mortality also warrant exploration.
This study has several notable strengths. First, it uses data from the MiD study, a multicenter prospective cohort with detailed session-level arrhythmia data as has rarely previously been obtained. Furthermore, our analysis includes the full intradialytic and interdialytic periods and accounts for SBIC, which to our knowledge has not previously been done using these data. A limitation of this study includes its assessment of a modest sample of patients. This precluded adjustment for or subgroup analysis by country of origin and thus country of origin remains a potential confounder. In addition, the inclusion/exclusion criteria of this cohort could limit generalizability. Furthermore, the modest sample size and concerns for overfitting precluded extensive adjustment for many potential confounders. Therefore, the possibility of residual confounding remains, and the results must be interpreted with caution. The added power that binomial regression, compared with logistic regression, gave by incorporating assessment of multiple episodes of an arrhythmia per session rather than simply asking if an episode occurred versus not in association with a given session may explain why significant results were found with binomial regression but not with the logistic regression model. However, multiple comparisons were examined, and the fact that our analyses did not correct for the multiple tests performed must be considered as a limitation. Another issue is that dialysate prescriptions reflect nephrologists' orders rather than the measured concentration of DBIC in dialysate baths. Furthermore, the inclusion and exclusion criteria in this study may have led to selection of healthier patients, which could limit the generalizability of the present findings to a sicker patient population.
In conclusion, this study suggests that, contrary to our original hypothesis, there was no consistent association of higher DBIC which is a higher risk of arrhythmia among participants of the MiD study. Owing to small numbers of events and multiple hypothesis testing, these results should be considered hypothesis generating. Larger studies and randomized controlled trials that assess variable DBIC and arrhythmia are required to fully understand how our management of acid-base in the maintenance hemodialysis patient population may affect a patient's risk of arrhythmia.
Supplementary Material
Acknowledgments
The authors would like to thank Ven Manda, John Burnes, and Amy Roettger from Medtronic for support and collaboration on the MiD study.
Footnotes
MiD Investigators and Committees: Nephrology Investigators—Don Williamson, MD (Southeastern Clinical Research Institute, Augusta, GA, Augusta, GA), Prabir Roy-Chaudhury, MD, (University of Cincinnati Medical Center Cincinnati, OH; now at the University of Arizona Tuscon, AZ), James Tumlin, MD (Nephronet Clinical Research Institute, Atlanta, GA), Vijay Kher, MD (Medanta - The Medicity- Kidney and Urology Institute, Gurgaon, India), Vikranth Reddy, MD (CARE Hospital Hyderabad, India), Kowdle Chandrasekhar Prakash, MD, (Apollo Hospitals–Chennai, Chennai, India), David Charytan, MD MSc (Brigham and Women's Hospital, Boston, MA; now at NYU Langone Medical Center, New York, NY), Suresh Chandra Tiwari, MD (Fortis Vasant Kunj Hospital Delhi, India), Saurabh Pokhariyal, MD (Fortis Memorial Research Institute Gurgaon, India), Amber Podoll, MD (University of Texas, Houston, Houston, TX), Sanjeev Jasuja, MD (Apollo Hospitals–Delhi, Delhi, India). Cardiology Investigators—G. Leslie Walters, MD (Augusta Cardiology Clinic, Augusta, GA), Kraig Wangsnes, MD (Cardiovascular Associates, Augusta, GA), Alexandru Costea, MD (University of Cincinnati Medical Center, Cincinnati, OH), Selcuk Tombul, MD (Diagnostic Cardiology Group, Chattanooga, TN), Balbir Singh, MD (Medanta - The Medicity- Heart Institute, Gurgaon, India), Brajesh Mishra, MD (Medanta - The Medicity- Heart Institute, Gurgaon, India), Sachin Yalagudri, MD (CARE Hospital, Hyderabad, India), Abhijeet Shelke, MD (CARE Hospital Hyderabad, India), Calambur Narasimhan, MD (CARE Hospital, Hyderabad, India), A.M. Karthigesan, MD (Apollo Hospitals–Chennai, Chennai, India), Abraham Oomman, MD (Apollo Hospitals–Chennai, Chennai, India), K P Pramod Kumar, MD (Apollo Hospitals–Chennai, Chennai, India), Bruce Koplan, MD (Brigham and Women's Hospital, Boston, MA), Upendra Kaul, MD (Fortis Vasant Kunj Hospital, Delhi, India), Tapan Ghose, MD (Fortis Vasant Kunj Hospital, Delhi, India), Ripen Gupta, MD (Fortis Vasant Kunj Hospital, Delhi, India), Arvind Sethi, MD (Fortis Escorts Hospital, Delhi, India), Nikhil Kumar, MD (Fortis Memorial Research Institute, Gurgaon, India), Ramesh Hariharan, MD, (University of Texas, Houston, Houston, TX), Rajnish Sardana, MD (Apollo Hospitals–Delhi, Delhi, India), Arif Wahab, MD (Apollo Hospitals–Delhi, Delhi, India) N.N Khanna, MD (Apollo Hospitals–Delhi, Delhi, India). Nephrology Co-investigators—Mark Smith, MD (Southeastern Clinical Research Institute, Augusta, GA), Suresh Kamath, MD (University of Cincinnati Medical Center, Cincinnati, OH), Claude Galphin, MD (South East Renal Research Institution (SERRI), Chattanooga, TN), Puneet Sodhi, MD (Medanta–The Medicity- Heart Institute, Gurgaon, India), Rajsekara Chakravarthy, MD (CARE Hospital, Hyderabad, India), Subba Rao Budithi, MD (Apollo Hospitals–Chennai, Chennai, India), Finnian Mc Causland, MBBCh, MMSc (Brigham and Women's Hospital, Boston, MA), Sanjeev Gulati, MD (Fortis Vasant Kunj Hospital, Delhi, India), Munawer Dijoo, MD (Fortis Vasant Kunj Hospital, Delhi, India), Upendra Singh, MD (Fortis Escorts Hospital, Delhi, India), Salil Jain, MD (Fortis Memorial Research Institute, Gurgaon, India), Vishal Saxena, MD (Fortis Memorial Research Institute, Gurgaon, India), Gaurav Sagar, MD (Apollo Hospitals, Delhi, India). Advisory Committee—David Charytan, MD, MSc, (Brigham and Women's Hospital, Boston, MA; now at NYU Langone Medical Center, New York, NY), Rachel Fissell, MD (Vanderbilt University, Nashville, TN), Robert Foley, MD (Hennepin County Medical Center, Minneapolis, MN), Charles A. Herzog, MD (Hennepin County Medical Center, University of Minnesota, Minneapolis, MN), Peter McCullough, MD (Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX), John D. Rogers, MD (Scripps Clinic-Torrey Pines, La Jolla, CA), James A. Tumlin, MD (South East Renal Research Institution (SERRI), Chattanooga, TN), Peter Zimetbaum, MD (Beth Israel Deaconess Medical Center, Boston, MA). Adverse Events Committee—Manish Assar, MD (Baylor University Medical Center, Dallas, TX), Mark Kremers, MD (Mid Carolina Cardiology Charlotte, NC), Wolfgang C. Winkelmayer, MD, ScD (Baylor College of Medicine, Houston, TX).
Contributor Information
Collaborators: Don Williamson, Prabir Roy-Chaudhury, James Tumlin, Vijay Kher, Vikranth Reddy, Kowdle Chandrasekhar Prakash, David Charytan, Suresh Chandra Tiwari, Saurabh Pokhariyal, Amber Podoll, Sanjeev Jasuja, G. Leslie Walters, Kraig Wangsnes, Alexandru Costea, Selcuk Tombul, Balbir Singh, Brajesh Mishra, Sachin Yalagudri, Abhijeet Shelke, Calambur Narasimhan, A.M. Karthigesan, Abraham Oomman, K.P. Pramod Kumar, Bruce Koplan, Upendra Kaul, Tapan Ghose, Ripen Gupta, Arvind Sethi, Nikhil Kumar, Ramesh Hariharan, Rajnish Sardana, Arif Wahab, N.N. Khanna, Mark Smith, Suresh Kamath, Claude Galphin, Puneet Sodhi, Rajsekara Chakravarthy, Subba Rao Budithi, Finnian McCausland, Sanjeev Gulati, Munawer Dijoo, Upendra Singh, Salil Jain, Vishal Saxena, Gaurav Sagar, David Charytan, Rachel Fissell, Robert Foley, Charles A. Herzog, Peter McCullough, John D. Rogers, James A. Tumlin, Peter Zimetbaum, Manish Assar, Mark Kremers, and Wolfgang C. Winkelmayer
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A623.
Funding
K.S. Ravi: Foundation for the National Institutes of Health (DK 127248) and Medtronic. V. Kher: Novartis India and Sanofi Aventis India. D.M. Charytan: Gilead Sciences, NovoNordisk, and Amgen. F.R. Mc Causland: National Institute of Diabetes and Digestive and Kidney Diseases, Satellite Healthcare, Fifth Eye, and Lexicon.
Author Contributions
Conceptualization: David M. Charytan, Finnian R. Mc Causland, Katherine Scovner Ravi.
Data curation: Alexandru I. Costea, Vijay Kher, Bruce A. Koplan, Prabir Roy-Chaudhury, James A. Tumlin, Don Williamson.
Formal analysis: David M. Charytan, Finnian R. Mc Causland, Candace K. McClure, Katherine Scovner Ravi.
Methodology: David M. Charytan, Finnian R. Mc Causland, Katherine Scovner Ravi.
Writing – original draft: David M. Charytan, Candace K. McClure, Katherine Scovner Ravi.
Writing – review & editing: Alexandru I. Costea, Vijay Kher, Bruce A. Koplan, Finnian R. Mc Causland, Prabir Roy-Chaudhury, James A. Tumlin, Don Williamson.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A622.
Supplemental Table 1. Characteristics of the participants at baseline according to country of origin.
Supplemental Table 2. Characteristics of the dialysis prescription at baseline according to country of origin.
Supplemental Table 3. Laboratory values by hemodialysis sessions.
Supplemental Table 4. Characteristics of the hemodialysis prescriptions by hemodialysis sessions.
References
- 1.United States Renal Data System. End-stage renal disease (ESRD) in the United States CI, incidence, prevalence, patient characteristics, and treatment modalities. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Health; 2020. [Google Scholar]
- 2.University of California San Francisco, The Kidney Project. Creating a bioartificial kidney as a permanent solution to kidney failure. Statistics. https://pharm.ucsf.edu/kidney/need/statistics.
- 3.Henry RMA Kostense PJ Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn Study. Kidney Int. 2002;62(4):1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x [DOI] [PubMed] [Google Scholar]
- 4.Makar MS, Pun PH. Sudden cardiac death among hemodialysis patients. Am J Kidney Dis. 2017;69(5):684–695. doi: 10.1053/j.ajkd.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein M, Roy-Chaudhury P. Arrhythmias and sudden cardiac death in hemodialysis patients. Temporal profile, electrolyte abnormalities, and potential targeted therapies. Nephrol News Issues 2016;30(4):suppl 23–26. PMID: 27254902 [PubMed] [Google Scholar]
- 6.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. New Engl J Med. 2011;365(12):1099–1107. doi: 10.1056/NEJMoa1103313 [DOI] [PubMed] [Google Scholar]
- 7.McGill RL, Weiner DE. Dialysate composition for hemodialysis: changes and changing risk. Semin Dial. 2017;30(2):112–120. doi: 10.1111/sdi.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramowitz MK. Bicarbonate balance and prescription in ESRD. J Am Soc Nephrol. 2017;28(3):726–734. doi: 10.1681/ASN.2016070780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tentori F Karaboyas A Robinson BM, et al. Association of dialysate bicarbonate concentration with mortality in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2013;62(4):738–746. doi: 10.1053/j.ajkd.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basile C, Rossi L, Lomonte C. The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int. 2016;89(5):1008–1015. doi: 10.1016/j.kint.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 11.Correa S Scovner KM Tumlin JA, et al.; MiD Investigators and Committees. Electrolyte changes in contemporary hemodialysis: a secondary analysis of the monitoring in dialysis (MiD) study. Kidney360. 2021;2(4):695–707. doi: 10.34067/KID.0007452020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charytan DM Foley R McCullough PA, et al.; MiD Investigators and Committees. Arrhythmia and sudden death in hemodialysis patients: protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol. 2016;11(4):721–734. doi: 10.2215/CJN.09350915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy-Chaudhury P Tumlin JA Koplan BA, et al.; MiD investigators and committees. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93(4):941–951. doi: 10.1016/j.kint.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 14.Tumlin JA Roy-Chaudhury P Koplan BA, et al.; MiD investigators and Committees. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol. 2019;20(1):80. doi: 10.1186/s12882-019-1212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi KS Espersen C Curtis KA, et al. Temporal changes in electrolytes, acid-base, QTc duration, and point-of-care ultrasound during inpatient hemodialysis sessions. Kidney360. 2022;3(7):1217–1227. doi: 10.34067/KID.0001652022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacher F Jesel L Borni-Duval C, et al. Cardiac rhythm disturbances in hemodialysis patients: early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol. 2018;4(3):397–408. doi: 10.1016/j.jacep.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 17.Zhang H Schaubel DE Kalbfleisch JD, et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int. 2012;81(11):1108–1115. doi: 10.1038/ki.2011.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69(12):2268–2273. doi: 10.1038/sj.ki.5000446 [DOI] [PubMed] [Google Scholar]
- 19.Soomro QH Bansal N Winkelmayer WC, et al.; MiD Investigators. Association of bradycardia and asystole episodes with dialytic parameters: an analysis of the monitoring in dialysis (MiD) study. Kidney360. 2022;3(11):1871–1880. doi: 10.34067/KID.0003142022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22(11):1981–1989. doi: 10.1681/ASN.2011040414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Akchar M, Siddique MS. Long QT Syndrome. StatPearls. StatPearls Publishing LLC.; 2020. [PubMed] [Google Scholar]
- 22.Beaubien ER, Pylypchuk GB, Akhtar J, Biem HJ. Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis. 2002;39(4):834–842. doi: 10.1053/ajkd.2002.32005 [DOI] [PubMed] [Google Scholar]
- 23.Gabutti L, Ferrari N, Giudici G, Mombelli G, Marone C. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrol Dial Transplant. 2003;18(11):2369–2376. doi: 10.1093/ndt/gfg383 [DOI] [PubMed] [Google Scholar]
- 24.Sam R, Vaseemuddin M, Leong WH, Rogers BE, Kjellstrand CM, Ing TS. Composition and clinical use of hemodialysates. Hemodial Int. 2006;10(1):15–28. doi: 10.1111/j.1542-4758.2006.01170.x [DOI] [PubMed] [Google Scholar]
- 25.Gabutti L, Ross V, Duchini F, Mombelli G, Marone C. Does bicarbonate transfer have relevant hemodynamic consequences in standard hemodialysis? Blood Purif. 2005;23(5):365–372. doi: 10.1159/000087193 [DOI] [PubMed] [Google Scholar]
- 26.Sethi D, Curtis JR, Topham DL, Gower PE. Acute metabolic alkalosis during haemodialysis. Nephron. 1989;51(1):119–120. doi: 10.1159/000185265 [DOI] [PubMed] [Google Scholar]
- 27.Kaye M, Somerville PJ, Lowe G, Ketis M, Schneider W. Hypocalcemic tetany and metabolic alkalosis in a dialysis patient: an unusual event. Am J Kidney Dis. 1997;30(3):440–444. doi: 10.1016/s0272-6386(97)90292-4 [DOI] [PubMed] [Google Scholar]
- 28.Harris DC, Yuill E, Chesher DW. Correcting acidosis in hemodialysis: effect on phosphate clearance and calcification risk. J Am Soc Nephrol. 1995;6(6):1607–1612. doi: 10.1681/ASN.V661607 [DOI] [PubMed] [Google Scholar]
- 29.Ibels LS. The pathogenesis of metastatic calcification in uraemia. Prog Biochem Pharmacol. 1980;17:242–250. PMID: 7208503 [PubMed] [Google Scholar]
- 30.Uribarri J. Moderate metabolic acidosis and its effects on nutritional parameters in hemodialysis patients. Clin Nephrol. 1997;48(4):238–240. PMID: 9352158 [PubMed] [Google Scholar]
- 31.Vermeulen M Giordano M Trevani AS, et al. Acidosis improves uptake of antigens and MHC class I-restricted presentation by dendritic cells. J Immunol. 2004;172(5):3196–3204. doi: 10.4049/jimmunol.172.5.3196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and/or supporting information.