Abstract
A 37-year-old man with a history of Kawasaki disease presented with total occlusion of the right coronary artery. The patient underwent percutaneous coronary intervention (PCI) with excimer laser coronary angioplasty (ELCA) and plain old balloon angioplasty (POBA). Three months after PCI, a coronary aneurysm with restenosis was detected at the PCI site, and PCI was performed again using a small balloon. The aneurysm healed three months after the second PCI procedure. This is the first report describing the long-term outcome after an aneurysm caused by PCI with ELCA and POBA.
Keywords: Kawasaki disease, percutaneous coronary intervention, coronary aneurysm, excimer laser catheter ablation
Introduction
Coronary artery disease (CAD) is a common sequelae of Kawasaki disease (KD) (1,2). CAD is often treated with percutaneous coronary intervention (PCI) in KD patients. However, aneurysm formation in the coronary artery after PCI is problematic (3).
Drug-eluting stents, which are often used in PCI, are not recommended for KD patients. Stent implantation is not recommended because of the high rate of adverse events, including the development of new aneurysms (4).
We herein report the long-term follow-up of a patient who developed a coronary aneurysm after undergoing PCI with excimer laser coronary angioplasty (ELCA) for treatment of an occluded right coronary artery (RCA).
Case Report
A 37-year-old man presented to our hospital with exertional chest pain. The patient had been diagnosed with KD at five years old. He was being treated with aspirin because of a coronary artery aneurysm in the mid-portion of the RCA. Coronary artery aneurysms had been undetectable at seven years old. At the time of this visit to our hospital, electrocardiography, chest radiography, and transthoracic echocardiography revealed normal findings. Blood test results, including inflammatory markers and coronary risk factors, were normal.
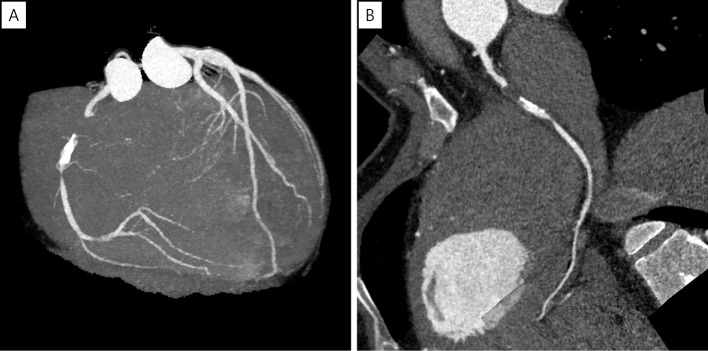
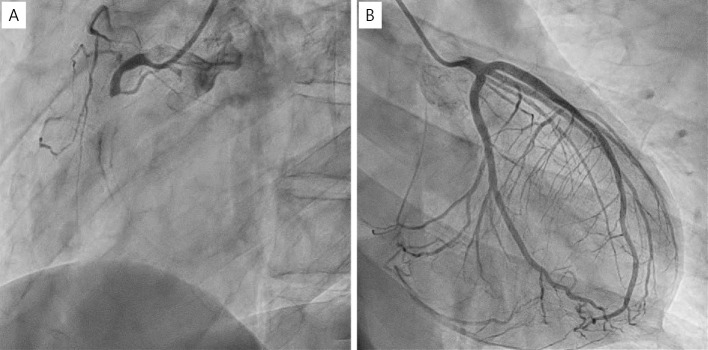
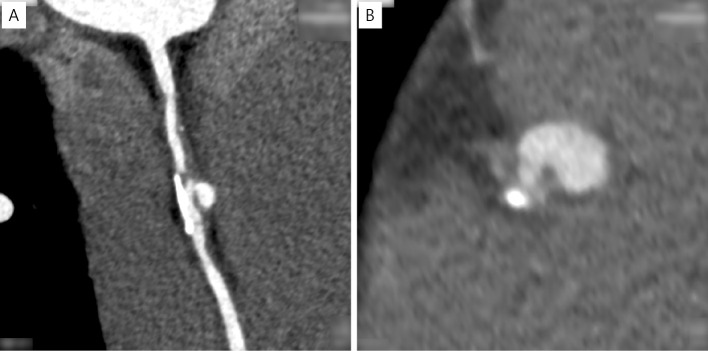
Coronary computed tomography angiography (CCTA) revealed total occlusion of the proximal portion of the RCA with eccentric calcification and severe stenosis of the middle portion of the RCA without an aneurysm (Fig. 1). The occluded site had low CT values, which are atypical for patients with a history of KD. Coronary angiography (CAG) revealed total occlusion of the proximal RCA (Fig. 2A) and collateral flow to the distal portion of the RCA (Fig. 2B). Left ventriculography revealed a normal wall motion in the left ventricle.
Figure 1.
CCTA showing total occlusion of the proximal part of RCA and eccentric calcification and severe stenosis of the mid-RCA. (A) Maximum intensity projection, (B) curved multi-planar reconstruction. CCTA: coronary computed tomography angiography, RCA: right coronary artery
Figure 2.
CAG showing the RCA occlusion and collateral flow from the LCA. (A) RCA, (B) LCA. CAG: coronary angiography, LCA: left coronary artery, RCA: right coronary artery
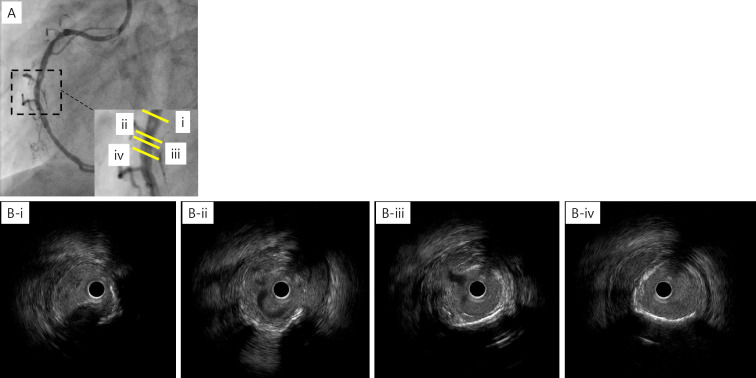
The patient underwent PCI for an RCA lesion. The conventional guidewire was passed smoothly through the occluded lesion. Intravascular ultrasonography (IVUS) revealed a low-echoic plaque at the occluded site. Owing to coronary sequelae in KD, a stentless strategy was selected. ELCA was chosen to reduce plaque volume. After aspiration of the thrombus, ablation with ELCA using 0.9-mm and 1.7-mm catheters was performed. Angioscopy after ELCA revealed a yellow plaque similar to an atherosclerotic lesion at the occluded site (Fig. 3). Long inflation using a 3.0-mm perfusion balloon achieved target vessel dilatation with Type C dissection (National Heart, Lung, and Blood Institute classification system for intimal tears). IVUS revealed dissection and blood speckles in the subintimal space (Fig. 4). After waiting for 10 min to confirm the coronary flow, PCI was performed (Fig. 5).
Figure 3.
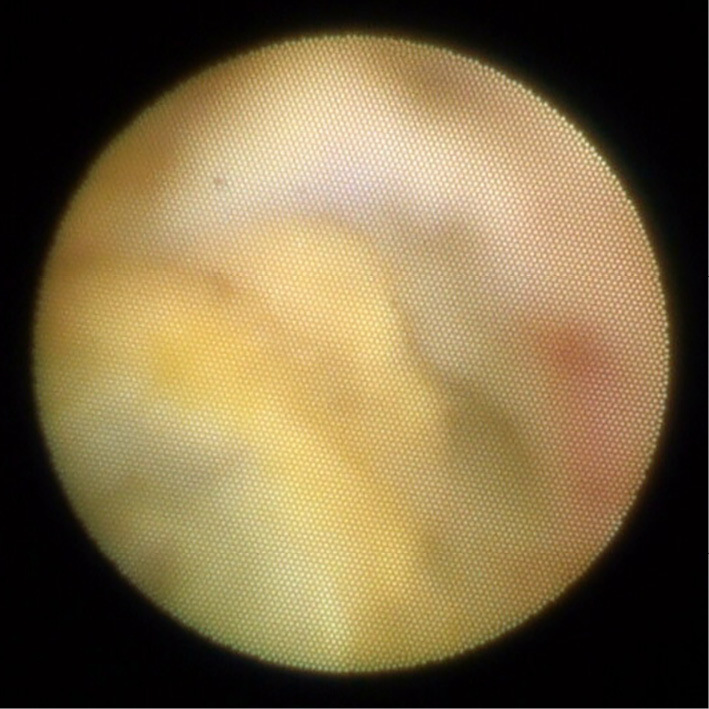
Angioscopy showing yellow plaque at the occluded site.
Figure 4.
IVUS after ELCA and balloon angioplasty showing coronary dissection and blood speckles in the subintimal space. (A) Right CAG, (B-i) eccentric calcification at 3 o’clock, (B-ii) the branch at 3 o’clock and the dissection at 9 o’clock, (B-iii) the dissection and blood speckles in the subintimal space, (B-iv) the hematoma at 4 to 11 o’clock. ELCA: excimer laser coronary angioplasty, IVUS: intravascular ultrasound
Figure 5.
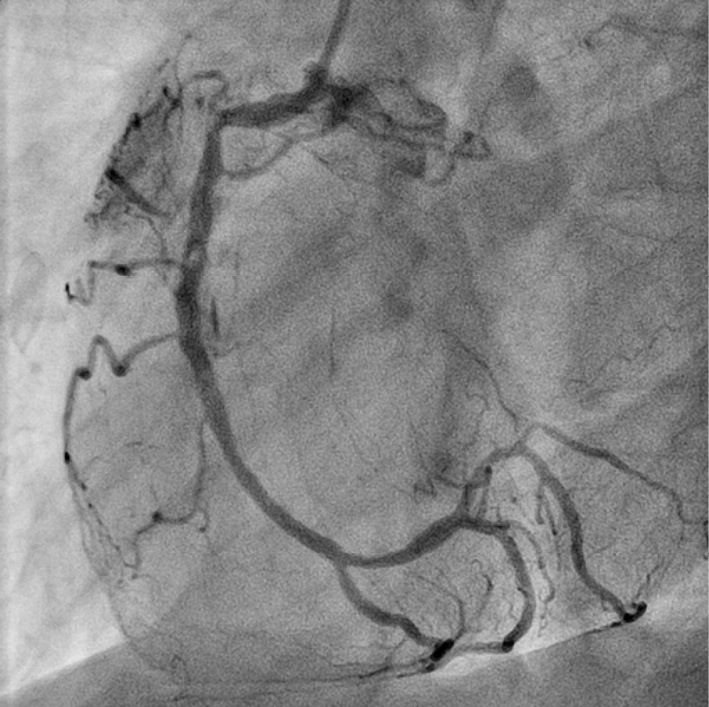
CAG showing coronary dissection at the middle portion of the RCA without any flow limit 10 min after balloon angioplasty. CAG: coronary angiography, RCA: right coronary artery
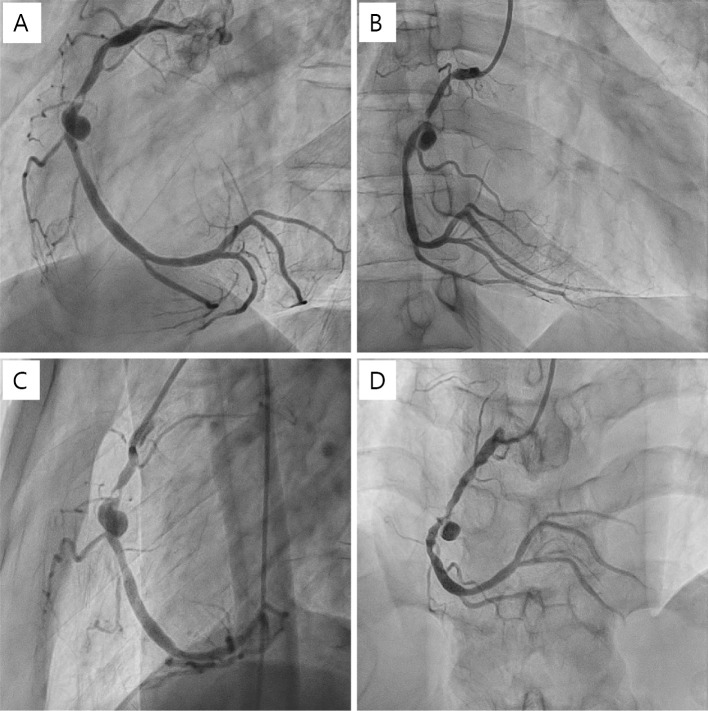
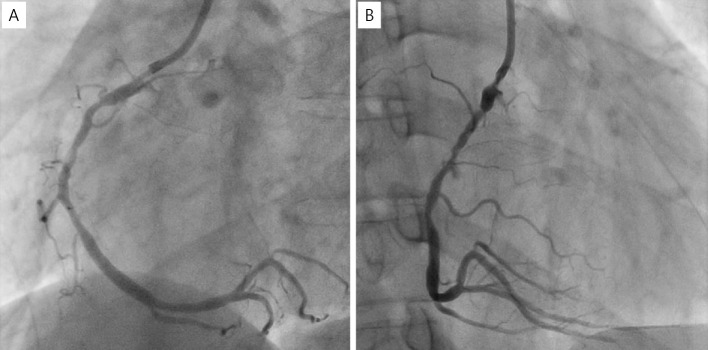
Since the coronary lesion had been pointed out, the patient was administered bisoprolol and rosuvastatin in addition to aspirin. Three months after PCI, the patient experienced chest pain again during effort. CAG and CCTA revealed restenosis at the former occlusion site and saccular aneurysm at the former dissection site (Fig. 6, 7). Surgical repair, covered stent implantation, and angioplasty with a small balloon have been discussed as treatment options. Surgical repair was not selected because the aneurysm size was not very big, and the patient did not wish to undergo surgical repair when we explained the surgical repair to him. If the aneurysm had grown any larger, we would have considered surgical repair again. Covered stents were not selected because of concerns about the risk of new aneurysm formation at the stent edge, since the stress on the vessel wall from covered stents is similar to that from other metal stents. We ultimately decided to perform PCI using a small balloon.
Figure 6.
Right CAG showing restenosis of part of the former CTO site and the saccular aneurysm of the former dissection site. (A) LAO view, (B) RAO view, (C) lateral view, (D) front view. CAG: coronary angiography, CTO: chronic total occlusion, LAO: left anterior oblique, RAO: right anterior oblique
Figure 7.
CCTA showing a stenotic lesion and saccular aneurysm. (A) Curved MPR image, (B) short-axis image. CCTA: coronary computed tomography angiography, MPR: multiplanar reconstruction
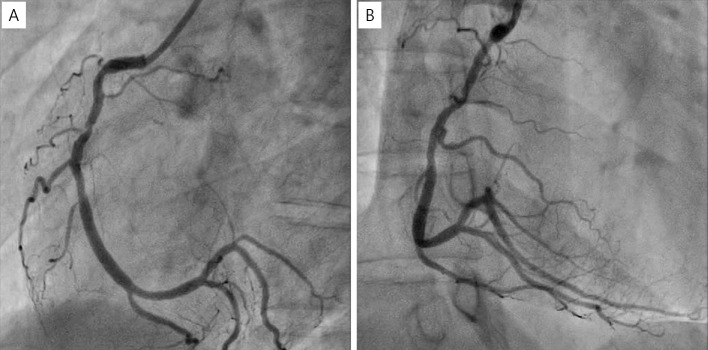
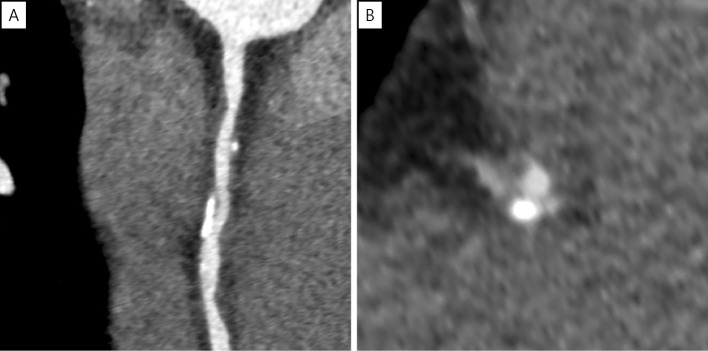
The patient thus underwent a second PCI procedure with balloon angioplasty using a 2.5-mm perfusion balloon. An undersized balloon was used to increase pressure in the true lumen without stressing the aneurysm. CAG revealed moderate stenosis without aneurysm enlargement (Fig. 8). Three months after the second PCI procedure, CAG and CCTA showed moderate stenosis without an aneurysm, and the patient felt no chest symptoms (Fig. 9, 10). The patient also reported no angina or ischemia on an exercise stress test performed two years after the second PCI procedure.
Figure 8.
Final CAG showing moderate stenosis without aneurysm enlargement. (A) LAO view, (B) RAO view. CAG: coronary angiography, LAO: left anterior oblique, RAO: right anterior oblique
Figure 9.
CAG showing moderate stenosis without an aneurysm. (A) LAO view, (B) RAO view. CAG: coronary angiography, LAO: left anterior oblique, RAO: right anterior oblique
Figure 10.
CCTA showing moderate stenosis without an aneurysm. (A) Curved MPR image, (B) short-axis image. CCTA: coronary computed tomography angiography, MPR: multiplanar reconstruction
Discussion
KD, which was first described in Japan in 1967, is an acute, self-limiting vasculitis of unknown etiology that occurs predominantly in infants and young children. The coronary arteries are one of the main targets of KD (3). In adults, KD is often detected as a coronary aneurysm with calcification on angiography. Coronary artery lesions have multiple pathological phases, including aneurysm formation, thrombotic occlusion, recanalization after thrombotic occlusion, and remodeling of the normal lumen with an abnormal arterial wall (3). Inflammation may disrupt the intima and tunica media structures (5).
Although KD can be acutely treated, with improvements seen in the long-term prognosis, coronary sequelae are still common in KD. Advances in devices and techniques for the treatment of CAD have led to the increased use of PCI for coronary arteries in KD. Balloon angioplasty for the stenotic lesion is effective, although balloon angioplasty with high pressure causes aneurysm formation (6,7). Stent implantation is not recommended because of the high rate of adverse events, including new aneurysm formation, stent migration, and thromboembolism (4,8). Although data concerning PCI with drug-eluting stents in patients with KD are limited, new aneurysmal formation has also been reported with this technique (4,9). In patients with KD, the structural integrity of the artery is compromised, and vascular support may be weak and easily disrupted by PCI with a high-pressure balloon and stent. A fragile vessel wall may not be able to withstand increased coronary pressure, and new aneurysms may form. Rotational atherectomy without balloon dilatation is suitable for severe localized stenoses with calcification (6,10). Excimer laser tissue ablation is mediated by photochemical, photothermal, and photomechanical mechanisms. Few reports have described PCI with ELCA in patients with KD. Kawamura et al. demonstrated good results using ELCA and a drug-coated balloon to treat PCI in patients with KD (11).
How coronary aneurysms heal remains unclear. As some possibilities, layered mural thrombus and luminal myofibroblastic proliferation are thought to be associated with aneurysm healing in KD coronary artery lesions (3). Occlusion by thrombus has been speculated in coronary aneurysm associated with coronary artery dissection (12).
In the present case, the coronary lesion had both KD-like and non-KD-like characteristics. CCTA and IVUS revealed a distal stenotic site with eccentric calcification in a KD-like lesion. IVUS showed a low echoic plaque proximal to the occluded site, and angioscopy revealed a yellow non-KD-like plaque that resembled an atherosclerotic plaque. Coronary artery dissection was performed using ELCA and balloon angioplasty. In patients with KD, the structural integrity of the artery is compromised, and vascular support may be weak. These features of KD may have contributed to coronary dissection in our case, with the dissected site becoming a new aneurysm. After a second PCI procedure for restenosis with a small balloon, the aneurysm healed within a few months. Using a small balloon for plain old balloon angioplasty (POBA) may prevent undue stress on the fragile vessel wall, and sealing the plaque may decrease flow into the aneurysm. POBA with a small balloon may be effective in treating complex KD coronary sequelae.
This is the first report describing the long-term follow-up of a patient with an aneurysm caused by PCI with ELCA.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Kato H, Ichinose E, Yoshioka F, et al. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol 49: 1758-1766, 1982. [DOI] [PubMed] [Google Scholar]
- 2. Fukazawa R, Kobayashi J, Ayusawa M, et al. ; the Japanese Circulation Society Joint Working Group. JCS/JSCS 2020 Guideline on Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease. Circ J 84: 1348-1407, 2020. [DOI] [PubMed] [Google Scholar]
- 3. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol 67: 1738-1749, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Tsuda E. Insights into stent implantation for coronary artery lesions caused by Kawasaki disease. Cardiol Young 30: 911-918, 2020. [DOI] [PubMed] [Google Scholar]
- 5. Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease - cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol 6: 394-401, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kato H, Ishii M, Akagi T, et al. Interventional catheterization in Kawasaki disease. J Interv Cardiol 11: 355-361, 1998. [Google Scholar]
- 7. Ino T, Akimoto K, Ohkubo M, et al. Application of percutaneous transluminal coronary angioplasty to coronary arterial stenosis in Kawasaki disease. Circulation 93: 1709-1715, 1996. [DOI] [PubMed] [Google Scholar]
- 8. Akagi T. Interventions in Kawasaki disease. Pediatr Cardiol 26: 206-212, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Sawai T, Tanigawa T, Masuda J, et al. New coronary aneurysm formation and malapposition after zotarolimus-eluting stent implantation in Kawasaki disease. J Cardiol Cases 8: 118-120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuda E, Asaumi Y, Noguchi T, Yasuda S. Long-term results of percutaneous transluminal coronary rotational atherectomy for localised stenosis caused by Kawasaki disease. Cardiol Young 32: 287-294, 2022. [DOI] [PubMed] [Google Scholar]
- 11. Kawamura I, Komiyama K, Fukamizu S, Shibui T, Ashikaga T, Sakurada H. Combination of drug-coated balloon angioplasty and excimer laser coronary angioplasty ablation for coronary restenosis of Kawasaki disease: a case report. J Cardiol Cases 15: 18-21, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eshima K, Takemoto M, Inoue S, Higo T, Tada H, Sunagawa K. Coronary aneurysm associated with coronary perforation after sirolimus-eluting stents implantation: close follow-up exceeding 2 years by coronary 3-dimensional computed tomography. J Cardiol 54: 115-120, 2008. [DOI] [PubMed] [Google Scholar]