Abstract
Objective
The current standard treatment for locally advanced, unresectable stage III non-small-cell lung cancer (NSCLC) is concurrent chemoradiation therapy (CCRT) and durvalumab administration. Although reports have indicated that the prognosis of squamous cell carcinoma is poorer than that of adenocarcinoma, real-world data are currently inadequate.
Methods
The present study analyzed patients with stage III NSCLC who received CCRT at the study center between April 2018 and February 2022. These patients were retrospectively classified into adenocarcinoma and squamous cell carcinoma groups for an analysis of the progression-free survival (PFS), overall survival (OS), and patient background factors, including the age, performance status, smoking history, and pre-CCRT laboratory data.
Results
A total of 109 patients were included for the analysis; 25 were excluded, and 44 and 40 patients were classified into the adenocarcinoma and squamous cell carcinoma groups, respectively. The median PFS was significantly longer in the adenocarcinoma group than in the squamous cell carcinoma group [27.9 (95% confidence interval (CI): 15.2-not achieved) vs. 9.63 (95% CI: 5.88-13.9) months; p<0.01]. Similarly, the median OS was significantly longer in the adenocarcinoma group than in the squamous cell carcinoma group [not achieved (95% CI: 48.1-not achieved) vs. 23.8 (95% CI; 14.6-not achieved) months; p<0.01]. In the multivariate Cox proportional hazard analysis, the histological type was the only prognostic factor for the PFS (p<0.05) and OS (p<0.05).
Conclusion
The median PFS and OS were poorer in patients with squamous cell carcinoma than in those with stage III NSCLC treated with CCRT and durvalumab. The histological type was an independent factor affecting the PFS and OS.
Keywords: chemoradiation therapy, immune checkpoint inhibitor, non-small-cell lung cancer, durvalumab
Introduction
Concurrent chemoradiation therapy (CCRT) is the standard treatment for locally advanced, unresectable stage III non-small-cell lung cancer (NSCLC) (1). The PACIFIC study published in 2017 reported that durvalumab, an immune checkpoint inhibitor, given every two weeks after CCRT for NSCLC until progressive disease (PD) or for one year significantly the prolonged progression-free survival (PFS) and overall survival (OS) compared to a placebo (2,3). Since then, adjuvant durvalumab therapy has become the standard treatment for unresectable stage III NSCLC.
A sub-analysis of the PACIFIC study reported that this treatment was effective for both squamous and non-squamous histological types, but squamous cell carcinoma tended to have a worse PFS than non-squamous cell carcinoma. Furthermore, other studies have reported that squamous cell carcinoma tends to have a worse prognosis than adenocarcinoma (4-6). However, some reports have indicated no marked differences in histology (7-9).
The terms “non-squamous cell carcinoma” and “non-adenocarcinoma” were used in those previous reports. Large-cell carcinoma, poorly differentiated carcinoma, polymorphous carcinoma, not otherwise specified (NOS), and NSCLC, for which the diagnosis of adenocarcinoma or squamous cell carcinoma could not be made owing to the lack of specimens, were included in those studies. Thus, there are insufficient real-world data on the histology-specific prognosis of adenocarcinoma and squamous cell carcinoma treated with CCRT and durvalumab.
Other reports have claimed that serum albumin (Alb), serum C-reactive protein (CRP), and blood hemoglobin (Hb) pretreatment values are useful prognostic factors for lung cancer (10-15). Furthermore, the Glasgow Prognostic Score (GPS), based on serum CRP and Alb values (16,17), can predict the prognosis of patients receiving immune checkpoint inhibitor therapy (18). However, the applicability of these factors in predicting the prognosis of patients receiving CCRT followed by adjuvant durvalumab is not well understood. In addition, although the PACIFIC study reported a better prognosis for patients who received durvalumab <14 days after receiving radiation therapy than for those who received durvalumab for ≥14 days (19), real-world data are needed to verify this.
Therefore, the present retrospective analysis of patients with unresectable stage III NSCLC investigated the prognostic value of adenocarcinoma versus squamous cell carcinoma and other factors.
Materials and Methods
Patients and study design
The present study enrolled patients with unresectable stage III NSCLC who received CCRT at the Tokyo Metropolitan Cancer and Infectious Diseases Center of Komagome Hospital between April 2018 and February 2022. The patients were followed-up until May 2023. The clinical data of each patient were obtained from medical records. The enrolled patients were divided into adenocarcinoma or squamous cell carcinoma groups, and clinical variables, including the PFS and OS, were compared between the groups. In addition, the PFS and OS were compared to identify factors other than histology. For continuous variables, receiver operating characteristic (ROC) curves were used to identify and classify the locations where the greatest differences were found in terms of progressive disease (PD) and death. A multivariate analysis was performed for factors that differed significantly in the univariate analysis.
In accordance with Japanese guidelines, intensity-modulated radiation therapy with a target radiation dose of 60 Gy was administered. The anticancer drugs combined with radiation therapy included cisplatin plus vinorelbine, cisplatin plus S-1, carboplatin plus paclitaxel, carboplatin plus nab-paclitaxel, carboplatin plus weekly paclitaxel, and low-dose carboplatin. After CCRT, patients were evaluated for the treatment effect by the Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1 (20), and received durvalumab if they do not have PD. Patients who were deemed eligible for durvalumab by the attending physician received durvalumab as soon as possible. The ethics committee of the Tokyo Metropolitan Cancer and Infectious Diseases Center at Komagome Hospital approved this study (approval number: 3143) and waived the requirement for informed consent. Refusal to participate was permitted using the opt-out method.
Data collection
The age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), serum Alb level, serum CRP level, GPS (16,17), blood Hb level, smoking history, programmed cell death ligand 1 (PD-L1) tumor proportion score (TPS), TNM classification (21), and effect of treatment according to RECIST were investigated. Blood tests were performed on the morning of the day of CCRT or on the previous day. Lung cancer was diagnosed based on lung or lymph node biopsy findings. The PD-L1 TPS was evaluated only in patients who were measured. Furthermore, the CCRT efficacy and number of patients who were able to receive durvalumab, were able to receive durvalumab for one year, discontinued durvalumab for reasons other than PD, had PD, had PD during durvalumab administration, and were unable to receive second-line therapy after PD were investigated. The time from the end of radiation therapy to durvalumab administration was also examined.
Statistical analyses
All values are expressed as the mean±standard deviation (SD). Fisher's exact test, the Mann-Whitney U test, and t-test were used to compare baseline patient characteristics. The OS and PFS were assessed using the Kaplan-Meier curve, and differences were compared using the log-rank test. The OS was defined as the time from the start of chemoradiotherapy to the time of death, and the PFS was defined as the time from the start of chemoradiotherapy to the time of death or PD. A multivariate Cox regression analysis was performed to analyze factors that differed significantly in the univariate analysis. Statistical significance was set at p<0.05.
All statistical analyses were performed using EZR version 1.54 (Jichi Medical University, Saitama Medical Center, Saitama, Japan) (22).
Results
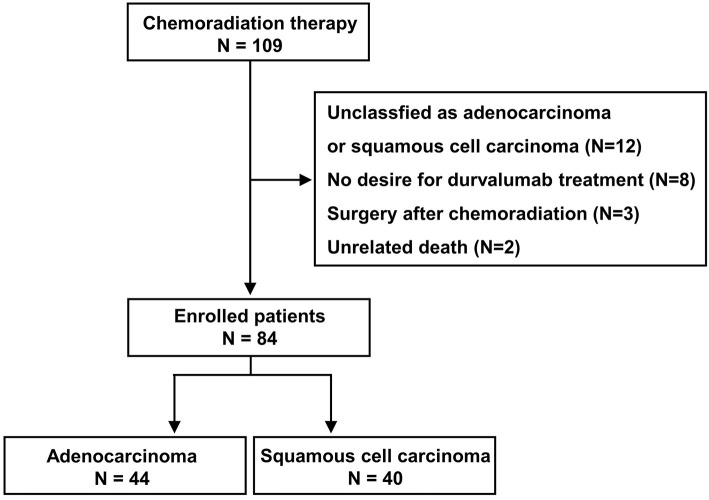
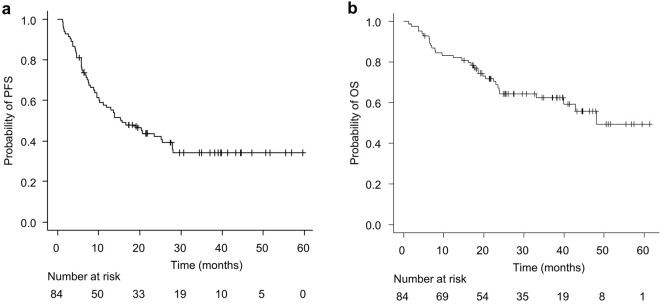
One hundred and nine patients were included in this study. Patients whose histology could not be classified as adenocarcinoma or squamous cell carcinoma, did not wish to be treated with durvalumab after CCRT, were treated surgically after CCRT, or died of a cause unrelated to lung cancer or treatment were excluded. Therefore, 84 patients were ultimately enrolled, and of these, 44 and 40 were classified into adenocarcinoma and squamous cell carcinoma groups, respectively (Fig. 1). The characteristics of the enrolled patients are shown in Table 1. The median age of the patients was 69 (45-89) years old; 66 patients (78.6%) were men, and 72 patients (85.7%) were treated with durvalumab after CCRT. Fig. 2 shows the Kaplan-Meier curves of the PFS and OS. The median PFS was 15.6 [95% confidence interval (CI): 10.2-25.4] months, and the median OS was 48.1 (95% CI: 39.9-not achieved) months.
Figure 1.
Flow chart of the study population.
Table 1.
Characteristics of Patients.
| Characteristics | |
| No. of patients | 84 |
| Age, y (range) | 69 (45-89) |
| Male, n (%) | 66 (78.6) |
| Current or former somoker, n (%) | 79 (94.0) |
| ECOG-PS, n (%) | |
| 0 | 35 (41.7) |
| 1 | 44 (52.4) |
| 2 or 3 | 5 (6.0) |
| Laboratory data, mg/dL (range) | |
| Alb | 3.8 (1.4-4.6) |
| CRP | 0.75 (0.02-13.3) |
| Hb | 12.9 (6.7-16.7) |
| GPS, n (%) | |
| 0 | 42 (50.0) |
| 1 | 22 (26.2) |
| 2 | 20 (23.8) |
| PD-L1 TPS, n (%) | |
| ≥50 | 22 (36.7) |
| 1-49 | 19 (31.7) |
| <1 | 19 (31.7) |
| TNM classification | |
| T factor | |
| T1-3, n (%) | 49 (58.3) |
| T4, n (%) | 35 (41.7) |
| N factor (N3) | |
| N1-2, n (%) | 65 (77.4) |
| N3, n (%) | 19 (22.6) |
| Treatment regimen | |
| CDDP+VNR, n (%) | 57 (67.9) |
| Low dose CBDCA, n (%) | 14 (16.7) |
| CBDCA+PTX, n (%) | 5 (6.0) |
| CBDCA+weekly PTX, n (%) | 5 (6.0) |
| CDDP+S-1, n (%) | 2 (2.4) |
| CBDCA+nab-PTX, n (%) | 1 (1.2) |
| RECIST after CCRT | |
| CR, n (%) | 1 (1.2) |
| PR, n (%) | 58 (69.0) |
| SD, n (%) | 20 (23.8) |
| PD, n (%) | 5 (6.0) |
| Durvalumab treatment, n (%) | 72 (85.7) |
| Last radiation to durvalumab treatment | |
| <14 days, n (%) | 47 (65.3) |
The median values for age and laboratory data were shown. Only PD-L1 TPS was measured by 60 patients; all other factors were measured by all patients. y: years, ECOG: Eastern Cooperative Oncology Group, PS: performance status, Alb: albumin, CRP: C-reactive protein, Hb: hemoglobin, GPS: Glasgow Prognostic Score, PD-L1: programmed cell death ligand 1, TPS: tumor proportion score, CDDP: cisplatin, VNR: vinorelbine, CBDCA: carboplatin, PTX: paclitaxel, CCRT: concurrent chemoradiation therapy, RECIST: response evaluation criteria in solid tumor, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease
Figure 2.
Kaplan-Meier curves showing the progression-free survival (a) and overall survival (b) in the study cohort.
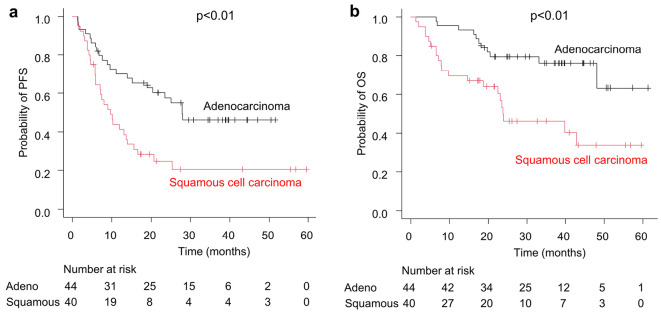
The age, ECOG-PS, serum albumin level, serum CRP level, blood Hb level, GPS, and T factor of 4 of TNM classification differed significantly between the adenocarcinoma and squamous cell carcinoma groups (Table 2). Fig. 3 shows the Kaplan-Meier curves for the PFS and OS in both groups. The median PFS of the squamous cell carcinoma group [9.63 (95% CI: 5.88-13.9) months] was significantly shorter than that of the adenocarcinoma group [27.9 (95% CI: 15.2-not achieved) months] (p<0.01), and the median OS of the squamous cell carcinoma group [23.8 (95% CI: 14.6-not achieved) months] was also significantly shorter than that of the adenocarcinoma group [not achieved (95% CI: 48.1-not achieved) months] (p<0.01).
Table 2.
Characteristics of Patients according to Histology.
| Adeno | Squamous | p value | |
|---|---|---|---|
| No. of patients | 44 | 40 | |
| Age, y (range) | 67 (45-89) | 71.5 (55-85) | <0.01 |
| Male, n (%) | 35 (79.5) | 31 (77.5) | 1 |
| Current or former smoker, n (%) | 39 (88.6) | 40 (100.0) | 0.057 |
| ECOG-PS, n (%) | |||
| 0 | 24 (54.5) | 11 (27.5) | <0.05 |
| 1 | 19 (43.2) | 25 (62.5) | 0.085 |
| 2 or 3 | 1 (0.23) | 4 (10.0) | 0.187 |
| Laboratory data, mg/dL (range) | |||
| Alb | 4.0 (1.4-4.6) | 3.6 (1.8-4.5) | <0.01 |
| CRP | 0.29 (0.02-7.07) | 1.13 (0.04-13.3) | <0.01 |
| Hb | 13.6 (6.7-16.7) | 12.1 (7.7-16.2) | <0.01 |
| GPS, n (%) | |||
| 0 | 27 (61.4) | 15 (37.5) | <0.05 |
| 1 | 12 (27.3) | 10 (25.0) | 1 |
| 2 | 5 (11.4) | 15 (37.5) | <0.01 |
| PD-L1 TPS, n (%) | |||
| ≥50 | 14 (42.4) | 8 (30.0) | 0.321 |
| 1-49 | 11 (33.3) | 8 (30.0) | 0.812 |
| <1 | 8 (24.2) | 11 (40.7) | 0.613 |
| TMN classification | |||
| T factor (T4), n (%) | 13 (29.5) | 22 (55.0) | <0.05 |
| N factor (N3), n (%) | 13 (29.5) | 6 (15.0) | 0.126 |
| Treatment regimen | |||
| CDDP+VNR, n (%) | 34 (77.3) | 23 (57.5) | 0.064 |
| Low dose CBDCA, n (%) | 5 (11.4) | 9 (22.5) | 0.243 |
| CBDCA+PTX, n (%) | 1 (2.3) | 4 (10.0) | 0.187 |
| CBDCA+weekly PTX, n (%) | 2 (4.6) | 3 (7.5) | 0.665 |
| CDDP+S-1, n (%) | 1 (2.3) | 1 (2.5) | 1 |
| CBDCA+nab-PTX, n (%) | 1 (2.3) | 0 (0) | 0.476 |
The median values for age and laboratory data were shown. Adeno: adenocarcinoma, Squamous: squamous cell carcinoma, y: years, ECOG: Eastern Cooperative Oncology Group, PS: performance status, Alb: albumin, CRP: C-reactive protein, Hb: hemoglobin, GPS: Glasgow Prognostic Score, PD-L1: programmed cell death ligand 1, TPS: tumor proportion score, CDDP: cisplatin, VNR: vinorelbine, CBDCA: carboplatin, PTX: paclitaxel
Figure 3.
Kaplan-Meier curves showing the progression-free survival (a) and overall survival (b) in the adenocarcinoma group (n=44) and squamous cell carcinoma group (n=40).
The CCRT efficacy and durvalumab treatment course were also investigated (Table 3). While the proportion of patients able to receive durvalumab did not differ markedly between the groups, the proportion of those able to receive durvalumab for one year did. Recurrence during durvalumab treatment did not differ markedly between the group, but the proportion of patients who were unable to receive second-line therapy did differ.
Table 3.
Clinical Manifestation according to Histology.
| Adeno | Squamous | p value | |
|---|---|---|---|
| No. of patients | 44 | 40 | |
| RECIST after CCRT, n (%) | |||
| PR or CR | 33 (75.0) | 26 (65.0) | 0.348 |
| PD | 2 (4.5) | 3 (7.5) | 0.665 |
| Durvalumab treatment, n (%) | 39 (88.6) | 33 (82.5) | 0.537 |
| Last radiation to durvalumab treatment | |||
| <14 days, n (%) | 25 (64.1) | 22 (66.7) | 1 |
| Administrated for one year | 23 (59.0) | 11 (33.3) | <0.05 |
| Discontinuation due to causes other than PD | 6 (15.3) | 10 (30.3) | 0.160 |
| PD in term of durvalumab treatment | 10 (25.6) | 12 (36.4) | 0.468 |
| Total of PD, n (%) | 22 (50.0) | 30 (75.0) | <0.05 |
| BSC after 1st line | 1 (4.5) | 11 (36.7) | <0.01 |
RECIST: response evaluation criteria in solid tumor, CCRT: concurrent chemoradiation therapy, PR: partial response, CR: complete response, PD: progressive disease, BSC: best supportive care
In addition to histology, other factors, such as the age, sex, ECOG-PS, serum albumin, serum CRP, blood Hb, PD-L1 expression, T factor, and N factor, were also classified into two groups and compared in terms of the PFS (Table 4). To compare the age, ECOG-PS, serum Alb, serum CRP, blood Hb, and PD-L1 expression, the groups were divided according to the point at which the ROC curves indicated the greatest difference. The groups differed significantly in terms of the histology (p<0.01) and Hb levels (p<0.05). A multivariate analysis revealed that the histology was a significant, independent, predictive factor of PD [hazard ratio (HR): 2.11; 95% CI: 1.18-3.78; p<0.05] after adjusting for the histology and Hb. Similarly, the OS was examined after dividing the groups by the point at which the ROC curves indicated the greatest difference (Table 5). The groups differed significantly in terms of the histology (p<0.01), age (p<0.05), ECOG-PS (p<0.05), serum Alb level (p<0.05), blood Hb level (p<0.01), and PD-L1 expression (p<0.05). A multivariate analysis revealed that histology was a significant independent predictive factor of death (HR: 2.32; 95% CI: 1.00-5.37; p<0.05) after adjusting for the histology, age, ECOG-PS, serum Alb, Hb, and PD-L1 expression.
Table 4.
Log-lank Test and Univariate and Multivariate Analysis for Progression Free Survival.
| Factor | Category | Median PFS, m (95% CI) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| p value | HR (95% CI) | p value | ||||
| Histology | Adeno | 27.9 (15.2-NA) | 1 | |||
| Squamous | 9.63 (5.88-13.9) | <0.01 | 2.11 (1.18-3.78) | <0.05 | ||
| Age (years) | <63 | 27.9 (9.63-NA) | ||||
| ≥63 | 13.9 (7.95-25.1) | 0.21 | ||||
| Gender | Female | 14.1 (7.20-27.9) | ||||
| Male | 19.1 (9.53-28.0) | 0.58 | ||||
| ECOG-PS | 0, 1 | 16.6 (10.2-27.9) | ||||
| 2, 3 | 4.57 (1.23-NA) | 0.28 | ||||
| Alb (mg/dL) | ≥3.8 | 19.1 (9.53-28.0) | ||||
| <3.8 | 13.0 (7.20-27.9) | 0.68 | ||||
| CRP (mg/dL) | ≤0.05 | NA (20.4-NA) | ||||
| >0.05 | 13.9 (9.03-25.1) | 0.06 | ||||
| GPS | 0 | 23.5 (11.1-NA) | ||||
| 1, 2 | 10.2 (5.82-25.1) | 0.09 | ||||
| Hb (mg/dL) | ≥12.9 | 28.0 (10.2-NA) | 1 | |||
| <12.9 | 11.9 (7.20-23.5) | <0.05 | 1.10 (0.95-1.28) | 0.27 | ||
| PD-L1 (%) | >1 | 15.6 (9.03-NA) | ||||
| ≤1 | 6.97 (4.40-13.9) | 0.11 | ||||
| T factor | T1-T3 | 20.4 (10.1-NA) | ||||
| T4 | 13.0 (6.21-27.9) | 0.21 | ||||
| N factor | N3 | NA (4.67-NA) | ||||
| N0-2 | 13.6 (8.84-20.4) | 0.06 | ||||
Age, ECOG-PS, Alb, CRP, Hb, PD-L1, T factor and N factor were divided into two groups where the most difference occurred with reference to ROC curve. m: months, HR: hazard ratio, CI: confidence interval, ECOG: Eastern Cooperative Oncology Group, PS: performance status, Alb: albumin, CRP: C-reactive protein, GPS: Glasgow Prognostic Score, Hb: hemoglobin, PD-L1: programmed cell death ligand 1
Table 5.
Log-lank Test and Univariate and Multivariate Analysis for Overall Survival.
| Factor | Category | Median OS, m (95% CI) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| p value | HR (95% CI) | p value | ||||
| Histology | Adeno | NA (48.1-NA) | 1 | |||
| Squamous | 23.8 (14.6-NA) | <0.01 | 2.32 (1.00-5.37) | <0.05 | ||
| Age (years) | <75 | NA (39.9-NA) | 1 | |||
| ≥75 | 23.9 (6.47-NA) | <0.05 | 1.02 (0.97-1.07) | 0.40 | ||
| Gender | Female | NA (22.4-NA) | ||||
| Male | 48.1 (39.9-NA) | 0.86 | ||||
| ECOG-PS | 0, 1 | 48.1 (39.9-NA) | 1 | |||
| 2, 3 | 5.02 (1.22-NA) | <0.05 | 1.62 (0.69-3.82) | 0.27 | ||
| Alb (mg/dL) | ≥3.3 | NA (39.9-NA) | 1 | |||
| <3.3 | 23.9 (6.41-NA) | <0.05 | 1.15 (0.42-3.06) | 0.80 | ||
| CRP (mg/dL) | ≤1.2 | NA (33.1-NA) | ||||
| >1.2 | 42.9 (16.8-NA) | 0.15 | ||||
| GPS | 0, 1 | NA (39.9-NA) | ||||
| 2 | 23.9 (7.06-NA) | 0.06 | ||||
| Hb (mg/dL) | ≥12.4 | NA (NA-NA) | 1 | |||
| <12.4 | 23.9 (16.8-48.1) | <0.01 | 1.22 (0.93-1.61) | 0.15 | ||
| PD-L1 TPS (%) | >10 | NA (23.9-NA) | 1 | |||
| ≤10 | 23.0 (14.6-48.1) | <0.05 | 1.01 (1.00-1.02) | 0.07 | ||
| T factor | T1-T3 | NA (42.9-NA) | ||||
| T4 | 39.9 (16.8-NA) | 0.09 | ||||
| N factor | N3 | NA (NA-NA) | ||||
| N0-2 | 48.1 (23.8-NA) | 0.15 | ||||
Age, ECOG-PS, Alb, CRP, Hb, PD-L1, T factor and N factor were divided into two groups where the most difference occurred with reference to ROC curve. m: months, HR: hazard ratio, CI: confidence interval, Adeno: adenocarcinoma, Squamous: squamous cell carcinoma, ECOG: Eastern Cooperative Oncology Group, PS: performance status, Alb: albumin, CRP: C-reactive protein, GPS: Glasgow Prognostic Score, Hb: hemoglobin, PD-L1: programmed cell death ligand 1, TPS: tumor proportion score
The PACIFIC study reported a better prognosis for patients who received durvalumab for <14 days after receiving radiation therapy than for those who received durvalumab for ≥14 days (19). Duration from the date of completion of radiotherapy to durvalumab treatment was classified into two groups and compared in terms of the PFS and OS (Table 6). To compare the duration groups, the patients were divided according to the point at which the ROC curves indicated the greatest difference. The greatest difference in PFS occurred when patients were classified by 11 days from the date of completion of radiotherapy to the date of durvalumab administration. The greatest difference in OS occurred when patients were classified by 19 days from the date of completion of radiotherapy to the date of durvalumab administration. When the duration from radiation therapy to durvalumab treatment was classified as 19 days, there was a significant difference in the OS (p<0.05). There was no marked difference in the prognosis when patients were classified by 14 days, when a difference was reported in the previous report (19).
Table 6.
Log-lank Test for Progression-free Survival and Overall Survival about the Term of Last Radiation to Durvalumab Treatment.
| Last radiation to durvalumab treatment (days) | Median PFS, m (95% CI) | p value | Median OS, m (95% CI) | p value |
|---|---|---|---|---|
| <11 | 25.4 (13.0-NA) | NA (NA-NA) | ||
| ≥11 | 20.4 (9.63-NA) | 0.753 | 48.1 (23.9-NA) | 0.315 |
| <19 | 20.7 (11.9-NA) | NA (42.9-NA) | ||
| ≥19 | 23.5 (5.75-NA) | 0.808 | 33.1 (6.5-NA) | <0.05 |
| <14 | 23.5 (10.2-NA) | NA (48.1-NA) | ||
| ≥14 | 20.7 (10.2-NA) | 0.765 | 42.9 (20.4-NA) | 0.173 |
The term of last radiation therapy to durvalumab treatment was divided into two groups where the most difference occurred with reference to ROC curve and 14 days. m: months, HR: hazard ratio, CI: confidence interval, PFS: progression-free survival, OS: overall survival
In summary, the median PFS and OS were poorer in patients with squamous cell carcinoma than in those with adenocarcinoma in unresectable stage III NSCLC cases treated with CCRT and durvalumab. The histological type was an independent factor affecting the PFS and OS.
Discussion
The median PFS was shorter in the present study than in the PACIFIC-R study, which aimed to validated real-world data concerning the use of the PACIFIC regimen (15.6 months vs. 21.7 months) (6). The possible reasons for this discrepancy were that the median age in the PACIFIC-R study was 66.0 years old, while in our study, it was 69.0 years old, and the percentage of never smokers in the PACIFIC-R study was 7.9%, while in our study, it was 6.0%. The median PFS by histology in the PACIFIC-R study was 25.3 months for non-squamous cell carcinoma and 14.6 months for squamous cell carcinoma, whereas the values were 27.9 months for adenocarcinoma and 9.63 months for squamous cell carcinoma in the present study. Furthermore, the percentage of squamous cell carcinoma in the PACIFIC-R study was 36.0%, while in the present study, it was 47.6%. The prognosis of squamous cell carcinoma was poor in both studies. However, the PFS was poorer in the present study, possibly because of the higher proportion of squamous cell carcinoma cases.
Although the PACIFIC-R study and the present study found similar trends, the terms, “non-squamous cell carcinoma” and “non-adenocarcinoma” have been used in several reports, including the PACIFIC-R study, making a simple comparison with the present study difficult (2-9). Clinically, it is incongruous to include adenocarcinoma, poorly differentiated carcinoma, polymorphous carcinoma, and large-cell carcinoma in the same non-squamous cell carcinoma population. In the present study, the prognosis was also compared between the squamous cell carcinoma and non-squamous cell carcinoma groups, and the results were almost the same as those of squamous cell carcinoma and adenocarcinoma (data not shown). The small number of patients in this study who could not be classified as either adenocarcinoma or squamous cell carcinoma may have resulted in similar prognoses for the classification of squamous cell carcinoma and adenocarcinoma and for the classification of squamous cell carcinoma and non-squamous cell carcinoma, and the prognosis of pure linear carcinoma and squamous cell carcinoma needs to be clarified. This study is valuable for its novelty in presenting the prognosis of unresectable stage III adenocarcinoma and squamous cell carcinoma.
The difference in the prognosis between adenocarcinoma and squamous cell carcinoma was thought to have emerged after the start of durvalumab administration, as no marked difference was found between the groups in the post-CCRT efficacy assessment using RECIST (Table 3). The Kaplan-Meier curves for the PFS and OS also demonstrated that the difference between the groups was more pronounced after CCRT, which lasted approximately two months (Fig. 3a, b). In terms of the prognosis, patients with squamous cell carcinoma were in poor condition compared with those with adenocarcinoma in terms of the age, ECOG-PS, Alb, CRP, GPS, Hb, and T factor at the start of CCRT (Table 2), possibly explaining the differences observed in the PFS and OS. Furthermore, although the groups did not differ markedly in the proportion of patients receiving durvalumab, they did differ in the proportion of patients receiving durvalumab for one year. The groups also differed in the proportion of patients with the best supportive care who were unable to receive second-line therapy (Table 3). Several reports suggested that squamous cell carcinoma may be less responsive to immunotherapy than adenocarcinoma (23,24). In summary, squamous cell carcinoma had worse values than adenocarcinoma in several factors before the start of chemoradiation therapy, and squamous cell carcinoma was less likely to respond to immunotherapy than adenocarcinoma, which might have influenced the difference in the prognosis shown in this study.
The multivariate analysis demonstrated that histology was an independent prognostic factor for the PFS and OS. A univariate analysis of the PFS demonstrated differences in the histology and Hb levels (Table 4); indeed, significant differences in median Hb levels were also observed when classified between adenocarcinoma and squamous cell carcinoma (Table 2). The lack of a significant difference in the Hb level in the multivariate analysis indicated that the Hb level had no independent prognostic impact. In contrast, a univariate analysis of the OS demonstrated differences in the histology, age, ECOG-PS, Alb, Hb, and PD-L1 TPS, although a multivariate analysis demonstrated no marked differences in terms of any of these factors (Table 5). Table 2 shows that, with the exception of the PD-L1 TPS, certain differences were observed between the adenocarcinoma and squamous cell carcinoma groups. These factors were considered to have influenced each other and did not differ markedly in the multivariate analysis. The GPS, which is reportedly able to predict the prognosis of various malignancies, was not a significant factor in the present study, possibly in part because no marked difference was found in the CRP levels in the univariate analysis (Tables 4, 55).
Significant differences in the OS were observed when the time from the end of radiation therapy to the start of durvalumab administration was set as 19 days (Table 6). In contrast, no marked difference in the PFS was observed when the ROC curve was used to determine the period of greatest difference. Further studies are needed to determine whether or not the time between the end of radiation therapy and administration of durvalumab affects the prognosis in a larger number of patients. However, the prognosis tended to be better in those with a shorter duration, as previously reported (19).
Several limitations associated with the present study warrant mention. First, it was a monocentric, non-randomized, retrospective study. Therefore, examining all possible prognostic factors and excluding patient selection bias is not feasible. Second, the type of chemotherapy used in combination with radiation therapy varied, and its potentially variable effect on the PFS and OS could not be ruled out. Third, only a few patients were analyzed for PD-L1 expression. If the OS analysis had included a larger cohort, multivariate analysis may have detected some differences. However, the strength of the present study is that it was based on real-world data concerning unresectable stage III NSCLC treated with CCRT and durvalumab.
In conclusion, the median PFS and OS were poorer in patients with squamous cell carcinoma than in those with adenocarcinoma among unresectable stage III NSCLC patients treated with CCRT and durvalumab. The histological type was an independent factor affecting the PFS and OS. A prospective, large-scale, multicenter study with a validation cohort is necessary to verify the present findings. The development of more effective therapies for unresectable stage III NSCLC, particularly squamous cell carcinoma, is warranted.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med 125: 723-729, 1996. [DOI] [PubMed] [Google Scholar]
- 2. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377: 1919-1929, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379: 2342-2350, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Desilets A, Blanc-Durand F, Lau S, et al. Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non-small cell lung cancer: a real-world multicentre study. Eur J Cancer 142: 83-91, 2021. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto T, Tsukita Y, Katagiri Y, et al. Durvalumab after chemoradiotherapy for locally advanced non-small cell lung cancer prolonged distant metastasis-free survival, progression-free survival and overall survival in clinical practice. BMC Cancer 22: 364, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Girard N, Bar J, Garrido P, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol 18: 181-193, 2023. [DOI] [PubMed] [Google Scholar]
- 7. Wang CC, Chiu LC, Ju JS, et al. Durvalumab as consolidation therapy in post-concurrent chemoradiation (CCRT) in unresectable stage III non-small cell lung cancer patients: a multicenter observational study. Vaccines 9: 1122, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verschueren MV, Dijs T, Gulikers JL, et al. Durvalumab after chemoradiotherapy in patients with stage III non-small-cell lung cancer: real-world outcomes versus clinical trial results. Immunotherapy 15: 839-851, 2023. [DOI] [PubMed] [Google Scholar]
- 9. Matsuura S, Serizawa S, Yamashita R, et al. The prognostic nutritional index before durvalumab after chemoradiation predict the overall survival in patients with stage III non-small cell lung cancer. Ann Med 55: 2196089, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topkan E, Bolukbasi Y, Ozdemir Y, Besen AA, Mertsoylu H, Selek U. Prognostic value of pretreatment Glasgow prognostic score in stage IIIB geriatric non-small cell lung cancer patients undergoing radical chemoradiotherapy. J Geriatr Oncol 10: 567-572, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Yamauchi Y, Safi S, Muley T, et al. C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung Cancer 114: 62-67, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Laurie SA, Ding K, Whitehead M, et al. The impact of anemia on outcome of chemoradiation for limited small-cell lung cancer: a combined analysis of studies of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol 18: 1051-1055, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka H, Ono T, Manabe Y, et al. Anemia is a prognostic factor for overall survival rate in patients with non-small cell lung cancer treated with stereotactic body radiation therapy. Cancer Manag Res 13: 7447-7453, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klümper N, Saal J, Berner F, et al. C reactive protein flare predicts response to checkpoint inhibitor treatment in non-small cell lung cancer. J Immunother Cancer 10: e004024, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jia-Min Z, Wei D, Ye L, Xiang-Tao P. Correlation between C-reactive protein/albumin ratio and prognosis in patients with lung adenocarcinoma. J Int Med Res 50: 3000605221105372, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. BR J Cancer 89: 1028-1030, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 90: 1704-1706, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Chen S, Chen H, Li W. A comprehensive analysis of Glasgow Prognostic Score (GPS)/the modified Glasgow Prognostic Score (mGPS) on immune checkpoint inhibitor efficacy among patients with advanced cancer. Cancer Med 12: 38-48, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 40: 1301-1311, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247, 2009. [DOI] [PubMed] [Google Scholar]
- 21. Goldstraw P, Chansky K, Crowley J, et al.; the International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11: 39-51, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol 41: 1213-1227, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahashabde R, Bhatti SA, Martin BC, et al. Real-world survival of first-line immune checkpoint inhibitor treatment versus chemotherapy in older patients with non-small-cell lung cancer and synchronous brain metastases. JCO Oncol Pract 19: 1009-1019, 2023. [DOI] [PubMed] [Google Scholar]