Abstract
A 70-year-old man was admitted to our hospital for restoration of sinus rhythm from atrial fibrillation by direct current counter shocks. On admission, he had a coronavirus disease 2019 (COVID-19) infection and syncope during bed rest. Electrocardiography revealed polymorphic ventricular tachycardia after ST-segment elevation with a normal QT interval. Coronary angiography revealed coronary vasospasm. Coronary vasospasm may be a cause of polymorphic ventricular tachycardia in COVID-19 patients.
Keywords: arrythmia, infection, spasm
Introduction
Coronavirus disease 2019 (COVID-19) is associated with cardiovascular complications, including acute myocardial infarction, arrhythmia, cardiomyopathy, pericarditis, myocarditis, and thromboembolic and pulmonary vascular diseases (1,2). Coronary vasospasm has also been reported in COVID-19 (3-7). However, there have been no reports of polymorphic ventricular tachycardia (VT) associated with coronary vasospasm in patients with COVID-19.
We herein report a patient with COVID-19 and syncope due to polymorphic VT associated with coronary vasospasm and myocarditis.
Case Report
A 70-year-old man was admitted to our hospital for restoration of sinus rhythm from atrial fibrillation (AF) by direct current counter shocks. The patient had been treated for hypertension for 30 years. He had been admitted to the Department of Plastic Surgery in our hospital because of an ulcer in both lower legs 6 months previously. He then had heart failure with cardiac hypertrophy and AF and was transferred to our ward. At that time, cardiac coronary angiography revealed 50% stenosis in segment 6 of the left coronary artery, and a pressure study revealed only mild pulmonary hypertension (pulmonary artery pressure (systolic/diastolic/mean) of 30/8/20 mmHg, cardiac output of 5.6 L/min, and cardiac index of 2.9 L/min/m2). An endomyocardial biopsy of the right ventricle revealed myocyte hypertrophy only, suggesting hypertensive heart disease. Thus, the patient was treated with sacubitril/valsartan 200 mg/day, cilnidipine 10 mg/day, eplerenone 25 mg/day, empagliflozin 10 mg/day, and edoxaban 60 mg/day.
However, he experienced dyspnea on exertion after blood pressure control, and intervention for AF was considered. As the AF had lasted for more than 10 years, we decided to perform direct current counter-shock to AF before catheter ablation to check the sinus node function and sustainability of the sinus rhythm. His mother, brother, and sister also had hypertension.
A physical examination on admission revealed the following findings: blood pressure, 87/65 mmHg; irregular pulse rate, 83 beats per minute; body temperature, 36.7°C; body mass index, 26.9 kg/m2; and no abnormal findings, except for bilateral pretibial edema. Laboratory data revealed mildly high levels of high-sensitivity troponin T (0.035 ng/mL) and N-terminal pro-brain natriuretic peptide (NT-proBNP) (1,282 pg/mL) (Table 1).
Table 1.
Laboratory Data.
| WBC | 4,500 | /μL | BUN | 22 | mg/dL | |
| RBC | 4.28×106 | /μL | Cre | 1.0 | mg/dL | |
| Hb | 12.4 | g/dL | TP | 6.4 | g/dL | |
| Hct | 41.3 | % | Alb | 3.7 | g/dL | |
| Plt | 190×103 | /μL | UA | 5.9 | mg/dL | |
| T-Bil | 0.6 | mg/dL | FPG | 109 | mg/dL | |
| AST | 19 | IU/L | LDL-C | 129 | mg/dL | |
| ALT | 20 | IU/L | HDL-C | 67 | mg/dL | |
| ALP | 68 | IU/L | TG | 91 | mmol/L | |
| LDH | 203 | IU/L | HbA1c | 5.8 | % | |
| γ-GTP | 11 | IU/L | CRP | 0.07 | mg/dL | |
| CK | 242 | IU/L | NT-proBNP | 1,282 | pg/mL | normal range (<55) |
| hs-TnT | 0.035 | ng/mL | FT3 | 2.29 | pg/mL | normal range (2.3-4.0) |
| Na | 143 | mEq/L | FT4 | 1.04 | ng/dL | normal range (0.9-1.7) |
| K | 3.9 | mEq/L | TSH | 4.32 | μIU/mL | normal range (0.5-5.0) |
| Cl | 109 | mEq/L |
Alb: albumin, ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, BUN: blood urea nitrogen, CK: creatine kinase, Cre: creatinine, CRP: C-reactive protein, FPG: fasting plasma glucose, FT3: free triiodothyronine, FT4: free thyroxine, γ-GTP: γ-glutamyl transpeptidase, Hb: hemoglobin, HbA1c: hemoglobin A1c, Hct: hematocrit, HDL-C: high-density lipoprotein cholesterol , hs-TnT: high sensitive-troponin T, LDH: lactate dehydrogenase, LDL-C: low-density lipoprotein cholesterol, NT-proBNP: N-terminal pro brain natriuretic peptide, Plt: platelet count, RBC: red blood cell, T-Bil: total bilirubin, TG: triglyceride, TP: total protein, TSH: thyroid stimulating hormone, UA: uric acid, WBC: white blood cell
Chest radiography showed cardiomegaly (cardiothoracic ratio, 59%) (Fig. 1), and electrocardiography (ECG) revealed atrial fibrillation with normal ST-T and QT intervals (Fig. 1). Transthoracic echocardiography revealed concentric left ventricular hypertrophy (interventricular septum, 15 mm; posterior wall, 12 mm; end-diastolic dimension, 36 mm), a normal left ventricular systolic function (ejection fraction, 62%) without asynergy, and left atrial enlargement (left atrial diameter, 42 mm). Transesophageal echocardiography revealed no thrombi in the cardiac cavities, including the left atrial appendage.
Figure 1.
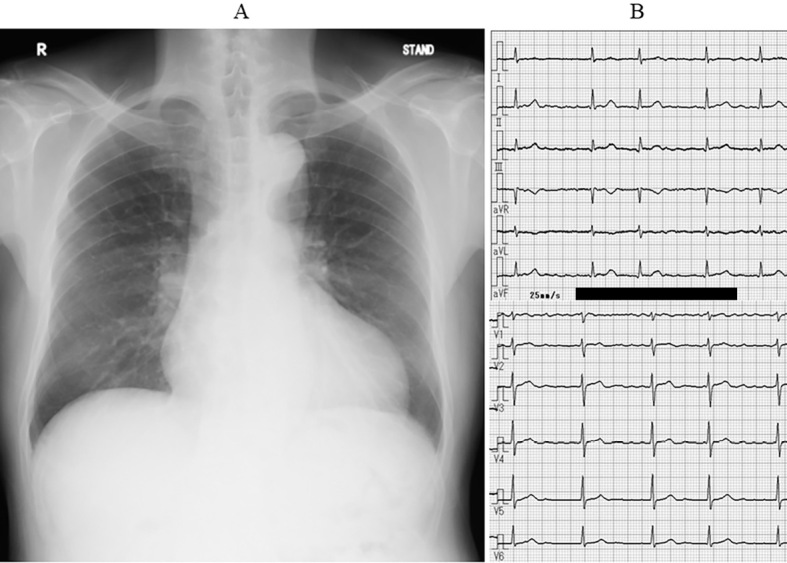
Chest radiography and electrocardiography on admission. Chest radiography showed cardiomegaly (A), and electrocardiography revealed atrial fibrillation with normal ST-T and QT intervals (B).
On day 2 of hospitalization, direct current counter shock was administered while the patient was prescribed pilsicainide (50 mg 3 times daily). His AF recovered to sinus rhythm, but he subsequently developed a fever and hypoxia after shock. Furthermore, AF recurred two days later, and pilsicainide was discontinued.
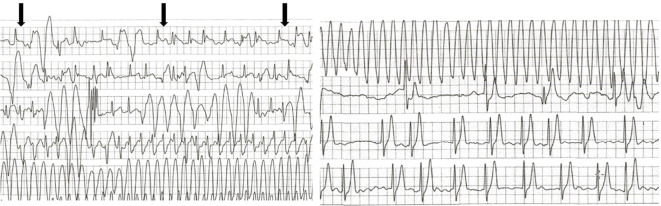
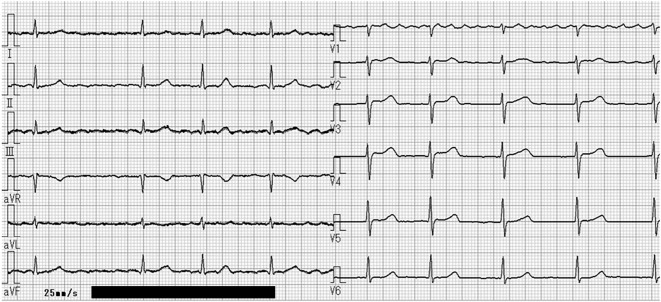
The COVID-19 antigen test was positive on day 3 of hospitalization, and the patient's infection was considered to have originated from his family before admission. He was quarantined, and remdesivir was initiated. On day 4 of hospitalization, the fever disappeared. However, on day 6 of hospitalization, he experienced a sudden onset of syncope during bed rest at approximately 8:00 pm. An ECG monitor revealed torsade de pointes for approximately 60 seconds after ST-segment elevation of the normal sinus beats and frequent premature ventricular contractions, which spontaneously resolved (Fig. 2). Immediately after the cessation of polymorphic VT, an ECG showed no ST-segment elevation or QT prolongation (Fig. 3). Thereafter, polymorphic VT was not observed.
Figure 2.
Electrocardiography monitor revealed torsade des pointe for approximately 60 seconds after ST-segment elevation (arrows) of the normal sinus beats and frequent premature ventricular contractions, which spontaneously recovered.
Figure 3.
Electrocardiography showed no ST-segment elevation and no QT prolongation (QTc interval, 446 ms) just after polymorphic ventricular tachycardia.
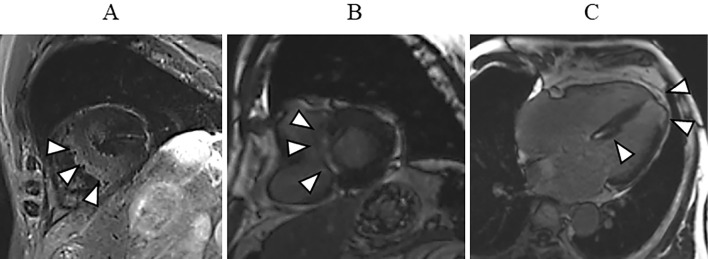
To investigate myocarditis due to COVID-19, cardiac magnetic resonance imaging (CMRI) was performed the day after 10-day quarantine. On CMRI, a T2-weighted black-blood MR image showed a high intensity in the interventricular septum (Fig. 4A). Delayed gadolinium enhancement was observed in the middle layer of the interventricular septum (Fig. 4B) and subendocardial wall of the left ventricular apex (Fig. 4C). These findings suggested myocarditis and/or ischemic change.
Figure 4.
In cardiac magnetic resonance imaging, a T2-weighted black-blood image showed high intensity in the interventricular septum (arrowheads) (A). Delayed gadolinium enhancement was seen in the middle layer of the interventricular septum (B, arrowheads; C, arrowheads), and subendocardial wall of the left ventricular apex (arrowheads) (C).
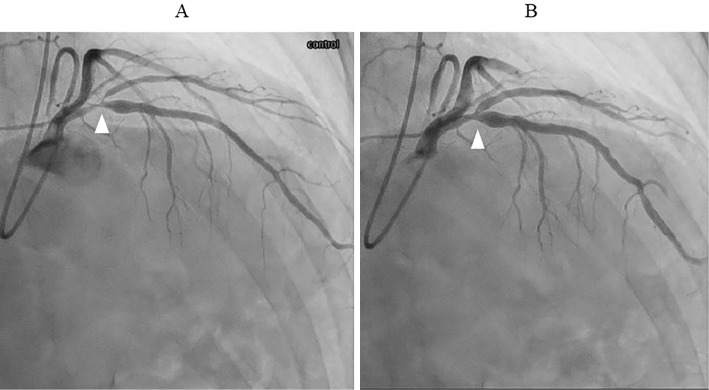
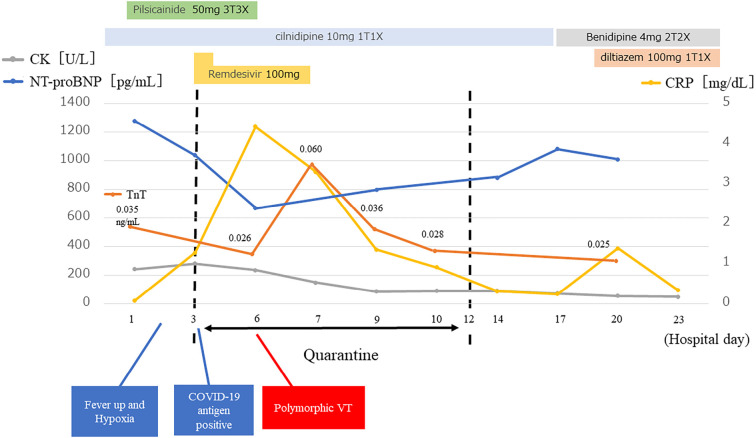
Coronary angiography two days after CMRI revealed 90% coronary stenosis in segment 6 of the left coronary artery without intracoronary nitroglycerine injection to evaluate coronary spasm, which was reduced by intracoronary nitroglycerine injection (Fig. 5). This indicated that coronary spasm occurred in the organic stenosis. Laboratory data showed that the C-reactive protein level was transiently elevated before polymorphic VT (Fig. 6). High-sensitivity troponin T also transiently increased after polymorphic VT, although creatine kinase levels did not change (Fig. 6). The NT-proBNP level decreased after AF recovery to sinus rhythm and increased after returning to AF (Fig. 6). Thus, we changed cilnidipine from 10 mg/day to benidipine 8 mg/day and diltiazem 100 mg/day.
Figure 5.
Coronary angiography revealed coronary stenosis in segment 6 of the left coronary artery (arrowhead) (A), and this stenosis disappeared after intracoronary nitroglycerine injection (arrowhead) (B).
Figure 6.
Time course of laboratory data. CK: creatine kinase, CRP: C-reactive protein, TnT: high-sensitivity troponin T, NT-proBNP: N-terminal pro brain natriuretic peptide, VT: ventricular tachycardia
The patient was diagnosed with polymorphic VT associated with coronary vasospasm and myocarditis due to COVID-19. His condition was stable, and he was discharged 19 days after admission. After discharge, the patient had no polymorphic VT for at least five months.
Discussion
We encountered a patient with COVID-19 who had polymorphic VT, coronary vasospasm, and myocarditis. Several mechanisms of coronary spasm have been proposed, including endothelial injury (2, 8), hyper-inflammatory response (cytokines) (9), hyperventilation (10), and myocarditis (11). However, the exact mechanism underlying coronary spasm in patients with COVID-19 remains unclear.
There have been five case reports of coronary spasm in patients with COVID-19 (Table 2) (3-7). Among the 6 patients, including our case (age, 37-75 years old; 5 men and 1 woman), 3 were Japanese, 3 had spontaneous coronary spasm, 4 had diffuse spasm, and 2, including our patient, had focal spasm. These previous reports have proposed that endothelial injuries through the angiotensin-converting enzyme 2 receptor and endothelial dysfunction due to increased inflammatory cytokines may induce coronary spasm (3-7).
Table 2.
Summary of Previous Reports and Our Report of Vasospastic Angina in Patients with COVID-19 Infection.
| Age (years) | Sex | Nationality | Coronary risk factor | MRI | VSA | Stenosis pattern | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | Spanish | None | n.e. | Provocative (Ergo) | Segmental | 3 |
| 2 | 61 | M | Turkish | n.m. | n.e. | Spontaneous | Diffuse | 4 |
| 3 | 62 | M | American | HT | n.e. | Spontaneous | LAD Segmental | 5 |
| 4 | 37 | M | Japanese | n.m. | T2 high intensity (+) LGE (+) | Provocative (Ach) | Diffuse | 6 |
| 5 | 65 | F | Japanese | HT, DM, smoking | BB T2 high intensity (+) LGE (+) | Provocative (Ach) | Diffuse | 7 |
| 6 | 75 | M | Japanese | HT | BB T2 high intensity (+) LGE (+) | Spontaneous | LAD Segmental | Present case |
Ach: acetylcholine, BB T2: black-blood T2, DM: diabetes mellitus, Ergo: ergonovine, F: female, HT: hypertension, LAD: left anterior descending branch of the left coronary artery, LGE: late gadolinium enhancement, M: male, n.e.: not examined, n.m.: not mentioned, VSA: vasospastic angina
In our patient, polymorphic VT related to coronary spasm occurred two days after the onset of COVID-19, although coronary spasm without polymorphic VT was later confirmed by coronary angiography. This suggests that the severe coronary spasm that induces polymorphic VT may be related to endothelial dysfunction due to a hyper-inflammatory response or cytokines.
Coronary spasm may occur in patients with pre-existing atherosclerotic coronary lesions (12). In the present patient, vasospasm in the pre-existing lesion was confirmed because coronary angiography had been performed one year before because of heart failure. Thus, COVID-19 infection, in addition to pre-existing atherosclerosis, may have induced vasospasm in the present patient. Furthermore, CMRI in three of these six patients, including our patient, suggested myocarditis or myocardial infarction and myocardial edema due to coronary spasm (6,7). Thus, there may be a relationship between coronary spasm and myocarditis in patients with COVID-19.
Stress CMRI would have been an excellent method to prove microvascular dysfunction/myocardial infarction with nonobstructive coronary arteries/coronary vasospasm (13). Unfortunately, stress CMRI cannot be performed in our hospital. Thus, we could not determine the direct cause of torsade de pointes in our patient. However, an ECG monitor revealed torsade de pointes just after transient ST-segment elevation of the normal sinus beats. This indicated that torsade des pointe was due to coronary spasm rather than myocarditis.
Cardiac arrhythmias are more common in critically ill patients with COVID-19. In a previous study, atrial fibrillation was the most frequent tachyarrhythmia, followed by premature ventricular contraction/non-sustained VT, paroxysmal supraventricular tachycardia, sustained VT/ventricular fibrillation, and polymorphic ventricular tachycardia/torsade de pointes (14). The most frequently reported bradyarrhythmias are sinus bradycardia and complete heart block (14). The potential mechanisms that could result in arrhythmogenesis among patients with COVID-19 include hypoxia caused by direct viral tissue involvement in the lungs, myocarditis, an abnormal host immune response, myocardial ischemia, myocardial strain, electrolyte derangements, intravascular volume imbalances, and drug side effects (15).
The most worrisome arrhythmogenic mechanism is the QT-prolonging effect of various anti-COVID pharmacotherapies that can lead to polymorphic VT in the form of torsade de pointes and sudden cardiac death (16). In patients with myocarditis, polymorphic and irregular ventricular arrhythmias are more common during the active inflammatory phase, whereas monomorphic and regular ventricular arrhythmias are associated with healed myocarditis (17).
Nishizaki et al. (18) reported that vasospastic attacks, even if asymptomatic, immediately precede the development of polymorphic VT, which may be associated with a repolarization abnormality and an increased risk of sudden death. There has been only one case report of polymorphic VT with a normal QTc interval in a patient with COVID-19 (19). This report suggested a correlation between a fever and VT (19). However, there have been no reports of polymorphic VT associated with coronary vasospasm in COVID-19 patients. In our patient, the transient ST-segment elevation preceding polymorphic VT indicated that coronary vasospasm induced polymorphic VT.
In conclusion, coronary vasospasm should be considered as a potential cause of polymorphic VT in patients with COVID-19.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 141: 1903-1914, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giustino G, Pinney SP, Lala A, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol 76: 2011-2023, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivero F, Antuña P, Cuesta J, Alfonso F. Severe coronary spasm in a COVID-19 patient. Catheter Cardiovasc Interv 97: E670-E672, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sümerkan M, Er Kara A, Doğan GM, Alyan Ö. Spontaneous, severe, and diffuse coronary vasospasm in a patient with COVID-19. Anatol J Cardiol 25: E36-E37, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Talon A, Saririan M. COVID-19 cardiac arrest due to Prinzmetal's angina in a previously normal heart. Clin Case Rep 9: e04205, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aikawa T, Ogino J, Oyama-Manabe N, Funayama N. Vasospastic angina: a cause of post-acute COVID-19 syndrome. Intern Med 61: 2693-2695, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azuma M, Kato S, Murohashi K, et al. Severe multivessel coronary vasospasm in a patient with coronavirus disease 2019. J Cardiol Cases 28: 147-149, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res 116: 2177-2184, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033-1034, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman LJ, Nixon PG. Chest pain and the hyperventilation syndrome--some aetiological considerations. Postgrad Med J 61: 957-961, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yilmaz A, Mahrholdt H, Athanasiadis A, et al. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 94: 1456-1463, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Ong P, Aziz A, Hansen HS, Prescott E, Athanasiadis A, Sechtem U. Structural and functional coronary artery abnormalities in patients with vasospastic angina pectoris. Circ J 79: 1431-1438, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Patel AR, Salerno M, Kwong RY, Singh A, Heydari B, Kramer CM. Stress cardiac magnetic resonance myocardial perfusion imaging: JACC review topic of the week. J Am Coll Cardiol 78: 1655-1668, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol 59: 329-336, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol 6: 1193-1204, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med 30: 451-460, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peretto G, Sala S, Rizzo S, et al. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J Am Coll Cardiol 75: 1046-1057, 2020. [DOI] [PubMed] [Google Scholar]
- 18. Nishizaki M, Arita M, Sakurada H, et al. Polymorphic ventricular tachycardia in patients with vasospastic angina - clinical and electrocardiographic characteristics and long-term outcome. Jpn Circ J 65: 519-525, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Babapoor-Farrokhran S, Port Z, Wiener PC, Amanullah A, Mainigi SK. Polymorphic ventricular tachycardia with a normal QTc interval in a patient with COVID-19 and fever: case report. SN Compr Clin Med 2: 2387-2390, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]