Abstract
Like other multicellular organisms, the fruit fly Drosophila melanogaster must maintain homeostasis of the internal milieu, including the maintenance of constant ion and water concentrations. In mammals, the with no lysine (K) (WNK)-Ste20-proline/alanine rich kinase/oxidative stress response 1 kinase cascade is an important regulator of epithelial ion transport in the kidney. This pathway regulates SLC12 family cotransporters, including sodium-potassium-2-chloride, sodium chloride, and potassium chloride cotransporters. The WNK-Ste20-proline/alanine rich kinase/oxidative stress response 1 kinase cascade also regulates epithelial ion transport via regulation of the Drosophila sodium-potassium-2-chloride cotransporter in the Malpighian tubule, the renal epithelium of the fly. Studies in Drosophila have contributed to the understanding of multiple regulators of WNK pathway signaling, including intracellular chloride and potassium, the scaffold protein Mo25, hypertonic stress, hydrostatic pressure, and macromolecular crowding. These will be discussed together, with implications for mammalian kidney function and BP control.
Keywords: BP, cell and transport physiology, cell signaling, ion transport, kinase, tubular physiology
Introduction
The first with no lysine (K) (WNK) kinase, WNK1, was cloned in 2000 by Cobb and colleagues in a screen for new members of the mitogen-activated protein/extracellular signal-related protein kinase family.1 Even in this initial characterization, there was a clue linking WNKs and ions: exposing HEK293 cells to increased concentrations of extracellular sodium chloride activated WNK1 activity.1 A second important observation of this study was that a catalytic lysine, typically found in subdomain 2 of other serine-threonine kinases, was instead found in subdomain 1, prompting the name WNK.1 Although unexplained at the time, the authors presciently speculated that this unusual placement reflected a yet-to-be-discovered regulatory or functional adaptation.1
The following year, Lifton and colleagues published a seminal paper that excited the interest of the renal physiology and nephrology communities, showing that mutations in WNK1 and its paralog, WNK4, resulted in a syndrome of high BP and hyperkalemia in humans.2 This stimulated intensive effort into understanding the role of WNKs in the regulation of renal epithelial ion transport and their role in maintaining homeostasis of ions, water, and BP. This review will highlight the role of studies in the fly, Drosophila melanogaster, in contributing to this understanding.
The Drosophila Renal System
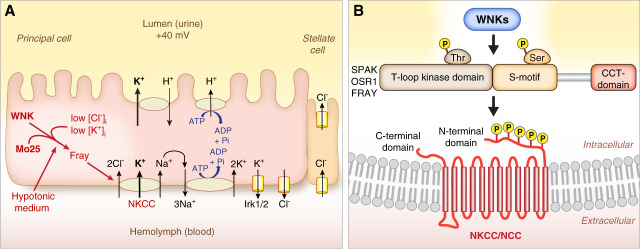
Like other multicellular organisms, Drosophila maintain homeostasis of the internal milieu and, therefore, require mechanisms to maintain constancy of ion and water concentrations in the face of varying intakes and losses of ions and water. The two central ionoregulatory and osmoregulatory epithelia are the Malpighian tubules (sometimes referred to as renal tubules) and the hindgut, which includes the ileum and rectal pads (Figure 1A).3 Although Drosophila have podocyte-like cells, called nephrocytes,4 these are anatomically separate from the tubules and hindgut. The Malpighian tubule is segmented, and urine generation occurs through the isosmotic secretion of a potassium chloride-rich fluid, which also contains sodium, by the main segment.5–8 The urine then moves through the lower segment and hindgut, where it is further modified (Figure 1B).9–11
Figure 1.
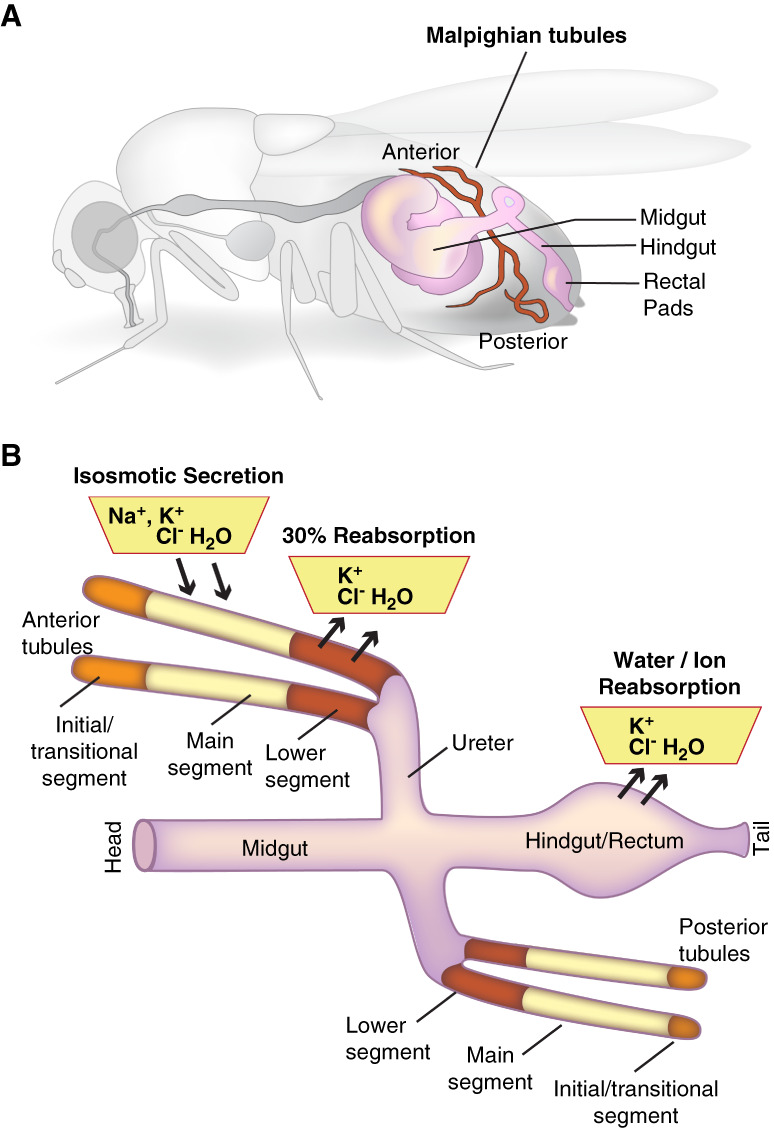
The renal system of Drosophila melanogaster. (A) The ionoregulatory and osmoregulatory epithelia are found in the abdomen of the fly. They consist of the four Malpighian tubules, an anterior pair and a posterior pair, and the hindgut, which includes the ileum and the distal rectal pads. (B) Ions, water, and nutrients consumed by the fly are absorbed through the midgut epithelium into the hemolymph, the plasma of the fly. The primary urine is generated through isosmotic secretion of fluid rich in potassium and chloride and also containing sodium. Secretion occurs in the main segment of the tubule. The pro-urine then flows through the downstream lower segment, where absorption of potassium, chloride, and water occurs, and then through the ureter. Further modification occurs during transit through the hindgut ileum and rectal pads, where additional ion and water reabsorption occurs. Reproduced from Rodan.12 Cl-, chloride; H2O, water; K+, potassium; Na+, sodium.
A simplified cell model of the fluid-secreting Malpighian tubule main segment is shown in Figure 2A (a more complete model is discussed in previous work).12 Transepithelial fluid secretion and ion fluxes can be measured in dissected Malpighian tubules ex vivo.13 The principal cells are cation-secreting, secreting potassium or sodium, and the stellate cells are chloride-secreting.14–16 Aquaporins are present in both principal and stellate cells, with transepithelial water secretion occurring primarily through the stellate cells.17 The apical vacuolar proton ATPase, homologous to the vacuolar proton ATPase found in mammalian collecting duct intercalated cells, energizes secretion.5,18–21 A secretory sodium-potassium-2-chloride cotransporter (NKCC) contributes to transepithelial potassium and fluid secretion by the principal cell.8 A basal sodium-potassium ATPase lowers intracellular sodium to allow ongoing sodium, potassium, and chloride uptake by the NKCC.8
Figure 2.
Role of WNK-SPAK/OSR1/Fray signaling in epithelial ion transport. (A) Cell model of the fluid-secreting main segment of the Malpighian tubule. The principal cell secretes cations (potassium and sodium). The stellate cell secretes chloride and water, via aquaporins (not shown). Ion transport is powered by the apical V-ATPases, which secrete protons into the lumen to generate a lumen-positive charge of approximately 40 mV. This drives exchange of protons for cations. A basolateral NKCC allows uptake of sodium, potassium, and chloride from the hemolymph (plasma). The basal sodium-potassium ATPase lowers intracellular sodium to provide the driving force for ion uptake through the NKCC. Thus, loss of the NKCC decreases transepithelial potassium secretion by approximately 30%–50% (depending on the condition) without affecting sodium secretion, whereas the sodium-potassium ATPase inhibitor, ouabain, increases transepithelial sodium secretion.8,23 The WNK-Fray kinase cascade positively regulates (stimulates) transepithelial potassium secretion via regulation of the NKCC. Regulators of WNK signaling include intracellular chloride, intracellular potassium, and the scaffold protein Mo25. Low intracellular chloride, low intracellular potassium, and Mo25 stimulate the pathway. (B) Mammalian and Drosophila WNK kinases phosphorylate the downstream Ste20 kinases, SPAK, OSR1, and Fray, on a conserved T-loop threonine. WNK phosphorylation of SPAK/OSR1/Fray on the T-loop threonine is required for activation. A second phosphorylation event occurs on a conserved serine in the S-motif. The CCT domain of SPAK/OSR1/Fray physically interacts with RFxV/I motifs in WNKs and in substrate proteins, including the NKCCs and NCC. SPAK/OSR1/Fray phosphorylate conserved serine and threonine residues in the N-terminal domains of NKCCs and NCC to activate the transporters. (A) Reproduced from Sun et al.24 (B) Reproduced from Goldsmith and Rodan.25 CCT, conserved C-terminal domain; Cl-, chloride; Fray, Frayed; H+, proton; NCC, sodium chloride cotransporter; NKCC, sodium-potassium-2-chloride cotransporter; OSR1, oxidative stress response 1; Pi, phosphate; Ser, serine; SPAK, Ste20-proline/alanine rich kinase; Thr, threonine; V-ATPase, vacuolar proton ATPase; WNK, With No Lysine (K).
WNK Regulation of SLC12 Cotransporters
The SLC12 cotransporter family contains the NKCCs, the sodium chloride cotransporter (NCC) in the distal convoluted tubule of the mammalian kidney, and the potassium chloride cotransporters (KCCs). The ion transport activity of all of these transporters is regulated by Ste20-proline/alanine rich kinase (SPAK) and oxidative stress response 1 (OSR1) kinase, two paralogous kinases.22 Phosphorylation of NKCCs and NCCs by SPAK or OSR1 increases ion transport activity.22 SPAK and OSR1 themselves are activated by WNKs via phosphorylation of a conserved threonine in the T-loop of SPAK and OSR1 (Figure 2B).22 SPAK/OSR1/Fray can interact via their CCT domain (Figure 2B) with RFxV/I motifs in WNKs and in substrates, such as the SLC12 cotransporters.26 Interestingly, the Drosophila WNK RFxV motif is dispensable for WNK function in development and in Malpighian tubule ion transport.27 This suggests additional modes of interaction between WNKs and SPAK/OSR1/Fray.
Mammals have four WNKs, WNKs 1–4, of which WNK4 is predominant in the distal convoluted tubule.28–30 WNK4, acting primarily through SPAK in the distal convoluted tubule (though OSR1 also plays a role, particularly in the absence of SPAK), regulates sodium chloride reabsorption through NCC.28–34 The WNK-SPAK/OSR1 kinase cascade also regulates sodium chloride reabsorption through NKCC2 in the thick ascending limb, although its role in the thick ascending limb is not as prominent as in the distal convoluted tubule.34–40 There is a single ancestral WNK homolog in Drosophila, Drosophila WNK, which phosphorylates and activates the SPAK/OSR1 homolog, Fray.41–43 Fray in turn phosphorylates and activates the Drosophila NKCC.23,44 The WNK-Fray kinase cascade regulates transepithelial potassium and fluid secretion in the Drosophila Malpighian tubule principal cell via regulation of the NKCC (Figure 2A).23 Thus, WNK-SPAK/OSR1 regulation of NCC/NKCC transporters is conserved in renal epithelia from flies to mammals.
Intracellular Chloride Regulates WNK Kinases
Studies in the 1990s in a variety of cell types showed that lowering of intracellular chloride increases NKCC activity.45 After discovery of the WNK-SPAK/OSR1 pathway, experiments in Xenopus oocytes and cultured cells showed that conditions that promote lowering of intracellular chloride, such as bathing cells in hypotonic low chloride medium or lowering extracellular potassium, increased WNK activity and NCC/NKCC phosphorylation.28,46–50 The molecular mechanism of chloride regulation of WNK kinases was solved by Goldsmith and colleagues, who demonstrated direct binding of chloride to the active site of WNKs using x-ray crystallography (Figure 3).51 This also explains the mysterious displacement of the catalytic lysine (lysine 233), which would otherwise interfere with chloride binding (Figure 3). Chloride binding inhibits WNK autosphosphorylation, which is required for activation. Thus, chloride directly inhibits WNK kinases.
Figure 3.
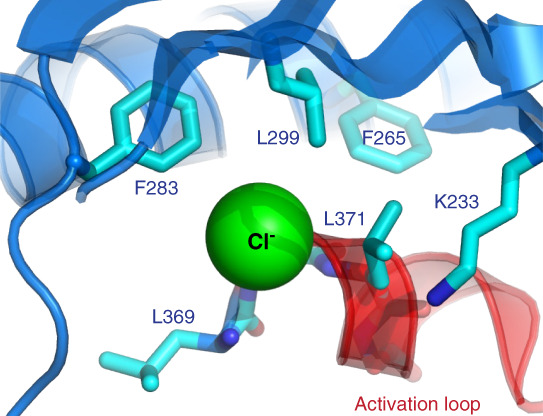
Chloride binds directly to WNK kinases. Chloride binds to the active site of WNK kinases and inhibits WNK autophosphorylation and activation. Mutation of WNK1 leucine 369 (or the homologous leucine in other WNKs) to phenylalanine decreases the inhibitory effects of chloride on WNK1 activation. The atypical lysine, lysine 233, is also shown. Its placement in subdomain 1, rather than subdomain 2, allows for chloride binding. Reproduced from Goldsmith and Rodan.25
Terker et al. proposed that lowering of extracellular potassium decreases intracellular chloride, relieving chloride inhibition of WNK4-SPAK signaling in the distal convoluted tubule to increase NCC phosphorylation and sodium chloride reabsorption.28 This could contribute to the hypertensive effect of the modern low potassium, high sodium diet.28 Studies in the Malpighian tubule allowed assessment of changes in intracellular chloride and the effects on both WNK activity and transepithelial ion transport. Consistent with other studies in mammalian cells,28,48,52–55 bathing the tubule in low potassium medium increased tubule DmWNK activity, whereas high potassium inhibited activity.24 The Malpighian tubule has basolateral potassium and chloride conductances56 (Figure 2A), so low extracellular potassium could lower intracellular chloride by hyperpolarizing the membrane potential, as also proposed for the distal convoluted tubule.28,57–59 To further investigate the effects of lowering intracellular chloride, tubules were bathed in hypotonic medium, which resulted in a drop in intracellular chloride from approximately 30 mM to 16 mM, as measured using a transgenic chloride sensor.24 Endogenous tubule DmWNK activity increased, and transepithelial ion and fluid flux increased in a WNK-, Fray-, and NKCC-dependent manner.23,24 Thus, these studies in the Malpighian tubule directly correlated changes in intracellular chloride with WNK activity and ion flux in a transporting renal epithelium.
A subsequent study in Drosophila demonstrated that intracellular chloride also regulates WNK signaling in central pacemaker neurons to regulate circadian period.60 Chloride rises in the pacemaker neurons over the course of the morning in an NKCC-dependent manner, restraining WNK-Fray signaling and the activity of an inwardly rectifying potassium channel.60 This could link WNK-Fray signaling to circadian oscillations in membrane potential.61
Intracellular Potassium Regulation of WNK Kinases
The first clues into the existence of a chloride-regulated kinase came from studies of NKCC phosphorylation and activation in diverse cell types, including the squid giant axon, the shark rectal gland, salivary gland epithelial cells, and tracheal epithelial cells.62–66 Notably, many of these are chloride-secreting epithelia, leading to the chloride-coupling hypothesis62 in which apical chloride secretion lowers intracellular chloride. This in turn relieves inhibition of a chloride-regulated kinase (which later studies identified as WNKs), NKCC phosphorylation, and basolateral chloride uptake by the NKCC.
The Drosophila principal cell secretes potassium (Figure 2A), raising the question of whether WNKs could also be regulated by potassium via potassium-coupling. Indeed, potassium, like chloride, inhibited autophosphorylation of both Drosophila and mammalian WNKs in vitro, as well as WNK3 and WNK4 phosphorylation of SPAK. Importantly, the greatest effects of potassium on WNK inhibition were observed within the physiological range of intracellular potassium, that is potassium concentrations of 100–140 mM. Bathing Malpighian tubules expressing Drosophila WNK or mammalian WNK3 or WNK4 (in place of Drosophila WNK) in high potassium baths inhibited WNK phosphorylation of tubule-expressed SPAK. This was not due to an effect on intracellular chloride because extracellular chloride was manipulated to keep measured intracellular chloride constant. Ouabain, an inhibitor of the sodium-potassium ATPase expected to lower intracellular potassium, activated tubule Drosophila WNK activity. Low potassium bath also stimulated WNK4 expressed in place of Drosophila WNK. The inhibitory effects of the high potassium bath were observed in tubules with both lower and higher intracellular chloride concentrations and in tubules expressing WNK3 and WNK4 with the leucine-to-phenylalanine mutations that render WNKs resistant to the inhibitory effects of chloride.67 These results indicated that potassium has direct inhibitory effects on WNK kinases that are independent of the effects of chloride.
Studies in rats fed low or high potassium diets have demonstrated changes in renal epithelial cell intracellular potassium.68–71 On the basis of these measurements and the relationships between chloride, potassium, and WNK activity,67,72 changes in intracellular potassium in the distal convoluted tubule could have similar effects on WNK4 activity as changes in intracellular chloride (discussed further in a previous work).25 However, additional study is needed to understand the potassium binding site(s) in WNKs and the physiological role of potassium regulation of WNK kinases.
Role of the Scaffold Protein Mo25
Mutation of the SPAK/OSR1/Fray T-loop threonine phosphorylated by WNKs (Figure 2B) to a negatively charged glutamate, mimicking phosphorylation, increases kinase activity relative to the wild-type kinases.23,73 Thus, the threonine-to-glutamate mutation mimics phosphorylation and activation by the upstream WNK kinases.74 Alessi and colleagues showed that the scaffolding protein Mo25 (mouse protein 25, also known as calcium-binding protein 39/Cab39) further stimulated the kinase activity of SPAK and OSR1 with these T-loop mutations.75 These data suggested that WNKs and Mo25 cooperatively increase activity of SPAK and OSR1, which increased subsequent phosphorylation of NKCC1, NKCC2, and NCC in vitro. Mo25 was also required for NKCC1 phosphorylation and activation in HEK293 cells.75
Mo25 is expressed in the thick ascending limb and distal convoluted tubule, with maximal signal observed apically/subapically in these nephron segments.31 This raised the question of the role of Mo25 in renal epithelial ion transport. As with the mammalian proteins, Drosophila Mo25 potently stimulated the activity of Fray with a phospho-mimicking T-loop mutation, FrayT206E, increasing phosphorylation of NKCC in vitro. Knockdown of Drosophila Mo25 in the Malpighian tubule did not affect ion transport in isotonic conditions, but prevented the increase in ion transport observed in hypotonic conditions, when intracellular chloride decreases. Drosophila Mo25 overexpression did not increase ion transport when overexpressed on its own or together with wild-type Drosophila WNK.24 Chloride has decreased inhibitory effects on WNK1 in which leucine 369 in the chloride-binding pocket (Figure 3) is mutated to phenylalanine.51 This region is perfectly conserved in Drosophila WNK, and leucine 421 is homologous to WNK1 leucine 369. Overexpression of Drosophila WNKL421F also did not increase ion transport in the Malpighian tubule. However, co-overexpression of Drosophila WNKL421F together with Mo25 increased ion transport, whereas co-overexpression of FrayT206E and Mo25 did not.24 These results suggest that maximal ion transport in the tubule requires both lowering of intracellular chloride to activate WNK and Mo25 (Figure 2A).
A recent study by Delpire and colleagues demonstrated that knockout of the two partially redundant mammalian Mo25 paralogs in the distal convoluted tubule strongly decreased NCC phosphorylation in mice fed a normal or low potassium diet, resulting in hypocalciuria and hypokalemia,76 as also seen in patients with Gitelman syndromem in whom NCC is mutated.77 Interestingly, SPAK phosphorylation was not decreased in the Mo25 knockout mice, although substantially higher levels of WNK4 were required to maintain SPAK phosphorylation. However, although SPAK was primarily localized to the apical/subapical membrane in the distal convoluted tubule of control mice, both phosphorylated and total SPAK were trapped in cytoplasmic puncta in the knockout mice.76 These puncta are reminiscent of WNK bodies, which are observed under hypokalemic conditions in which the WNK-SPAK pathway is activated to increase NCC phosphorylation.28,31,35,78–81 Like WNK bodies, the SPAK-containing puncta in the Mo25 knockout mice also contain WNK1, most likely the truncated kidney-specific isoform that is required for WNK body formation.79 These results suggest a role for Mo25 in recruiting SPAK from WNK bodies to the apical membrane to phosphorylate NCC. How this occurs requires future study.
WNK Activation by Hypertonic Stress
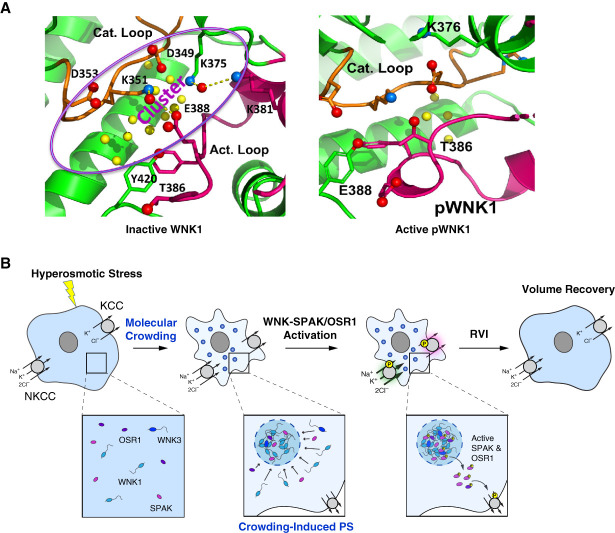
In the first article describing WNK kinases, it was observed that WNK1 activity is stimulated by hypertonic stress.1 Since then, two mechanisms have been proposed for WNK activation by hypertonic stress. Inactive, unphosphorylated WNK is found in an asymmetric dimer that is stabilized by chloride.25,51,82 The serine residue in the activation loop which is autophosphorylated to allow WNK activation is trapped in the dimeric interface. In vitro demands on solvent induced by polyethylene glycol 400 or ethylene glycol shift the equilibrium toward the autophosphorylation-competent monomer.83 Interestingly, inactive WNK (for example, WNK in which the autophosphorylation site serine is mutated to alanine) contains large amounts of bound water between the catalytic and activation loop in an area defined by a cluster of evolutionarily conserved charged amino acids. The active phosphorylated WNK contains fewer bound waters (Figure 4A).83 Thus, bound water may be part of the osmosensing mechanism. WNKs are also activated by hydrostatic pressure (including WNK3 expressed in the Drosophila Malpighian tubule), which, in principle, would favor conformations with less bound water, as seen in phosphorylated WNK monomers. WNK activation was observed at pressures of 80–190 kPa or 600–1425 mm Hg.84 Whether WNK activation can occur at more physiological levels of hydrostatic pressure remains to be determined.
Figure 4.
Mechanisms for hypertonic stress activation of WNK kinases. (A) An inactive isoform of WNK1 (left), in which the activation loop autophosphorylated serine (serine 382 in WNK1) is mutated to alanine, contains many bound water molecules (yellow balls) amid a cluster of charged amino acids (D, aspartate; K, lysine; E, glutamate). Inactive WNK1 exists as an asymmetric dimer in which the activation loop serine is buried in the dimer interface. The dimer is stabilized by chloride, whereas osmotic and hydrostatic pressure favors the phosphorylated monomer. There are notably fewer bound water molecules in phosphorylated WNK1 (right). (B) Cell shrinkage in response to hypertonic stress results in macromolecular crowding and Drosophila and mammalian WNK phase separation (PS) into biomolecular condensates, which contain WNKs, SPAK, and OSR1. After phosphorylation and activation, SPAK and OSR1 leave the condensates to phosphorylate NKCC (activating) and KCC (inhibitory), resulting in net ion and water influx and cell volume recovery (RVI, regulatory volume increase). (A) Reproduced from Akellaet al.83 (B) Reproduced from Boyd-Shiwarski et al.85 KCC, potassium chloride cotransporter; pWNK1, phosphorylated with no lysine (K) kinase 1; WNK1, with no lysine (K) kinase 1.
A second mechanism for WNK activation in the face of extracellular hypertonic stress is the formation of WNK-containing biomolecular condensates (Figure 4B). These form through a process called liquid-liquid phase separation in response to the crowding of macromolecules that occurs during cell shrinkage when extracellular tonicity is increased. SPAK and OSR1 are also recruited into WNK condensates and are phosphorylated. Activated SPAK and OSR1 then leave the condensates through unknown mechanisms to phosphorylate NKCC1 and activate the transporter. Simultaneous phosphorylation of KCC decreases its activity, leading to net ion and water influx and restoration of cell volume. Drosophila WNK also undergoes phase separation in response to cellular hypertonic stress. Phase separation of both mammalian and Drosophila WNK is mediated by segments of their large C-terminal domains. Although these have low sequence identity (22%), they share high disorder tendency and the presence of prion-like domains.85 Whether condensate formation influences the bound waters in the WNK kinase domain is an interesting question that requires further study.
To date, hypertonic activation of WNK kinases has been studied in vitro and in cultured cells on short timescales, consistent with the rapid timescales of cell volume responses to osmotic stress. For example, the formation of WNK condensates via liquid-liquid phase separation occurs within seconds, and biochemical activation of WNK kinase activity after hypertonic stress is typically studied within 30–60 minutes of stress application.85–89 Additional study is required to determine whether WNK condensate formation occurs in vivo in the kidney in response to hypertonic stress.
Why So Many WNKs?
Mammals have four WNK paralogs, WNKs 1–4. WNKs have roles in multiple cell types and tissues, as reviewed elsewhere.15,90–93 WNK1, WNK2, and WNK3 genetically buffer one another in a human cell line, that is, loss of WNK2 or WNK3 was detrimental to cell viability when WNK1 had been knocked out, and loss of WNK1 was detrimental to cell viability when WNK2 and WNK3 were knocked out.94 WNK3 protein was also increased in HEK293T cultured cells in which WNK1 was knocked out, although this was not sufficient to restore SPAK and OSR1 phosphorylation to wild-type levels. This could be due to nonequivalence of WNK1 and WNK3 or perhaps in part because WNK2 and WNK4 protein levels were also decreased in WNK1 knockout cells.89
There is evidence that different WNK paralogs differ in their regulation by chloride, potassium, and hypertonic stress. For example, when WNK3 and WNK4 were expressed in Xenopus laevis oocytes, WNK3 was activated by hypertonic stress, whereas WNK4 was activated by bathing oocytes in hypotonic low chloride medium, which lowers intracellular chloride.88 WNK4 is inhibited by lower concentrations of chloride in vitro compared with WNK1 or WNK3,72 which may make it more sensitive to changes in intracellular chloride concentrations in the physiological range in epithelial cells of the distal convoluted tubule. This may explain why WNK4 knockout in mice results in a salt-wasting tubulopathy that is not compensated by increased expression of WNK1 in the kidney.29,30 WNK3 and WNK4 have differential responses to intracellular potassium, with WNK4 expressed in the Malpighian tubule demonstrating more activation in response to low potassium compared with WNK3 and differential effects of potassium on in vitro WNK3 versus WNK4 activity.67
Effects on BP
Thiazide-type diuretics, which inhibit NCC, are a cornerstone of antihypertensive therapy. NCC knockout mice are resistant to the hypertensive effect of the high sodium, low potassium diet, which activates WNK4-SPAK signaling.28 Increased WNK signaling generally increases BP, whereas loss of WNK4 or SPAK can decrease BP (further discussed in additional works).95,96
Of the mechanisms discussed above, the role of chloride regulation of WNKs has been most clearly linked to BP. Terker et al. initially proposed that low extracellular potassium could lower intracellular chloride and increase WNK4 activity to increase NCC phosphorylation and sodium chloride reabsorption in the distal convoluted tubule.28 Further evidence in favor of this idea came from studies in which the leucine-to-phenylalanine mutation, which decreases chloride inhibition of WNKs (discussed above), was introduced into mouse WNK4. These knock-in mice were hypertensive.97
BP was not measured in the Mo25 double knockout mice, but given the profound decreases in total and phosphorylated NCC in these mice, loss of Mo25 would be expected to have an antihypertensive effect.76
Whether WNK regulation by potassium, hypertonic stress or macromolecular crowding influences BP is so far unknown. Potassium regulates WNK4,67 but whether this has implications for BP requires further study. Studies on hypertonic stress, macromolecular crowding, and hydrostatic pressure regulation of WNKs have thus far focused on WNK1 and WNK3.83–85 WNK3 knockouts have no differences in BP on normal diet and a modestly lower BP on low salt diet.98,99 However, this may be due to extrarenal loss of WNK3 because WNK3 mRNA was not detected in microdissected segments of the mouse nephron.100 Interestingly, though, renal WNK1 and SPAK/OSR1 abundance was increased in both the thick ascending limb and distal convoluted tubule of WNK3 knockout mice.99 Mutations that increase WNK1 increase BP, and one study demonstrated a decrease in BP in WNK1 heterozygous knockout mice, although this could be due to vascular effects.101–104 Effects of WNK1 knockout in the mammalian kidney tubular epithelium have not been reported. It will be interesting to examine whether the activating effects of hypertonic stress, macromolecular crowding, and hydrostatic pressure are seen with WNK4 and whether WNK1 regulation by these stressors affects BP or other aspects of kidney function.
Conclusion
WNK-SPAK/OSR1/Fray kinase regulation of the SLC12 family of sodium transporters, which includes NKCC1, NKCC2, and NCC, is evolutionarily conserved in renal epithelia from Drosophila to humans. The ease of genetic manipulation in the fly, its short life cycle and physiological accessibility, and the availability of Drosophila cell culture models have allowed detailed examination of the regulation of WNKs by chloride, potassium, the scaffold protein Mo25/Cab39, hypertonic stress, macromolecular crowding, and hydrostatic pressure. Some of these mechanisms (chloride, Mo25) have proven important for regulation of WNK4 in the distal convoluted tubule, whereas the roles of other mechanisms (potassium, hypertonic stress, macromolecular crowding and hydrostatic pressure) in mammalian kidney function remain to be explored. The tiny but mighty fruit fly is likely to continue to play a role in understanding these fascinating kinases.
Acknowledgments
The author thanks Diana Lim for assistance with figure preparation.
Disclosures
Disclosure forms, as provided by each author, are available with the online version of the article at http://links.lww.com/KN9/A658.
Funding
A.R. Rodan: National Institute of Diabetes and Digestive and Kidney Diseases (DK110358 and DK098145).
Author Contributions
Conceptualization: Aylin R. Rodan.
Funding acquisition: Aylin R. Rodan.
Visualization: Aylin R. Rodan.
Writing – original draft: Aylin R. Rodan.
Writing – review & editing: Aylin R. Rodan.
References
- 1.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275(22):16795–16801. doi: 10.1074/jbc.275.22.16795 [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH Disse-Nicodème S Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. doi: 10.1126/science.1062844 [DOI] [PubMed] [Google Scholar]
- 3.Cohen E, Sawyer JK, Peterson NG, Dow JAT, Fox DT. Physiology, development, and disease modeling in the Drosophila excretory system. Genetics. 2020;214(2):235–264. doi: 10.1534/genetics.119.302289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koehler S, Huber TB. Insights into human kidney function from the study of Drosophila. Pediatr Nephrol. 2023;38(12):3875–3887. doi: 10.1007/s00467-023-05996-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol. 1994;197:421–428. doi: 10.1242/jeb.197.1.421 [DOI] [PubMed] [Google Scholar]
- 6.Linton SM, O’Donnell MJ. Contributions of K+:Cl- cotransport and Na+/K+-ATPase to basolateral ion transport in malpighian tubules of Drosophila melanogaster. J Exp Biol. 1999;202(Pt 11):1561–1570. doi: 10.1242/jeb.202.11.1561 [DOI] [PubMed] [Google Scholar]
- 7.Rheault MR, O’Donnell MJ. Analysis of epithelial K(+) transport in Malpighian tubules of Drosophila melanogaster: evidence for spatial and temporal heterogeneity. J Exp Biol. 2001;204(Pt 13):2289–2299. doi: 10.1242/jeb.204.13.2289 [DOI] [PubMed] [Google Scholar]
- 8.Rodan AR, Baum M, Huang C-L. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am J Physiol Cell Physiol. 2012;303(8):C883–C894. doi: 10.1152/ajpcell.00201.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen EH Deaton LE Onken H, et al. Osmoregulation and excretion. Compr Physiol. 2014;4(2):405–573. doi: 10.1002/cphy.c130004 [DOI] [PubMed] [Google Scholar]
- 10.Luan Z, Quigley C, Li H-S. The putative Na+/Cl--dependent neurotransmitter/osmolyte transporter inebriated in the Drosophila hindgut is essential for the maintenance of systemic water homeostasis. Sci Rep. 2015;5:7993. doi: 10.1038/srep07993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yerushalmi GY, Misyura L, MacMillan HA, Donini A. Functional plasticity of the gut and the Malpighian tubules underlies cold acclimation and mitigates cold-induced hyperkalemia inDrosophila melanogaster. J Exp Biol. 2018;221(Pt 6):jeb174904. doi: 10.1242/jeb.174904 [DOI] [PubMed] [Google Scholar]
- 12.Rodan AR. The Drosophila Malpighian tubule as a model for mammalian tubule function. Curr Opin Nephrol Hypertens. 2019;28(5):455–464. doi: 10.1097/MNH.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellinger JN, Rodan AR. Use of the ramsay assay to measure fluid secretion and ion flux rates in the Drosophila melanogaster malpighian tubule. J Vis Exp. 2015(105):53144. doi: 10.3791/53144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrero P, Terhzaz S, Romero MF, Davies SA, Blumenthal EM, Dow JAT. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc Natl Acad Sci U S A. 2014;111(39):14301–14306. doi: 10.1073/pnas.1412706111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell MJ, Dow JA, Huesmann GR, Tublitz NJ, Maddrell SH. Separate control of anion and cation transport in malpighian tubules of Drosophila Melanogaster. J Exp Biol. 1996;199(Pt 5):1163–1175. doi: 10.1242/jeb.199.5.1163 [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell MJ Rheault MR Davies SA, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol. 1998;274(4):R1039–R1049. doi: 10.1152/ajpregu.1998.274.4.R1039 [DOI] [PubMed] [Google Scholar]
- 17.Cabrero P Terhzaz S Dornan AJ, et al. Specialized stellate cells offer a privileged route for rapid water flux in Drosophila renal tubule. Proc Natl Acad Sci U S A. 2020;117(3):1779–1787. doi: 10.1073/pnas.1915943117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du J Kean L Allan AK, et al. The SzA mutations of the B subunit of the Drosophila vacuolar H+ ATPase identify conserved residues essential for function in fly and yeast. J Cell Sci. 2006;119(Pt 12):2542–2551. doi: 10.1242/jcs.02983 [DOI] [PubMed] [Google Scholar]
- 19.Allan AK, Du J, Davies SA, Dow JAT. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics. 2005;22(2):128–138. doi: 10.1152/physiolgenomics.00233.2004 [DOI] [PubMed] [Google Scholar]
- 20.Davies SA Goodwin SF Kelly DC, et al. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem. 1996;271(48):30677–30684. doi: 10.1074/jbc.271.48.30677 [DOI] [PubMed] [Google Scholar]
- 21.Dow JA, Davies SA, Guo Y, Graham S, Finbow ME, Kaiser K. Molecular genetic analysis of V-ATPase function in Drosophila melanogaster. J Exp Biol. 1997;200(Pt 2):237–245. doi: 10.1242/jeb.200.2.237 [DOI] [PubMed] [Google Scholar]
- 22.Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014;7(334):re3. doi: 10.1126/scisignal.2005365 [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Schellinger JN, Huang C-L, Rodan AR. Hypotonicity stimulates potassium flux through the WNK-SPAK/OSR1 kinase cascade and the Ncc69 sodium-potassium-2-chloride cotransporter in the Drosophila renal tubule. J Biol Chem. 2014;289(38):26131–26142. doi: 10.1074/jbc.M114.577767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q Wu Y Jonusaite S, et al. Intracellular chloride and scaffold protein Mo25 cooperatively regulate transepithelial ion transport through WNK signaling in the malpighian tubule. J Am Soc Nephrol. 2018;29(5):1449–1461. doi: 10.1681/ASN.2017101091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldsmith EJ, Rodan AR. Intracellular ion control of WNK signaling. Annu Rev Physiol. 2023;85:383–406. doi: 10.1146/annurev-physiol-031522-080651 [DOI] [PubMed] [Google Scholar]
- 26.Taylor CA, Cobb MH. CCT and CCT-like modular protein interaction domains in WNK signaling. Mol Pharmacol. 2022;101(4):201–212. doi: 10.1124/molpharm.121.000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarikipati P Jonusaite S Pleinis JM, et al. Unanticipated domain requirements for Drosophila Wnk kinase in vivo. PLoS Genet. 2023;19(10):e1010975. doi: 10.1371/journal.pgen.1010975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terker AS Zhang C McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21(1):39–50. doi: 10.1016/j.cmet.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castañeda-Bueno M Cervantes-Pérez LG Vázquez N, et al. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci U S A. 2012;109(20):7929–7934. doi: 10.1073/pnas.1200947109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi D Mori T Nomura N, et al. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep. 2014;34(3):e00107. doi: 10.1042/BSR20140047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm PR Taneja TK Liu J, et al. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem. 2012;287(45):37673–37690. doi: 10.1074/jbc.M112.402800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S-S Lo Y-F Wu C-C, et al. SPAK-knockout mice manifest gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21(11):1868–1877. doi: 10.1681/ASN.2009121295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferdaus MZ Barber KW López-Cayuqueo KI, et al. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol. 2016;594(17):4945–4966. doi: 10.1113/JP272311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiqi FH Zuber AM Glover M, et al. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2(2):63–75. doi: 10.1002/emmm.200900058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick JA Mutig K Nelson JH, et al. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab. 2011;14(3):352–364. doi: 10.1016/j.cmet.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin S-H Yu I-S Jiang S-T, et al. Impaired phosphorylation of Na+-K+-2Cl- cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A. 2011;108(42):17538–17543. doi: 10.1073/pnas.1107452108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terker AS Castañeda-Bueno M Ferdaus MZ, et al. With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am J Physiol Renal Physiol. 2018;315(4):F781–F790. doi: 10.1152/ajprenal.00485.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng C-J, Yoon J, Baum M, Huang C-L. STE20/SPS1-related proline/alanine-rich kinase (SPAK) is critical for sodium reabsorption in isolated, perfused thick ascending limb. Am J Physiol Renal Physiol. 2015;308(5):F437–F443. doi: 10.1152/ajprenal.00493.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferdaus MZ Miller LN Agbor LN, et al. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight. 2017;2(24):e96700. doi: 10.1172/jci.insight.96700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeoka Y Nguyen LT Sharma A, et al. Dysregulation of the WNK4-SPAK/OSR1 pathway has a minor effect on baseline NKCC2 phosphorylation. Am J Physiol Renal Physiol. 2024;326(1):F39–F56. doi: 10.1152/ajprenal.00100.2023 [DOI] [PubMed] [Google Scholar]
- 41.Sato A, Shibuya H. WNK signaling is involved in neural development via Lhx8/Awh expression. PLoS One. 2013;8(1):e55301. doi: 10.1371/journal.pone.0055301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serysheva E Berhane H Grumolato L, et al. Wnk kinases are positive regulators of canonical Wnt/β-catenin signalling. EMBO Rep. 2013;14(8):718–725. doi: 10.1038/embor.2013.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leiserson WM, Harkins EW, Keshishian H. Fray, a Drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheathment. Neuron. 2000;28(3):793–806. doi: 10.1016/s0896-6273(00)00154-9 [DOI] [PubMed] [Google Scholar]
- 44.Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011;59(2):320–332. doi: 10.1002/glia.21103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haas M, III, Forbush B, III. BF: the Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515 [DOI] [PubMed] [Google Scholar]
- 46.Richardson C Rafiqi FH Karlsson HKR, et al. Activation of the thiazide-sensitive Na+-Cl– cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–684. doi: 10.1242/jcs.025312 [DOI] [PubMed] [Google Scholar]
- 47.Richardson C Sakamoto K de los Heros P, et al. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci. 2011;124(Pt 5):789–800. doi: 10.1242/jcs.077230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naito S Ohta A Sohara E, et al. Regulation of WNK1 kinase by extracellular potassium. Clin Exp Nephrol. 2011;15(2):195–202. doi: 10.1007/s10157-010-0378-9 [DOI] [PubMed] [Google Scholar]
- 49.Moriguchi T Urushiyama S Hisamoto N, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280(52):42685–42693. doi: 10.1074/jbc.M510042200 [DOI] [PubMed] [Google Scholar]
- 50.Ponce-Coria J San-Cristobal P Kahle KT, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A. 2008;105(24):8458–8463. doi: 10.1073/pnas.0802966105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014;7(324):ra41. doi: 10.1126/scisignal.2005050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy M, O’Shaughnessy KM. Modified HEK cells simulate DCT cells in their sensitivity and response to changes in extracellular K. Physiol Rep. 2019;7(22):e14280. doi: 10.14814/phy2.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penton D Czogalla J Wengi A, et al. Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl−‐dependent and independent mechanisms. J Physiol. 2016;594(21):6319–6331. doi: 10.1113/JP272504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee A, Yang C-L, McCormick JA, Martz K, Sharma A, Ellison DH. Roles of WNK4 and SPAK in K+-mediated dephosphorylation of the NaCl cotransporter. Am J Physiol Renal Physiol. 2021;320(5):F719–F733. doi: 10.1152/ajprenal.00459.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murali SK Little R Poulsen SB, et al. Potassium effects on NCC are attenuated during inhibition of cullin E3–ubiquitin ligases. Cells. 2021;11(1):95. doi: 10.3390/cells11010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ianowski JP, O’Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl- cotransport and Cl- conductance. J Exp Biol. 2004;207(Pt 15):2599–2609. doi: 10.1242/jeb.01058 [DOI] [PubMed] [Google Scholar]
- 57.Wang M-X Cuevas CA Su X-T, et al. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int. 2018;93(4):893–902. doi: 10.1016/j.kint.2017.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuevas CA Su X-T Wang M-X, et al. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol. 2017;28(6):1814–1825. doi: 10.1681/ASN.2016090935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C Wang L Zhang J, et al. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci U S A. 2014;111(32):11864–11869. doi: 10.1073/pnas.1411705111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schellinger JN Sun Q Pleinis JM, et al. Chloride oscillation in pacemaker neurons regulates circadian rhythms through a chloride-sensing WNK kinase signaling cascade. Curr Biol. 2022;32(6):1429–1438.e6. doi: 10.1016/j.cub.2022.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodan AR. Circadian rhythm regulation by pacemaker neuron chloride oscillation in flies. Physiology. 2024;39(3):0. doi: 10.1152/physiol.00006.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lytle C, Forbush B. The Na-K-Cl cotransport protein of shark rectal gland. II. Regulation by direct phosphorylation. J Biol Chem. 1992;267(35):25438–25443. doi: 10.1016/s0021-9258(19)74060-5 [DOI] [PubMed] [Google Scholar]
- 63.Breitwieser GE, Altamirano AA, Russell JM. Elevated [Cl-]i, and [Na+]i inhibit Na+, K+, Cl- cotransport by different mechanisms in squid giant axons. J Gen Physiol. 1996;107(2):261–270. doi: 10.1085/jgp.107.2.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foskett JK. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol. 1990;259(6 Pt 1):C998–C1004. doi: 10.1152/ajpcell.1990.259.6.C998 [DOI] [PubMed] [Google Scholar]
- 65.Haas M, McBrayer D, Lytle C. [Cl-]i-dependent phosphorylation of the Na-K-Cl cotransport protein of dog tracheal epithelial cells. J Biol Chem. 1995;270(48):28955–28961. doi: 10.1074/jbc.270.48.28955 [DOI] [PubMed] [Google Scholar]
- 66.Lytle C, Forbush B. Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am J Physiol. 1996;270(2 Pt 1):C437–C448. doi: 10.1152/ajpcell.1996.270.2.C437 [DOI] [PubMed] [Google Scholar]
- 67.Pleinis JM Norrell L Akella R, et al. WNKs are potassium-sensitive kinases. Am J Physiol Cell Physiol. 2021;320(5):C703–C721. doi: 10.1152/ajpcell.00456.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cemerikić D, Wilcox CS, Giebisch G. Intracellular potential and K+ activity in rat kidney proximal tubular cells in acidosis and K+ depletion. J Membr Biol. 1982;69(2):159–165. doi: 10.1007/BF01872275 [DOI] [PubMed] [Google Scholar]
- 69.Khuri RN, Agulian SK, Kalloghlian A. Intracellular potassium in cells of the distal tubule. Pflugers Archiv: Eur J Physiol. 1972;335(4):297–308. doi: 10.1007/BF00586220 [DOI] [PubMed] [Google Scholar]
- 70.Beck F, Dörge A, Mason J, Rick R, Thurau K. Element concentrations of renal and hepatic cells under potassium depletion. Kidney Int. 1982;22(3):250–256. doi: 10.1038/ki.1982.162 [DOI] [PubMed] [Google Scholar]
- 71.Beck F-X, Dörge A, Rick R, Schramm M, Thurau K. Effect of potassium adaptation on the distribution of potassium, sodium and chloride across the apical membrane of renal tubular cells. Pflugers Archiv: Eur J Physiol. 1987;409(4-5):477–485. doi: 10.1007/BF00583804 [DOI] [PubMed] [Google Scholar]
- 72.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang C-L, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89(1):127–134. doi: 10.1038/ki.2015.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vitari AC Thastrup J Rafiqi FH, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397(1):223–231. doi: 10.1042/BJ20060220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DMF. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–1711. doi: 10.1126/science.1178377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filippi BM de los Heros P Mehellou Y, et al. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J. 2011;30(9):1730–1741. doi: 10.1038/emboj.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferdaus MZ, Koumangoye R, Welling PA, Delpire E. Kinase scaffold Cab39 is necessary for phospho-activation of the thiazide-sensitive NCC. Hypertension. 2024;81(4):801–810. doi: 10.1161/HYPERTENSIONAHA.123.22464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blanchard A Bockenhauer D Bolignano D, et al. Gitelman syndrome: consensus and guidance from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2017;91(1):24–33. doi: 10.1016/j.kint.2016.09.046 [DOI] [PubMed] [Google Scholar]
- 78.Schumacher F Siew K Zhang J, et al. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med. 2015;7(10):1285–1306. doi: 10.15252/emmm.201505444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyd-Shiwarski CR Shiwarski DJ Roy A, et al. Potassium-regulated distal tubule WNK bodies are kidney-specific WNK1 dependent. Mol Biol Cell. 2018;29(4):499–509. doi: 10.1091/mbc.E17-08-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomson MN Cuevas CA Bewarder TM, et al. WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia. Am J Physiol Renal Physiol. 2020;318(1):F216–F228. doi: 10.1152/ajprenal.00232.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bahena-Lopez JP Vergara L de la-Peña V, et al. KS-WNK1 is required for the renal response to extreme changes in potassium intake. Am J Physiol Renal Physiol. 2024;326(3):F460–F476. doi: 10.1152/ajprenal.00235.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Min X, Lee B-H, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure. 2004;12(7):1303–1311. doi: 10.1016/j.str.2004.04.014 [DOI] [PubMed] [Google Scholar]
- 83.Akella R Humphreys JM Sekulski K, et al. Osmosensing by WNK kinases. Mol Biol Cell. 2021;32(18):1614–1623. doi: 10.1091/mbc.E20-01-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Humphreys JM Teixeira LR Akella R, et al. Hydrostatic pressure sensing by WNK kinases. Mol Biol Cell. 2023;34(11):ar109. doi: 10.1091/mbc.E23-03-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boyd-Shiwarski CR Shiwarski DJ Griffiths SE, et al. WNK kinases sense molecular crowding and rescue cell volume via phase separation. Cell. 2022;185(24):4488–4506.e20. doi: 10.1016/j.cell.2022.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengupta S Tu S-W Wedin K, et al. Interactions with WNK (with No lysine) family members regulate oxidative stress response 1 and ion Co-transporter activity. J Biol Chem. 2012;287(45):37868–37879. doi: 10.1074/jbc.M112.398750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zagórska A Pozo-Guisado E Boudeau J, et al. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol. 2007;176(1):89–100. doi: 10.1083/jcb.200605093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pacheco-Alvarez D Carrillo-Pérez DL Mercado A, et al. WNK3 and WNK4 exhibit opposite sensitivity with respect to cell volume and intracellular chloride concentration. Am J Physiol Cell Physiol. 2020;319(2):C371–C380. doi: 10.1152/ajpcell.00488.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roy A Goodman JH Begum G, et al. Generation of WNK1 knockout cell lines by CRISPR/Cas-mediated genome editing. Am J Physiol Renal Physiol. 2015;308(4):F366–F376. doi: 10.1152/ajprenal.00612.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab. 2017;25(2):285–299. doi: 10.1016/j.cmet.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 91.Rodan AR, Jenny A. Current topics in developmental biology. In: Jenny A, ed. Protein Kinases in Development and Disease; 2017:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gallolu Kankanamalage S, Karra AS, Cobb MH. WNK pathways in cancer signaling networks. Cell Commun Signal. 2018;16(1):72. doi: 10.1186/s12964-018-0287-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murillo-de-Ozores AR, Chávez-Canales M, de Los Heros P, Gamba G, Castañeda-Bueno M. Physiological processes modulated by the chloride-sensitive WNK-SPAK/OSR1 kinase signaling pathway and the cation-coupled chloride cotransporters. Front Physiol. 2020;11:585907. doi: 10.3389/fphys.2020.585907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao Y-X Lee SY Aguilera-Uribe M, et al. The TSC22D, WNK, and NRBP gene families exhibit functional buffering and evolved with Metazoa for cell volume regulation. Cell Rep. 2024;43(7):114417. doi: 10.1016/j.celrep.2024.114417 [DOI] [PubMed] [Google Scholar]
- 95.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431 [DOI] [PubMed] [Google Scholar]
- 96.Omage K, McCormick JA. Cullin3/WNK/SPAK signaling: impact on NaCl cotransporter activity in blood pressure regulation. Kidney360. 2024. doi: 10.34067/KID.0000000000000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J-C, Lo Y-F, Lin Y-W, Lin S-H, Huang C-L, Cheng C-J. WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci U S A. 2019;116(10):4502–4507. doi: 10.1073/pnas.1817220116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oi K Sohara E Rai T, et al. A minor role of WNK3 in regulating phosphorylation of renal NKCC2 and NCC co-transporters in vivo. Biol Open. 2012;1(2):120–127. doi: 10.1242/bio.2011048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mederle K Mutig K Paliege A, et al. Loss of WNK3 is compensated for by the WNK1/SPAK axis in the kidney of the mouse. Am J Physiol Renal Physiol. 2013;304(9):F1198–F1209. doi: 10.1152/ajprenal.00288.2012 [DOI] [PubMed] [Google Scholar]
- 100.Chen L, Chou C-L, Knepper MA. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol. 2021;32(4):897–912. doi: 10.1681/ASN.2020101406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vidal-Petiot E Elvira-Matelot E Mutig K, et al. WNK1-related Familial Hyperkalemic Hypertension results from an increased expression of L-WNK1 specifically in the distal nephron. Proc Natl Acad Sci U S A. 2013;110(35):14366–14371. doi: 10.1073/pnas.1304230110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chávez-Canales M Zhang C Soukaseum C, et al. WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension. 2014;64(5):1047–1053. doi: 10.1161/HYPERTENSIONAHA.114.04036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zambrowicz BP Abuin A Ramirez-Solis R, et al. Wnk1 kinase deficiency lowers blood pressure in mice: a gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci U S A. 2003;100(24):14109–14114. doi: 10.1073/pnas.2336103100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Susa K Kita S Iwamoto T, et al. Effect of heterozygous deletion of WNK1 on the WNK-OSR1/SPAK-NCC/NKCC1/NKCC2 signal cascade in the kidney and blood vessels. Clin Exp Nephrol. 2012;16(4):530–538. doi: 10.1007/s10157-012-0590-x [DOI] [PubMed] [Google Scholar]