Abstract
Locally advanced cervical carcinoma (LACC) remains a significant global health challenge owing to its high recurrence rates and poor outcomes, despite current treatments. This study aimed to develop a comprehensive risk stratification model for LACC by integrating Cox regression and competing risk analyses. This was done to improve clinical decision making. We analyzed data from 3428 patients with LACC registered in the Surveillance, Epidemiology, and End Results program and diagnosed them between 2010 and 2015. Cox regression and competing risk analyses were used to identify the prognostic factors. We constructed and validated nomograms for overall survival (OS) and disease-specific survival (DSS). Multivariate Cox regression identified key prognostic factors for OS, including advanced International Federation of Gynecology and Obstetrics stage, age, marital status, ethnicity, and tumor size. Notably, International Federation of Gynecology and Obstetrics stages IIIA, IIIB, and IVA had hazard ratios of 2.227, 2.451, and 4.852, respectively, significantly increasing the mortality risk compared to stage IB2. Ethnic disparities were evident, with African Americans facing a 39.8% higher risk than Caucasians did. Competing risk analyses confirmed the significance of these factors in DSS, particularly tumor size. Our nomogram demonstrated high predictive accuracy, with area under the curve values ranging from 0.706 to 0.784 for DSS and 0.717 to 0.781 for OS. Calibration plots and decision curve analyses further validated the clinical utility of this nomogram. We present effective nomograms for LACC risk stratification that incorporate multiple prognostic factors. These models provide a refined approach for individualized patient management and have the potential to significantly enhance therapeutic strategies for LACC.
Keywords: competing risk analysis, Cox regression, locally advanced cervical carcinoma, nomogram, prognostic factors, SEER registry
1. Introduction
According to the 2020 Global Cancer Observatory statistics, cervical carcinoma remains a significant global health challenge with approximately 604,127 new cases and 341,831 deaths worldwide.[1] Locally advanced cervical carcinoma (LACC), classified as International Federation of Gynecology and Obstetrics (FIGO) stage IB-IVA, remains a critical concern.[2–5] Despite advancements in early detection and prevention, many patients are still being diagnosed with LACC.
Concurrent chemoradiotherapy (CCRT) is the standard treatment for LACC; CCRT, it often results in suboptimal outcomes, including high recurrence rates and severe side effects.[3–7] The complexity of treatment is further compounded by prognostic factors such as age, ethnicity, marital status, tumor size, FIGO staging, and histopathological evaluations.[8–11] Therefore, effective management of LACC is a multifaceted challenge.
Our study adopted a dual approach, employing Cox regression and competing risk analyses. Cox regression was chosen for its flexibility and effectiveness in identifying key survival factors in LACC. Competing risk analyses offer insights into the clinical outcomes.[12–18]
Our goal was to integrate these methodologies to create a comprehensive and nuanced risk stratification model that significantly enhances clinical decision making in LACC. Utilizing data from the Surveillance, Epidemiology, and End Results (SEER) registry, we aimed to identify independent prognostic factors and validate our predictive models, addressing the pressing need for more effective LACC treatment strategies.
2. Materials and methods
2.1. Ethical review
Our analysis utilized data released from an online publicly available SEER database. This study was exempt from local research ethics committee approval, considering that SEER data were de-identified and publicly available for research use.
2.2. Datasets and patients
We analyzed the incidence and outcomes of LACC from 2010 to 2015 using data from the SEER Research Plus Data 18 registry. We adhered to the 7th edition of the American Joint Committee on Cancer (AJCC7) Cancer Staging System, focusing on patients age ≥18 years who met FIGO 2009 guidelines, with confirmed diagnoses of either squamous cell carcinoma or adenocarcinoma (AC) as their primary malignancy.[19–22] Patients with distant metastases or those with incomplete data were excluded from the study. The study period was selected based on the available and specific variables. Ethical approval was not required owing to the de-identified nature of the SEER database, and the Helsinki Declaration guidelines were followed.
Patient demographics included race, age, marital status, histological grade, TNM stage, pathological subtypes, treatment modalities, survival time, vital status, and the cause of death. TNM staging was reassessed per FIGO 2009 standards, and AJCC7 cervical cancer grading guidelines were strictly followed. Treatment options included radiation monotherapy, combined chemoradiotherapy, and surgery, with the main radiation methods being external beam radiation, brachytherapy, or both. Patients with 0 or unknown survival times were excluded.
2.3. Outcomes
The primary outcome was overall survival (OS), defined as the time from LACC diagnosis to death from any cause. The secondary outcome was disease-specific survival (DSS), defined as the period from diagnosis to death due to LACC. Deaths from other causes were considered competing risk factors.
2.4. Incident analysis of LACC from 2010 to 2015
The incidence rate, adjusted for age using the 2000 US Standard Population, was calculated as the number of cases per million annually between 2010 and 2015.[23,24] The annual percentage changes (APC) were determined using weighted least squares analysis.
2.5. Nomogram construction and validation
The SEER dataset of 3428 patients was divided into a training cohort (n = 2399) and validation cohort (n = 1029) in a 7:3 ratio. Prognostic and competing risk analyses were conducted in the training cohort and were validated in the validation cohort.
2.6. Cox regression analysis and nomogram for OS
We used both univariate and multivariate Cox regression analyses to identify independent prognostic factors, focusing on those with P-values <0.05 in univariate analysis. We then developed nomograms to predict 1-, 3-, 5-, and 7-year survival risks by assigning unique points to each variable. X-tile software (version 3.6.1) was used to classify the patients into low-, medium-, or high-risk categories.[25,26]
2.7. Competing risk analysis and nomogram for DSS
Competing risks were evaluated using the cumulative incidence function and Fine-Gray competing risk regression, employing the “cmprsk” R package.[27] We focused on deaths directly caused by LACC.
2.8. Nomogram validation for OS and DSS
Model predictions were evaluated using calibration curves and decision curve analysis (DCA) in both cohorts. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive accuracy.
2.9. Online predictive tools for OS and DSS
Digital tools for OS and DSS assessments in LACC patients were developed using the “DynNom” and “Shiny” R packages and the Shiny website (https://www.shinyapps.io/).
2.10. Statistical analysis
Baseline categorical variables were presented as frequencies and percentages, and continuous variables were presented as mean ± standard deviation or interquartile range. The “surv_cutpoint” function in R (version 4.3.1)’s “survminer” package was applied to determine optimal cutoffs for age and tumor size. Categorical data comparisons were performed using Pearson chi-square test or Fisher exact test. Kaplan–Meier survival estimation and log-rank tests were used to assess OS and DSS. Cox regression and competing risk analyses were used to evaluate risk factors, with statistical significance set at P < .05.
3. Results
3.1. Incident and patient baseline characteristics
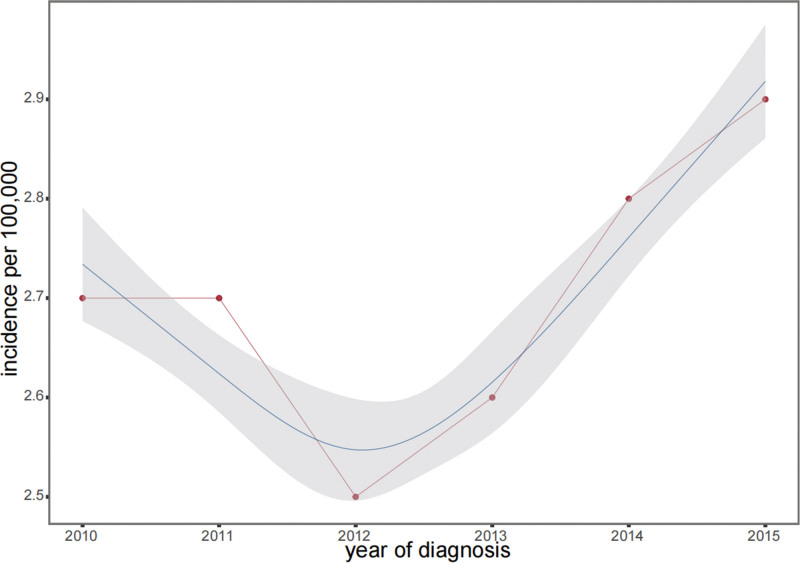
From 2010 to 2015, the incidence of LACC displayed a U-shaped trend (Fig. 1), initially decreasing and then increasing, with a significant APC (P < .05). Among the 3428 participants, the majority were White (73.0%), followed by Black (14.6%), and other races (12.3%), with no significant racial differences between the training and validation groups (P = .263). The median age was 49 years (IQR: 40–60), with similar age distributions in both the groups (P = .326). Surgery was not performed in 59.8% of patients, which was consistent across both groups (P = .849). Treatment modalities varied but showed no significant group differences (P > .2). The median tumor size was 51 mm (IQR: 40–68 mm), and the survival outcomes were comparable between the groups (P > .1). Detailed demographics are presented in Table 1.
Figure 1.
Trends in the annual age-adjusted incidence of LACC from 2010 to 2015. The vertical axis represents the number of cases per 100,000 individuals, while the horizontal axis shows the year of diagnosis. Data points are marked in red, depicting a “U-shaped” pattern over the 6-year timeframe. The incidence rate per 100,000 was 0.27 in 2010, dropped to 0.25 in 2012, and then steadily increased to 0.29 by 2015. The gray area in the graph indicates the confidence interval for the incidence rates, providing a visual representation of the range of data fluctuations. LACC = locally advanced cervical cancer.
Table 1.
Baseline demographic and clinical characteristics of patients with LACC.
| level | Overall | Training | Validation | P | |
|---|---|---|---|---|---|
| n | 3428 | 2399 | 1029 | ||
| Race (%) | White | 2504 (73.046) | 1733 (72.238) | 771 (74.927) | .263 |
| Black | 501 (14.615) | 360 (15.006) | 141 (13.703) | ||
| Other | 423 (12.340) | 306 (12.755) | 117 (11.370) | ||
| Age (median [IQR]) | 49.000 [40.000, 60.000] | 49.000 [40.000, 60.000] | 48.000 [39.000, 59.000] | .326 | |
| Marital status (%) | Married | 1414 (41.249) | 980 (40.850) | 434 (42.177) | .493 |
| Single | 2014 (58.751) | 1419 (59.150) | 595 (57.823) | ||
| Histology (%) | SCC | 2782 (81.155) | 1942 (80.950) | 840 (81.633) | .674 |
| AC | 646 (18.845) | 457 (19.050) | 189 (18.367) | ||
| Grade (%) | I | 236 (6.884) | 178 (7.420) | 58 (5.637) | .238 |
| II | 1546 (45.099) | 1082 (45.102) | 464 (45.092) | ||
| III | 1573 (45.887) | 1086 (45.269) | 487 (47.328) | ||
| IV | 73 (2.130) | 53 (2.209) | 20 (1.944) | ||
| FIGO (%) | IB2 | 505 (14.732) | 337 (14.048) | 168 (16.327) | .516 |
| IIA1 | 120 (3.501) | 83 (3.460) | 37 (3.596) | ||
| IIA2 | 188 (5.484) | 138 (5.752) | 50 (4.859) | ||
| IIB | 671 (19.574) | 477 (19.883) | 194 (18.853) | ||
| IIIA | 67 (1.954) | 51 (2.126) | 16 (1.555) | ||
| IIIB | 1743 (50.846) | 1219 (50.813) | 524 (50.923) | ||
| IVA | 134 (3.909) | 94 (3.918) | 40 (3.887) | ||
| Sequence (%) | No surgery | 2051 (59.831) | 1446 (60.275) | 605 (58.795) | .849 |
| RAS | 1224 (35.706) | 847 (35.306) | 377 (36.638) | ||
| RPTS | 131 (3.821) | 90 (3.752) | 41 (3.984) | ||
| RBAS | 22 (0.642) | 16 (0.667) | 6 (0.583) | ||
| Surgery (%) | Not recommended | 1894 (55.251) | 1326 (55.273) | 568 (55.199) | .222 |
| SP | 1486 (43.349) | 1034 (43.101) | 452 (43.926) | ||
| RBNP | 48 (1.400) | 39 (1.626) | 9 (0.875) | ||
| Radiation (%) | None | 338 (9.860) | 236 (9.837) | 102 (9.913) | .961 |
| Beam radiation | 1375 (40.111) | 964 (40.183) | 411 (39.942) | ||
| brachytherapy | 268 (7.818) | 191 (7.962) | 77 (7.483) | ||
| CBB | 1447 (42.211) | 1008 (42.018) | 439 (42.663) | ||
| Chemotherapy (%) | No | 604 (17.620) | 430 (17.924) | 174 (16.910) | .506 |
| Yes | 2824 (82.380) | 1969 (82.076) | 855 (83.090) | ||
| Tumor size (median [IQR]) | 51.000 [40.000, 68.000] | 50.000 [40.000, 68.000] | 55.000 [40.000, 70.000] | .127 | |
| Cause of death (%) | Alive | 2110 (61.552) | 1457 (60.734) | 653 (63.460) | .173 |
| Cervix Uteri | 992 (28.938) | 701 (29.221) | 291 (28.280) | ||
| other | 326 (9.510) | 241 (10.046) | 85 (8.260) | ||
| Time (median [IQR]) | 45.500 [21.000, 69.000] | 46.000 [21.000, 69.000] | 45.000 [21.000, 71.000] | .822 | |
| Death (%) | Alive | 2110 (61.552) | 1457 (60.734) | 653 (63.460) | .143 |
| Dead | 1318 (38.448) | 942 (39.266) | 376 (36.540) |
AC = adenocarcinoma; CI = confidence interval; CBB = combined beam with brachytherapy; FIGO = International Federation of Gynecology and Obstetrics; Grade I = well-differentiated; Grade II = moderately differentiated; Grade III = poorly differentiated; Grade IV = undifferentiated; HR = hazard ratio; LACC = locally advanced cervical carcinoma; P = P-value; RAS = radiation after surgery; RBAS = radiation before and after surgery; RBNP = recommended but not performed; RPTS = radiation prior to surgery; SCC = squamous cell carcinoma; SP = surgery performed.
3.2. Cutoff values for age and tumor size
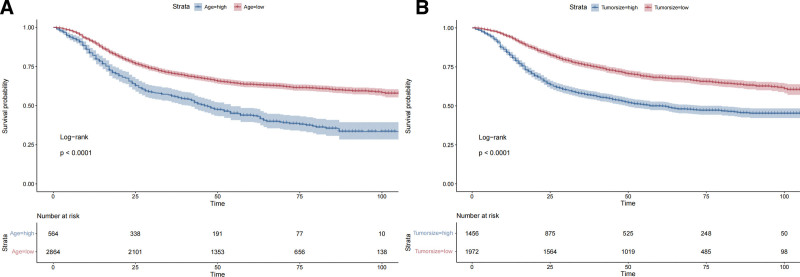
Age and tumor size cutoff values were established at 64 mm and 58 mm, respectively (Fig. 2). Kaplan–Meier curves using these thresholds showed significant differences (P < .0001).
Figure 2.
Optimal cutoff values for age and tumor size in LACC patients. (A) The survival curve for age and (B) for tumor size. Both curves demonstrate statistically significant differences, with P-values <.0001. Survival probability decreases over time, with lower survival rates observed in the older age group and the larger tumor size group. The “Strata” labels indicate different risk groups, with blue representing the low-risk group (younger age/smaller tumor) and red indicating the high-risk group (older age/larger tumor). The table below each point in time displays the number of patients at risk at that time. LACC = locally advanced cervical carcinoma.
3.3. Cox regression analysis for OS
The multivariate analysis identified several significant prognostic factors. Age, advanced FIGO stage, histological grade, marital status, ethnicity, and treatment modality significantly influenced survival risks (Table 2). Notably, advanced stages and poor histological grades contributed to the risk, whereas treatment modes such as beam radiation and brachytherapy reduced this risk. Each unit increase in tumor size also increased risk (hazard ratio (HR) = 1.004, P < .001).
Table 2.
Univariate and multivariate Cox proportional hazards analyses of factors affecting OS in LACC patients.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.017 (1.012–1.021) | <0.001 | 1.015 (1.01–1.019) | <.001 |
| Chemotherapy | ||||
| Yes vs no | 0.867 (0.737–1.02) | 0.085 | NA | NA |
| FIGO | ||||
| IIA1 vs IB2 | 1.186 (0.748–1.883) | 0.468 | 1.35 (0.843–2.161) | .211 |
| IIA2 vs IB2 | 1.456 (1.015–2.087) | 0.041 | 1.432 (0.994–2.063) | .054 |
| IIB vs IB2 | 1.297 (0.991–1.698) | 0.058 | 1.36 (1.025–1.804) | .033 |
| IIIA vs IB2 | 2.592 (1.657–4.054) | <0.001 | 2.173 (1.378–3.425) | .001 |
| IIIB vs IB2 | 2.099 (1.666–2.644) | <0.001 | 2.475 (1.941–3.155) | <.001 |
| IVA vs IB2 | 5.395 (3.923–7.421) | <0.001 | 4.552 (3.276–6.325) | <.001 |
| Grade | ||||
| II vs I | 1.245 (0.939–1.651) | 0.128 | 1.166 (0.875–1.555) | .295 |
| III vs I | 1.523 (1.152–2.014) | 0.003 | 1.4 (1.053–1.863) | .021 |
| IV vs I | 2.126 (1.362–3.317) | 0.001 | 2.087 (1.332–3.27) | .001 |
| Histology | ||||
| AC vs SCC | 0.82 (0.693–0.97) | 0.02 | 1.014 (0.849–1.212) | .874 |
| Marital status | ||||
| Single vs married | 1.257 (1.101–1.434) | 0.001 | 1.175 (1.026–1.346) | .02 |
| Race | ||||
| Black vs White | 1.499 (1.272–1.766) | <0.001 | 1.398 (1.179–1.657) | <.001 |
| Other vs White | 0.956 (0.78–1.172) | 0.666 | 0.972 (0.791–1.194) | .786 |
| Radiation | ||||
| Beam radiation vs none | 0.799 (0.649–0.984) | 0.035 | 0.554 (0.421–0.728) | <.001 |
| Brachytherapy vs none | 0.694 (0.517–0.933) | 0.015 | 0.433 (0.306–0.611) | <.001 |
| CBB vs none | 0.606 (0.491–0.749) | <0.001 | 0.392 (0.297–0.519) | <.001 |
| Sequence | ||||
| RAS vs no surgery | 0.504 (0.434–0.584) | <0.001 | 0.953 (0.74–1.228) | .711 |
| RPTS vs no surgery | 0.699 (0.49–0.997) | 0.048 | 1.605 (1.053–2.448) | .028 |
| RBAS vs no surgery | 1.549 (0.853–2.811) | 0.15 | 3.093 (1.623–5.894) | .001 |
| Surgery | ||||
| SP vs not recommended | 0.542 (0.473–0.621) | <0.001 | 0.528 (0.41–0.679) | <.001 |
| RBNP vs not recommended | 0.946 (0.592–1.512) | 0.817 | 0.808 (0.504–1.296) | .377 |
| Tumor size | 1.004 (1.003–1.005) | <0.001 | 1.004 (1.003–1.005) | <.001 |
AC = adenocarcinoma; CI = confidence interval; CBB = combined beam with brachytherapy; HR = hazard ratio; RAS = radiation after surgery; LACC = locally advanced cervical carcinoma; OS = overall survival; RBAS = radiation before and after surgery; RBNP = recommended but not performed; RPTS = radiation prior to surgery; SCC = squamous cell carcinoma; SP = surgery performed.
3.4. Competing risk analysis for DSS
Similar patterns were observed in DSS analysis, with advanced FIGO stages and racial disparities significantly impacting the risk. Radiation treatment notably reduced the risk, and tumor size remained an independent prognostic factor (Table 3).
Table 3.
Univariate and multivariate competing risks analyses of DSS in patients with LACC.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.001 (0.996–1.007) | .690 | – | – |
| Chemotherapy | ||||
| Yes vs no | 1.01 (0.829–1.232) | .920 | – | – |
| FIGO | ||||
| IIA1 vs IB2 | 1.071 (0.613–1.869) | .810 | 1.32 (0.742–2.349) | .340 |
| IIA2 vs IB2 | 1.442 (0.943–2.206) | .091 | 1.457 (0.941–2.258) | .092 |
| IIB vs IB2 | 1.188 (0.86–1.642) | .300 | 1.252 (0.884–1.772) | .210 |
| IIIA vs IB2 | 2.484 (1.445–4.27) | .001 | 2.227 (1.245–3.984) | .007 |
| IIIB vs IB2 | 2.226 (1.694–2.925) | <.001 | 2.451 (1.829–3.283) | <.001 |
| IVA vs IB2 | 5.382 (3.669–7.895) | <.001 | 4.852 (3.256–7.23) | <.001 |
| Grade | ||||
| II vs I | 1.092 (0.799–1.491) | .580 | 1.019 (0.729–1.424) | .910 |
| III vs I | 1.374 (1.01–1.87) | .043 | 1.261 (0.905–1.758) | .170 |
| IV vs I | 1.793 (1.1–2.923) | .019 | 1.705 (1.039–2.798) | .035 |
| Histology | ||||
| AC vs SCC | 0.768 (0.632–0.934) | .008 | 0.931 (0.75–1.157) | .520 |
| Marital status | ||||
| Single vs married | 1.204 (1.035–1.401) | .016 | 1.131 (0.964–1.326) | .130 |
| Race | ||||
| Black vs White | 1.429 (1.18–1.729) | <.001 | 1.334 (1.087–1.638) | .006 |
| Other vs White | 0.932 (0.736–1.182) | .560 | 1.027 (0.806–1.308) | .830 |
| Radiation | ||||
| Beam radiation vs none | 0.89 (0.692–1.144) | .360 | 0.641 (0.457–0.898) | .010 |
| Brachytherapy vs none | 0.876 (0.625–1.228) | .440 | 0.613 (0.407–0.922) | .019 |
| CBB vs none | 0.711 (0.552–0.915) | .008 | 0.493 (0.35–0.694) | <.001 |
| Sequence | ||||
| RAS vs no surgery | 0.543 (0.459–0.642) | <.001 | 0.959 (0.725–1.269) | .770 |
| RPTS vs no surgery | 0.662 (0.433–1.013) | .058 | 1.343 (0.838–2.153) | .220 |
| RBAS vs no surgery | 2.216 (1.352–3.632) | .002 | 3.843 (2.15–6.868) | <.001 |
| Surgery | ||||
| SP vs not recommended | 0.549 (0.469–0.642) | <.001 | 0.555 (0.417–0.738) | <.001 |
| RBNP vs not recommended | 1.071 (0.654–1.753) | .790 | 0.92 (0.545–1.552) | .750 |
| Tumor size | 1.004 (1.002–1.006) | <.001 | 1.004 (1.003–1.005) | <.001 |
AC = adenocarcinoma; CI = confidence interval; CBB = combined beam with brachytherapy; DSS = disease-specific survival; HR = hazard ratio; LACC = locally advanced cervical carcinoma; RAS = radiation after surgery; RBAS = radiation before and after surgery; RBNP = recommended but not performed; RPTS = radiation prior to surgery; SCC = squamous cell carcinoma; SP = surgery performed.
3.5. Developing nomograms and risk stratification
Using the training cohort, we developed nomograms to predict OS and DSS in patients with LACC. We stratified the patients into low-, intermediate-, and high-risk groups, each demonstrating distinct survival trajectories over 7 years (see Figs. 3 and 4). Significant variations in survival rates were observed among the risk categories. Table 4 details the median survival times alongside the critical 1-, 3-, 5-, and 7-year survival probabilities segmented by risk group.
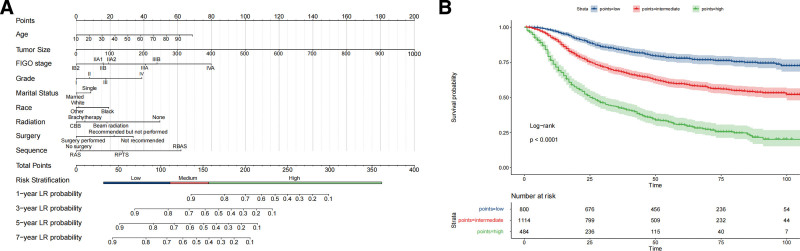
Figure 3.
Prognostic evaluation of OS in LACC using nomograms and risk stratification. (A) A detailed nomogram that estimates the probability of 1-, 3-, 5-, and 7-year OS based on a range of clinical and demographic variables. Points are assigned to age, tumor size, FIGO stage, histological grade, marital status, race, type of radiation therapy, surgical intervention, and sequence of treatment. This results in a total score that corresponds to a risk stratification category and survival probability. (B) Kaplan–Meier survival curves stratified into high, intermediate, and low-risk groups based on the total points calculated from the nomogram. The curves provide a visual representation of survival probabilities over time for each risk group. A log-rank test confirms the statistical significance of differences observed. The number of patients at risk at various time points is also indicated, providing context for the survival probabilities displayed. The low-risk group shows high survival resilience, with a 1-year survival rate of 96.9% and a 7-year rate of 75.0%. Intermediate-risk patients have a 1-year survival rate of 91.0%, which decreases to 54.5% at 7 years. The high-risk group starts at a 1-year survival rate of 71.6%, dropping dramatically to 24.8% at 7 years, demonstrating the most significant decline. OS = overall survival; FIGO = International Federation of Gynecology and Obstetrics; LACC = locally advanced cervical carcinoma.
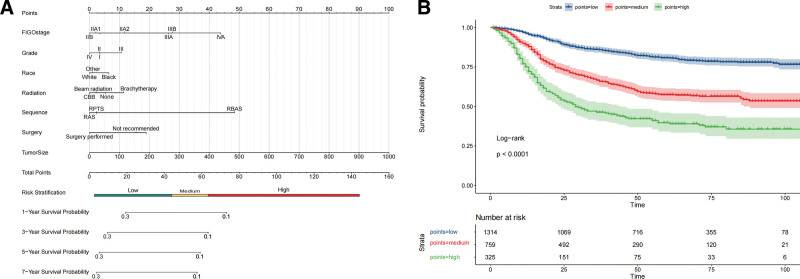
Figure 4.
Prognostic nomogram and survival curves for DSS in LACC. (A) A nomogram that assigns points to clinical variables such as FIGO stage, histological grade, race, type of radiation therapy, surgery sequence, and tumor size. This culminates in a total score that indicates a patient’s risk category and corresponding survival probabilities at 1, 3, 5, and 7 years. (B) Kaplan–Meier survival curves segmented into high, medium, and low-risk groups as determined by the nomogram’s scoring system. These curves visually represent the differences in survival rates over time among the risk categories, with statistical validation provided by the log-rank test. The number at risk at various time intervals is also noted, offering a detailed view of survival trends according to stratified risk groups. The low-risk cohort maintains excellent survival rates, starting at 96.5% at 1 year and declining to 78.0% at 7 years. The intermediate-risk cohort experienced a more pronounced decrease from 89.0% at 1 year to 56.4% at 7 years. The high-risk category showed the steepest decline, from 75.1% at 1 year to 35.6% at 7 years, highlighting the severity of risk associated with this group. DSS = disease-specific survival; FIGO = International Federation of Gynecology and Obstetrics; LACC = locally advanced cervical carcinoma.
Table 4.
Survival rates by risk stratification for OS and DSS in LACC (in months).
| Risk group | Median time | 1-year rate | 3-year rate | 5-year rate | 7-year rate | |
|---|---|---|---|---|---|---|
| OS | High risk | 26 (23–31) | 71.6 (67.6–75.7) | 42.5 (38.2–47.2) | 20.7 (26.5–35.5) | 24.8 (20.4–30.0) |
| Medium risk | Not reached (95% CI NA–NA) | 91.0 (89.3–92.7) | 68.7 (66.0–71.5) | 59.7 (56.8–62.9) | 54.5 (51.1–58.1) | |
| Low risk | Not reached (95% CI NA–NA) | 96.9 (95.7–98.1) | 84.0 (81.4–86.6) | 77.8 (74.7–80.9) | 75.0 (71.5–78.5) | |
| DSS | High risk | 29 (24–44) | 75.1 (70.4–80.0) | 46.3 (40.9–52.4) | 39.7 (34.2–46.2) | 35.6 (29.6–43.0) |
| Medium risk | Not reached (95% CI NA–NA) | 89.0 (86.8–91.3) | 66.7 (63.3–70.3) | 57.4 (53.7–61.5) | 56.4 (52.5–60.5) | |
| Low risk | Not reached (95% CI NA–NA) | 96.5 (95.4–97.5) | 86.1 (84.2–88.1) | 80.8 (78.5–83.2) | 78.0 (75.4–80.7) | |
CI = confidence interval; DSS = disease-specific survival; LACC = locally advanced cervical carcinoma; NA = unavailable; OS = overall survival.
3.6. Validation of OS and DSS nomograms
Multiple validation methods confirmed the reliability and performance of the nomograms. Figure S1, Supplemental Digital Content, http://links.lww.com/MD/N864 and Figure S2, Supplemental Digital Content, http://links.lww.com/MD/N864 depict the OS and DSS ROC curves, respectively, for both the training and validation cohorts, over 1 to 7 years. The ROC curves demonstrated high accuracy, with area under the curve (AUC) values ranging from 0.717 to 0.781 for OS and 0.706 to 0.784 for DSS, underscoring the precision of the nomograms. The calibration plots for these survival metrics are presented in Figure S3, Supplemental Digital Content, http://links.lww.com/MD/N864 and Figure S4, Supplemental Digital Content, http://links.lww.com/MD/N864 which further reinforce the predictive validity of the models. DCA across various time points substantiated the substantial clinical benefits of nomograms within a wide range of threshold probabilities (Figure S5, Supplemental Digital Content, http://links.lww.com/MD/N864 and Figure S6, Supplemental Digital Content, http://links.lww.com/MD/N864). In addition, we developed 2 online tools for efficient and user-friendly survival prediction for OS (https://zhangying123.shinyapps.io/DynNomapp_cox/) and DSS (https://zhangying123.shinyapps.io/DynNomapp_CRM/).
4. Discussion
Cervical cancer poses a significant global health challenge, with 604,127 new cases and 341,831 deaths annually.[1] Patients with LACC face even greater treatment complexity. Despite advancements in early screening and preventive measures, current treatment options, such as CCRT, exhibit limited efficacy owing to their high recurrence rates and severe adverse effects.[3–7] Moreover, prognostic factors, such as age, race, marital status, and tumor size, further complicate treatment strategies.[8–11] In our study, we used data from the SEER database and employed Cox regression and competing risk analyses to address these intricacies. Our objective was to establish a comprehensive risk stratification model to guide precise clinical interventions.
Our study revealed a distinctive pattern in LACC prevalence between 2010 and 2015. Utilizing data from the SEER database, we observed an initial decrease from 0.27 per million in 2010 to 0.25 per million in 2012. This was followed by an increase to 0.29 per million followed this. This trend was statistically significant, with APC significantly different from zero (P < .05). Notably, this period coincided with the Affordable Care Act enactment in 2010. The initial decline in LACC prevalence could potentially be attributed to Affordable Care Act’s enhancement of access to preventive healthcare services, such as cervical cancer screenings. However, the increase in prevalence from 2012 to 2015 suggests that other factors, such as rising healthcare costs, coverage gaps, or social determinants affecting risk, may have contributed to this observed trend.[28–32]
Our investigation pioneered the LACC prognosis by establishing apparent age and tumor size thresholds of 64 and 58 mm, respectively. This significantly affects patient outcomes. Through Kaplan–Meier analyses, we demonstrated the statistical significance of the survival outcomes associated with these thresholds (P < .0001), highlighting their clinical relevance. Unlike previous studies that predominantly relied on vague mean or median age ranges, our study innovatively defined a clinically operational age cutoff based on survival metrics.[33–36] This addition fills the existing knowledge gaps and has the potential to revolutionize personalized treatment, particularly for geriatric populations. Equally significant is the establishment of a 58 mm threshold for tumor size, serving as a measurable benchmark for therapeutic and prognostic assessments. Tumors exceeding this threshold indicate a more aggressive disease phenotype and necessitate tailored and intensive treatment. Prior studies offer descriptive measures, making our Kaplan–Meier-supported threshold a valuable clinical tool.[37–39]
Our analyses also revealed a multifaceted landscape of risk factors, including advanced FIGO stage, histological grade, and racial disparities. These factors significantly affected OS and DSS. Our study’s emphasis on the significance of advanced FIGO stages and age closely aligns with a recent multicenter study that focused on LACC. That study found that AC/adenosquamous histology was associated with a lower pathological complete response and a higher risk of recurrence and death than squamous cell carcinoma. This suggests that histological type may also play a crucial role in patient outcomes.[40] Significant racial disparities existed, with Black patients facing an increased risk of DSS disparities (HR = 1.334, P = .006). This aligns with findings from previous studies, which indicated that Black women with advanced cervical cancer are less likely to receive brachytherapy. This results in differences in survival rates among racial groups.[41] Therefore, it is imperative to adopt a comprehensive and equitable approach to patient care. Effective treatment modalities such as radiation therapy and brachytherapy, along with surgical interventions, significantly reduced the risk of both OS (HR = 0.528, P < .001) and DSS (HR = 0.641, both P < .02), as previously demonstrated.[42–46] These findings confirm the effectiveness of these treatments and suggest their applicability in a broader range of patients. These results underscore the importance of early intervention and advocate for a more holistic approach to patient care.
Our study introduced robust nomograms that accurately predicted OS and DSS in patients with LACC. These models underwent rigorous validation and demonstrated high discriminative capabilities, with AUCs ranging from 0.717 to 0.781 for OS and 0.706 to 0.784 for DSS.[47] The calibration curves and DCA support the reliability and clinical utility of these nomograms. Our risk stratification method categorized patients into low-, intermediate-, and high-risk cohorts, revealing distinct temporal survival patterns over 1-, 3-, 5-, and 7-year intervals, respectively. The low-risk group demonstrated a resilient survival trend, with a survival rate of 75.0% over year 7. Simultaneously, the high-risk cohort experienced a significant decline, with a survival rate of only 24.8% at the same interval. Similar patterns were observed in DSS outcomes, underscoring the importance of personalized risk-adapted strategies for managing LACC. The effectiveness of our nomogram was further enhanced by incorporating multiple independent prognostic factors, including the FIGO stage, age, histological grade, and treatment modality. This contributed to the predictive accuracy of the model. A multidimensional approach is essential for optimizing patient outcomes. We implemented these nomograms, accessible through user-friendly online tools for both OS and DSS, providing clinicians with a convenient interface for individual patient risk assessment and survival prediction.
This study has some limitations. First, the SEER dataset we utilized lacked essential biomarkers, including HPV DNA, P16INK4A, and Ki-67, which are critical for prognostic assessment.[48–54] Additionally, the database lacks complete treatment details, such as surgical techniques, chemotherapy regimens, radiotherapy doses, tumor morphology, comorbidities, and socioeconomic factors that influence survival.[55,56] The absence of imaging data limits the ability of deep learning approaches to enhance model accuracy. Owing to these unaccounted variables in our analysis, future studies should consider incorporating these key factors to improve their clinical relevance.
5. Conclusions
By analyzing data from the SEER database, our study delves into the intricate landscape of risk factors affecting OS and DSS rates among patients with LACC, yielding valuable insights. Our study introduces robust nomograms that have undergone rigorous validation, demonstrating a high predictive accuracy for survival outcomes. These nomograms pave the way for personalized risk-adapted treatment strategies. Our findings demonstrated the pivotal role of age, FIGO stage, histological grade, and racial disparity in predicting LACC prognosis. Importantly, our research established clinical, operational age, and tumor size cutoff values. This addresses a significant gap in the literature and provides a more nuanced approach to patient care.
Acknowledgments
The authors express their gratitude to the study participants and their families as well as the medical professionals involved in their treatment.
Author contributions
Conceptualization: Ying Zhang, Ya-Ping Meng.
Data curation: Ying Zhang, Xiao-Feng Xu.
Formal analysis: Ying Zhang, Xiao-Feng Xu.
Investigation: Ying Zhang.
Methodology: Ying Zhang.
Project administration: Ya-Ping Meng.
Validation: Ying Zhang.
Visualization: Ying Zhang.
Writing – original draft: Ying Zhang, Ya-Ping Meng.
Writing – review & editing: Ying Zhang, Xiao-Feng Xu, Qin Shi, Ya-Ping Meng.
Supplementary Material
Abbreviations:
- AC
- adenocarcinoma
- ACA
- Affordable Care Act
- AJCC7
- 7th edition of the American Joint Committee on Cancer (AJCC7)
- APC
- annual percentage changes
- AUC
- area under the curve
- CCRT
- concurrent chemoradiotherapy
- DCA
- decision curve analysis
- DSS
- disease-specific survival
- FIGO
- International Federation of Gynecology and Obstetrics
- HR
- hazard ratio
- LACC
- locally advanced cervical carcinoma
- OS
- overall survival
- ROC
- receiver operating characteristic
- SEER
- Surveillance, Epidemiology, and End Results
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Zhang Y, Meng Y-P, Xu X-F, Shi Q. Prognostic nomograms for locally advanced cervical cancer based on the SEER database: Integrating Cox regression and competing risk analysis. Medicine 2024;103:45(e40408).
YZ and Y-PM contributed equally to this work.
Contributor Information
Ya-Ping Meng, Email: ypingfly@126.com.
Xiao-Feng Xu, Email: 1xuxiaofeng@163.com.
Qin Shi, Email: shiqin0901@126.com.
References
- [1].Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11:e197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–82. [DOI] [PubMed] [Google Scholar]
- [3].Tian X, Wang X, Cui Z, et al. A fifteen-gene classifier to predict neoadjuvant chemotherapy responses in patients with stage IB to IIB squamous cervical cancer. Adv Sci (Weinh). 2021;8:2001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abu-Rustum NR, Yashar CM, Bean S, et al. NCCN guidelines insights: cervical cancer, version 1.2020. J Natl Compr Canc Netw. 2020;18:660–6. [DOI] [PubMed] [Google Scholar]
- [5].Dyer BA, Zamarin D, Eskandar RN, Mayadev JM. Role of immunotherapy in the management of locally advanced and recurrent/metastatic cervical cancer. J Natl Compr Canc Netw. 2019;17:91–7. [DOI] [PubMed] [Google Scholar]
- [6].Monk BJ, Toita T, Wu X, et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1334–48. [DOI] [PubMed] [Google Scholar]
- [7].Mileshkin LR, Moore KN, Barnes EH, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24:468–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sakuragi N, Kato T, Shimada C, et al. Oncological outcomes after Okabayashi-Kobayashi radical hysterectomy for early and locally advanced cervical cancer. JAMA Netw Open. 2020;3:e204307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rose PG, Java J, Whitney CW, et al. Nomograms predicting progression-free survival, overall survival, and pelvic recurrence in locally advanced cervical cancer developed from an analysis of identifiable prognostic factors in patients from NRG Oncology/Gynecologic Oncology Group randomized trials of chemoradiotherapy. J Clin Oncol. 2015;33:2136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pötter R, Tanderup K, Schmid MP, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22:538–47. [DOI] [PubMed] [Google Scholar]
- [11].Rufini V, Collarino A, Calcagni ML, et al. The role of (18)F-FDG-PET/CT in predicting the histopathological response in locally advanced cervical carcinoma treated by chemo-radiotherapy followed by radical surgery: a prospective study. Eur J Nucl Med Mol Imaging. 2020;47:1228–38. [DOI] [PubMed] [Google Scholar]
- [12].Pellino K, Kerridge S, Church C, et al. Social deprivation and prognosis in Scottish patients with pulmonary arterial hypertension. Eur Respir J. 2018;51:1700444. [DOI] [PubMed] [Google Scholar]
- [13].Christensen IE, Lillegraven S, Mielnik P, et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2022;81:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Back Nielsen M, Carlsson J, Køster Rimvall M, Petersen JH, Norredam M. Risk of childhood psychiatric disorders in children of refugee parents with post-traumatic stress disorder: a nationwide, register-based, cohort study. Lancet Public Health. 2019;4:e353–9. [DOI] [PubMed] [Google Scholar]
- [15].Maiwall R, Pasupuleti S, Bihari C, et al. Incidence, risk factors, and outcomes of transition of acute kidney injury to chronic kidney disease in cirrhosis: a prospective cohort study. Hepatology. 2020;71:1009–22. [DOI] [PubMed] [Google Scholar]
- [16].Jo J, Donahue J, Sarai G, Petroni G, Schiff D. Management of venous thromboembolism in high-grade glioma: does low molecular weight heparin increase intracranial bleeding risk? Neuro Oncol. 2022;24:455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsai M, Wang C, Lee W, et al. Tenofovir is superior to entecavir on tertiary prevention for BCLC stage 0/A hepatocellular carcinoma after curative resection. Liver Cancer. 2022;11:22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tinelli M, Rossini A, Scudeller L, et al. Dynamics of carbapenemase-producing Enterobacterales intestinal colonisation in the elderly population after hospital discharge, Italy, 2018–2020. Int J Antimicrob Agents. 2022;59:106594. [DOI] [PubMed] [Google Scholar]
- [19].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- [20].Petru E, Lück H, Stuart G, Gaffney D, Millan D, Vergote I. Gynecologic Cancer Intergroup (GCIG) proposals for changes of the current FIGO staging system. Eur J Obstet Gynecol Reprod Biol. 2009;143:69–74. [DOI] [PubMed] [Google Scholar]
- [21].Ballester M, Dubernard G, Lécuru F, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011;12:469–76. [DOI] [PubMed] [Google Scholar]
- [22].Sturdza AE, Pötter R, Kossmeier M, et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy including image-guided brachytherapy: a Retro-EMBRACE study. Int J Radiat Oncol Biol Phys. 2021;111:168–77. [DOI] [PubMed] [Google Scholar]
- [23].Gaddam S, Abboud Y, Oh J, et al. Incidence of pancreatic cancer by age and sex in the US, 2000-2018. JAMA. 2021;326:2075–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8:1690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tian MX, Liu WR, Wang H, et al. Tissue-infiltrating lymphocytes signature predicts survival in patients with early/intermediate stage hepatocellular carcinoma. BMC Med. 2019;17:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao F, Huang L, Wang Z, Wei F, Xiao T, Liu Q. Epidemiological trends and novel prognostic evaluation approaches of patients with stage II-IV colorectal neuroendocrine neoplasms: a population-based study with external validation. Front Endocrinol (Lausanne). 2023;14:1061187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Xu Y, Li W, et al. Cardiac MRI to predict sudden cardiac death risk in dilated cardiomyopathy. Radiology. 2023;307:e222552. [DOI] [PubMed] [Google Scholar]
- [28].Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- [30].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- [31].Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- [32].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [33].Moore KN, Java JJ, Slaughter KN, et al. Is age a prognostic biomarker for survival among women with locally advanced cervical cancer treated with chemoradiation? An NRG Oncology/Gynecologic Oncology Group ancillary data analysis. Gynecol Oncol. 2016;143:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Draghini L, Costantini S, Vicenzi L, et al. Positron emission tomography for staging locally advanced cervical cancer and assessing intensity-modulated radiotherapy approach. Radiol Med. 2019;124:819–25. [DOI] [PubMed] [Google Scholar]
- [35].Chang H, Wang M, Liu Y, Wu Y. Parametrial involvement and decreased survival of women with FIGO stage IIIC1 cervical cancer. J Gynecol Oncol. 2023;34:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elit LM, Fyles AW, Gu C, et al. Effect of positron emission tomography imaging in women with locally advanced cervical cancer: a randomized clinical trial. JAMA Netw Open. 2018;1:e182081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Casarin J, Buda A, Bogani G, et al. Predictors of recurrence following laparoscopic radical hysterectomy for early-stage cervical cancer: a multi-institutional study. Gynecol Oncol. 2020;159:164–70. [DOI] [PubMed] [Google Scholar]
- [38].Moussilmani T, Knight S, Mancini J, et al. Prognosis impact of posttreatment pelvic MRI in patients treated for stage IB2-IIB cervical cancer with chemoradiation therapy. Eur J Surg Oncol. 2021;47:1103–10. [DOI] [PubMed] [Google Scholar]
- [39].Seban R, Robert C, Dercle L, et al. Increased bone marrow SUVmax on 18F-FDG PET is associated with higher pelvic treatment failure in patients with cervical cancer treated by chemoradiotherapy and brachytherapy. Oncoimmunology. 2019;8:e1574197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Legge F, Bizzarri N, Gallotta V, et al. Locally advanced cervical carcinoma patients treated with chemoradiation followed by radical surgery: clinical response and oncological outcomes according to histotype after propensity score analysis. Eur J Surg Oncol. 2022;48:2045–52. [DOI] [PubMed] [Google Scholar]
- [41].Alimena S, Yang DD, Melamed A, et al. Racial disparities in brachytherapy administration and survival in women with locally advanced cervical cancer. Gynecol Oncol. 2019;154:595–601. [DOI] [PubMed] [Google Scholar]
- [42].Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87:111–9. [DOI] [PubMed] [Google Scholar]
- [43].Gill BS, Lin JF, Krivak TC, et al. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: the impact of new technological advancements. Int J Radiat Oncol Biol Phys. 2014;90:1083–90. [DOI] [PubMed] [Google Scholar]
- [44].Yeung AR, Pugh SL, Klopp AH, et al. Improvement in patient-reported outcomes with intensity-modulated radiotherapy (RT) compared with standard RT: a report from the NRG Oncology RTOG 1203 study. J Clin Oncol. 2020;38:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lin AJ, Kidd E, Dehdashti F, et al. Intensity-modulated radiation therapy and image-guided adapted brachytherapy for cervix cancer. Int J Radiat Oncol Biol Phys. 2019;103:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang P, Deng X, Qu A, et al. Image guidance volume-modulated arc radiation therapy concurrently with nab-paclitaxel plus cisplatin for patients with locally advanced cervical cancer: a single-arm dose escalation trial. Int J Radiat Oncol Biol Phys. 2023;115:1197–204. [DOI] [PubMed] [Google Scholar]
- [47].Zhang Y, Liu L, Zhang K, et al. Nomograms combining clinical and imaging parameters to predict recurrence and disease-free survival after concurrent chemoradiotherapy in patients with locally advanced cervical cancer. Acad Radiol. 2023;30:499–508. [DOI] [PubMed] [Google Scholar]
- [48].Youn JW, Hur S, Woo JW, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020;21:1653–60. [DOI] [PubMed] [Google Scholar]
- [49].Jeannot E, Latouche A, Bonneau C, et al. Circulating HPV DNA as a marker for early detection of relapse in patients with cervical cancer. Clin Cancer Res. 2021;27:5869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cabel L, Bonneau C, Bernard-Tessier A, et al. HPV ctDNA detection of high-risk HPV types during chemoradiotherapy for locally advanced cervical cancer. ESMO Open. 2021;6:100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].He Y, Shi J, Zhao H, et al. p16INK4A flow cytometry of exfoliated cervical cells: its role in quantitative pathology and clinical diagnosis of squamous intraepithelial lesions. Clin Transl Med. 2023;13:e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bergeron C, Ronco G, Reuschenbach M, et al. The clinical impact of using p16INK4a immunochemistry in cervical histopathology and cytology: an update of recent developments. Int J Cancer. 2015;136:2741–51. [DOI] [PubMed] [Google Scholar]
- [53].Duangkaew P, Tapaneeyakorn S, Apiwat C, et al. Ultrasensitive electrochemical immunosensor based on dual signal amplification process for p16(INK4a) cervical cancer detection in clinical samples. Biosens Bioelectron. 2015;74:673–9. [DOI] [PubMed] [Google Scholar]
- [54].Clarke MA, Cheung LC, Castle PE, et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 2019;5:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Corrado G, Anchora LP, Bruni S, et al. Patterns of recurrence in FIGO stage IB1-IB2 cervical cancer: comparison between minimally invasive and abdominal radical hysterectomy. Eur J Surg Oncol. 2023;49:107047. [DOI] [PubMed] [Google Scholar]
- [56].Pecorino B, D’Agate MG, Scibilia G, et al. Evaluation of surgical outcomes of abdominal radical hysterectomy and total laparoscopic radical hysterectomy for cervical cancer: a retrospective analysis of data collected before the LACC trial. Int J Environ Res Public Health. 2022;19:13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.