To the Editor: Gouty arthritis, an autoinflammatory disease, exhibits a rapid onset of clinical symptoms but often resolve spontaneously within 7–10 days. In the acute gouty inflammatory responses, monosodium urate (MSU) crystal is recognized as damage-associated molecular patterns by Toll-like receptors (TLRs) on macrophage membranes. Subsequently, the TLR-NF-kappa-B inhibitor alpha (IκBα)/nuclear factor kappa B (NF-κB) pathway is activated, producing many inflammatory factors, neutrophil chemokines, and cathepsin and participating in the assembly of nucleotide-binding oligomerization domain receptor protein 3 (NLRP3) inflammasomes.[1] The NLRP3 inflammasome consists of NLRP3, apoptosis-associated speck-like protein (ASC), and caspase-1. Synergism between the NLRP3 inflammasome and the NF-κB pathway plays a crucial role in gouty inflammation by activating the maturation of interleukin (IL)-1β and IL-18, inducing pyroptosis and releasing large amounts of proinflammatory mediators. Studies have shown that increased anti-inflammatory mediators, such as transforming growth factor β1 (TGF-β1) and IL-10, are involved in acute inflammatory spontaneous remission.[2] Decoy receptor 3 (DcR3) serves as an essential regulator of inflammation and the immune response. DCR3 exerts anti-inflammatory effects by blocking the human tumor necrosis factor-related ligand 1A (TL1A)/death receptor 3 (DR3) axis by competing with DR3 to bind to TL1A. The TL1A/DR3/DcR3 axis is a novel immune pathway that participates in the pathogenesis of several autoinflammatory disorders.[3] However, it is unclear whether the TL1A/DR3/DcR3 axis is involved in the occurrence, progression, and spontaneous remission of gouty arthritis. Here, we measured the expression levels of DcR3, TL1A, and DR3 in patients with acute gout (AG) or intercritical gout (IG). Subsequently we used THP-1 cells transfected with DcR3-specific small interfering RNA (si-DcR3) or a DcR3 overexpression plasmid (pcDNA-DcR3) to determine the function of DcR3 in MSU-induced gouty inflammation.
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of North Sichuan Medical College (No: 2022ER423-1). All participants gave informed consent. A total of 40 AG patients, 40 IG patients, and 50 healthy controls (HC) recruited from Affiliated Hospital of North Sichuan Medical College were enrolled in the study. Basic demographic information was collected, besides clinical and laboratory tests were also conducted [Supplementary Figure 1A, http://links.lww.com/CM9/C129].
THP-1 cells were transfected with si-DcR3, non-specific siRNA (negative control, si-NC), a DcR3 overexpression plasmid (pcDNA-DcR3), or the pcDNA vector. The cells were seeded into 6-well plates at a density of 3 × 106 cells/well. When the plated cells reached 80% confluency, they were induced to differentiate into macrophages by incubation with 100 ng/mL phorbol-12-myristate-13-acetate (PMA) for 24 h. The cells were then stimulated with MSU (100 μg/mL) or culture medium, and the culture supernatants and cells were collected at 0 h, 3 h, 6 h, 9 h, and 12 h post-stimulation [Supplementary Figures 1B and 2, http://links.lww.com/CM9/C129].
Total RNA was extracted from peripheral blood mononuclear cells (PBMCs) of gout patients and HC, as well as from THP-1 cells and was reverse transcribed into complementary DNC (cDNA). Then, quantitative real-time polymerase chain reaction (RT-qPCR) was performed to obtain a mean cycle threshold (CT) value for each sample. The CT values of the samples were compared using the 2−ΔΔCT method, and β-actin expression was used as an internal reference. The levels of DcR3 (Abcam, Cambridge, UK), IL-1β (Boster, Wuhan, China), TNF-α (Neobioscience, Shenzhen, China), TGF-β1 (Fine, Wuhan, China), and IL-10 (Fine) were quantified in serum samples of all subjects as well as supernatants of THP-1 cells by enzyme-linked immunosorbent assay (ELISA). Besides, western blot analyses were also conducted with primary antibodies against DcR3 (1:500, Affinity Biosciences, Jiangsu, China), TL1A (1:1000, Abcam), DR3 (1:500, Abcam), IL-1β (1:1000, CST, Boston, USA), NF-κB (1:1000, CST), p-NF-κB (1:1000, CST), IκBα (1:1000, CST), p-IκBα (1:1000, CST), NLRP3 (1:1000, CST), ASC (1:1000, CST), caspase-1 (1:1000, Affinity Biosciences, China), and GAPDH (1:1000, CST).
Quantitative data were expressed as the mean ± standard deviation (SD) or median (25th percentile, 75th percentile) and analyzed using appropriate statistical tests such as t test, one-way analysis of variance (ANOVA), Mann–Whitney U test, or Kruskal–Wallis H test. Multiple or pairwise comparisons were performed using the LSD-t or Mann–Whitney U tests. Gene expression during gout flares and after resolution was statistically analyzed using paired sample Student’s t tests. Furthermore, correlation analysis between variables was also performed using Spearman’s rank correlation test. The significance level was set at a two-sided P-value of less than 0.05. All analyses were performed using SPSS 26.0 (IBM Corp, Armonk, NY, USA).
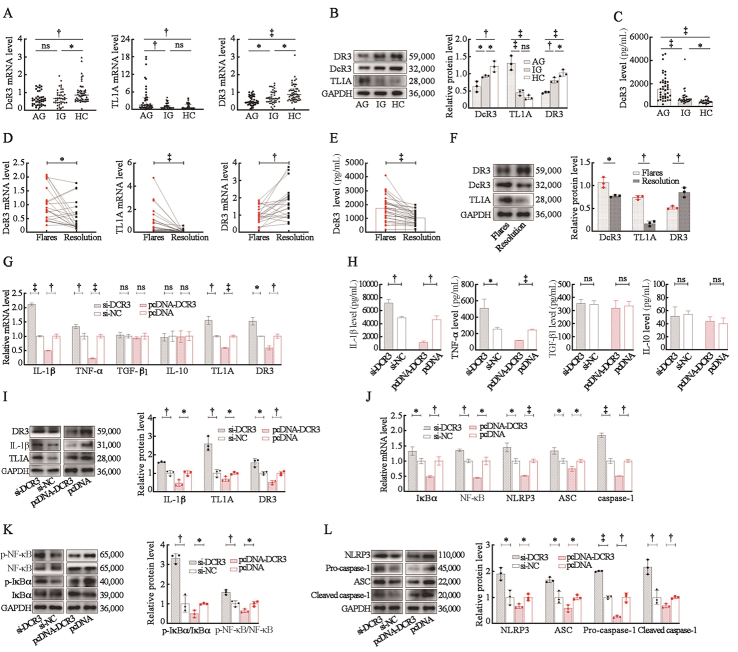
Clinical and laboratory data are shown in Supplementary Table 1, http://links.lww.com/CM9/C129, and no significant among-group differences were observed in age and sex. The DcR3 mRNA levels in PBMCs in the AG and IG groups were significantly lower than that in the HC group [Figure 1A]. Besides, the DcR3 protein level in PBMCs in the AG group was significantly lower than those in the IG and HC groups [Figure 1B]. Conversely, the DcR3 protein concentration in peripheral blood plasma in the AG group was significantly higher than those in peripheral blood plasma in the IG and HC groups [Figure 1C]. Compared with those in IG and HC groups, the mRNA and protein levels of DR3 in AG patients were significantly lower, while TL1A mRNA and protein were significantly higher [Figure 1A, B]. The mRNA and protein expression levels of DcR3 and TL1A decreased, while the expression level of DR3 increased after AG resolution when compared to their levels during gout flares [Figure 1D, E, F]. These results suggest that the TL1A/DR3/DcR3 axis may be involved in acute gouty inflammation. The levels of DcR3 and IL-1β mRNA and protein in THP-1 cells peaked at 9 h after MSU treatment in an in vitro study, with the highest concentration of IL-1β observed in the culture supernatants occurring 12 h after MSU treatment [Supplementary Figure 3, http://links.lww.com/CM9/C129]. Therefore, cells treated with MSU for 9 h were selected for subsequently downregulated or overexpressed experiments. Significantly increased TL1A, DR3, IL-1β, and tumor necrosis factor α (TNF-α) production was observed in si-DcR3 group, while contrasting results were observed after DcR3 overexpression; TGF-β1 and IL-10 production remained unchanged regardless of DcR3 downregulated or overexpressed [Figure 1G, H, I]. These results suggest that DcR3 might be involved in the pathogenesis of gouty inflammation remission by blocking the TL1A/DR3 axis and suppressing the production of IL-1β and TNF-α but not by regulating TGF-β1 and IL-10. Additionaly, the low expression of DcR3 in THP-1 cells treated with MSU increased the phosphorylation levels of IκBα and NF-κB and the NLRP3 inflammasome component expression, while contrasting results were observed in DcR3 overexpression group [Figure 1J, K, L].
Figure 1.
DcR3 suppresses the NF-κB pathway and the NLRP3 inflammasome activation in gouty inflammation. (A) Expression of DcR3, TL1A, and DR3 mRNA levels in gout patients’ PBMCs. (B) Expression of DcR3, TL1A, and DR3 protein levels in gout patients’ PBMCs. (C) Expression of DcR3 protein concentration in gout patients’ peripheral blood plasma. (D) Relative expression of DcR3, TL1A, and DR3 mRNA levels in PBMCs between the patients with gout flares and after resolution. (E) Relative expression of DcR3 protein concentration in peripheral blood plasma between the patients with gout flares and after resolution. (F) Relative expression of DcR3, TL1A, and DR3 protein levels in PBMCs between the patients with gout flare and after resolution. (G) Effect of DcR3 on the mRNA levels of IL-1β, TNF-α, TGF-β1, IL-10, TL1A, and DR3 in THP-1 cells treated with MSU. (H) Effect of DcR3 on the production of IL-1β, TNF-α, TGF-β1, and IL-10 in the supernatant of THP-1 cells treated with MSU. (I) Effect of DcR3 on the protein levels of IL-1β, TL1A, and DR3 in THP-1 cells treated with MSU. (J) Effect of DcR3 on the mRNA levels of IκBα, NF-κB, NLRP3, ASC, and caspase-1 in THP-1 cells treated with MSU. (K, L) Effect of DcR3 on the protein levels of p-IκBα, p-NF-κB, NLRP3, ASC, pro-caspase-1, and cleaved caspase-1 in THP-1 cells treated with MSU. *P <0.05; †P <0.01; ‡P <0.001; ns, no statistic significance. AG: Acute gout; ASC: Apoptosis-associated speck-like protein; DcR3: Decoy receptor 3; DR3: Death receptor 3; HC: Healthy controls; IG: Intercritical gout; IκBα: Inhibitor kappa B alpha; IL-10: Interleukin-10; IL-1β: Interleukin-1β; MSU: Monosodium urate; NF-κB: Nuclear factor kappa-B; NLRP3: Nucleotide-binding oligomerization domain receptor protein 3; PBMCs: Peripheral blood mononuclear cells; pcDNA-DcR3: Overexpression plasmid of DcR3; p-IκBα: Phosphorylated inhibitor kappa B alpha; p-NF-κB: Phosphorylated nuclear factor kappa-B; si-DcR3: DcR3-specific small interfering RNA; TGF-β1: Transforming growth factor-β1; TNF-α: Tumor necrosis factor; TL1A: Human tumor necrosis factor-related ligand 1A.
In MSU crystal-induced gouty inflammation, the dynamic balance of proinflammatory and anti-inflammatory factors plays an important role in the spontaneous remission mechanism of inflammation in gout. Studies suggest that IL-1 and TNF-α are typical proinflammatory mediators in gouty inflammation and that TGF-1 and IL-10 may be involved in self-limiting gouty inflammation.[1,2] The release of proinflammatory factors, such as IL-1β, IL-6, and TNF-α, is crucial in the amplification reaction of the gout inflammation cascade. IL-1β and TNF-α can stimulate the secretion of TL1A.[3] TL1A binds to DR3 to provide co-stimulation signals to lymphocytes, amplifing helper T cell 1 (Th1)- and Th17-mediated immune responses, promoting IL-2 signaling, and inducing the secretion of many proinflammatory factors.[4] In addition, IL-6 significantly upregulates the expression of DcR3.[5] According to the results of DcR3, TL1A, DR3, and inflammatory factors, we speculate that the dramatic increase in inflammatory cytokines in AG patients stimulates the secretion of TL1A, which combines with DR3 to further induce the secretion of proinflammatory factors, forming a vicious cycle of inflammatory responses. DcR3 is also increased correspondingly to combat the excessive inflammatory responses. A significant increase in the concentration of DcR3 protein secreted extracellularly inhibits the expression of mRNA, intracellular protein, and membrane protein by negative feedback.
In acute gouty inflammation, IL-1β is produced primarily through the TLR-IκBα/NF-κB pathway and the NLRP3 inflammasome. Under normal conditions, NF-κB dimers bind to IκBα in the cytoplasm, leading to NF-κB inactivation.[6] MSU crystals form complexes with cluster of differentiation (CD)14 and bind to TLRs on macrophages to result rapidly activation of the IκB kinase complex, leading to IκBα phosphorylation, degradation, and detachment from NF-κB. The degradation of IκBα exposes the nuclear localization signaling region of NF-κB, promoting NF-κB phosphorylation and nuclear transcription. The activated NF-κB pathway promotes the transcription, translation, and modification of NLRP3 mRNA.[7] Additionally, MSU crystals bind to NLRP3 receptors, recruiting ASC and pro-caspase-1 to assemble into signal transduction complexes. Activated caspase-1 (cleaved caspase-1) cleaves IL-1β and IL-18 into their active forms and cleaves Gasdermin D. The Gasdermin-N domain binds to phospholipid proteins on the cell membrane to form pores, inducing pyroptosis. Cell rupture and the release of proinflammatory contents induce a cascade-amplified inflammatory responses. Besides, the combination of TL1A and DR3 can rapidly degrade cytoplasmic IκBα and induce the activation of the NF-κB pathway.[8] This study showed that low expression of DcR3 increased the phosphorylation levels of IκBα and NF-κB and the NLRP3 inflammasome component expression, suggesting that DcR3 can negatively regulate the activation of IκBα/NF-κB pathway and the NLRP3 inflammasome in gouty inflammation.
In conclusion, our study suggest that DcR3 may provide negative feedback regulation of the development of gouty inflammation by blocking the TL1A/DR3 axis, suppressing the NF-κB pathway and the NLRP3 inflammasome activation, and inhibiting IL-1β and TNF-α production.
Conflicts of interest
None.
Supplementary Material
Footnotes
Yi Jiang, Xin Tu, Jianwei Guo, Jianxiong Zheng, and Quanbo Zhang contributed equally to this work.
How to cite this article: Jiang Y, Tu X, Guo JW, Zheng JX, Liao X, He YX, Xie Y, Zhang QB, Qing YF. DcR3 suppresses the NF-κB pathway and the NLRP3 inflammasome activation in gouty inflammation. Chin Med J 2024;137:2644–2646. doi: 10.1097/CM9.0000000000003274
References
- 1.So AK, Martinon F. Inflammation in gout: Mechanisms and therapeutic targets. Nat Rev Rheumatol 2017;13:639–647. doi: 10.1038/nrrheum.2017.155. [DOI] [PubMed] [Google Scholar]
- 2.Wu M, Tian Y, Wang Q, Guo C. Gout: A disease involved with complicated immunoinflammatory responses: A narrative review. Clin Rheumatol 2020;39:2849–2859. doi: 10.1007/s10067-020-05090-8. [DOI] [PubMed] [Google Scholar]
- 3.Siakavellas SI, Sfikakis PP, Bamias G. The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin Arthritis Rheum 2015;45:1–8. doi: 10.1016/j.semarthrit.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J Wang X Fahmi H Wojcik S Fikes J Yu Y, et al. Role of TL1A in the pathogenesis of rheumatoid arthritis. J Immunol 2009;183:5350–5357. doi: 10.4049/jimmunol.0802645. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh SL, Lin WW. Decoy receptor 3: An endogenous immunomodulator in cancer growth and inflammatory reactions. J Biomed Sci 2017;24:39. doi: 10.1186/s12929-017-0347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 8.Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem 2003;278:39251–39258. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]