Abstract
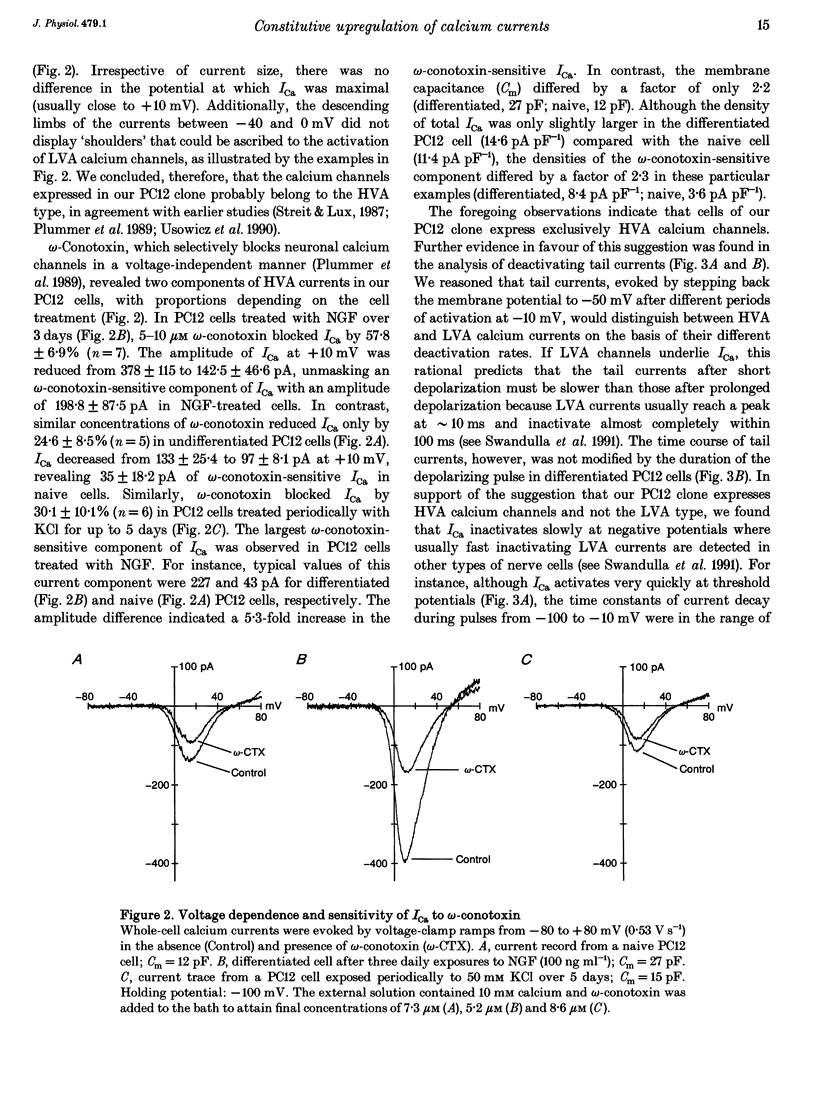
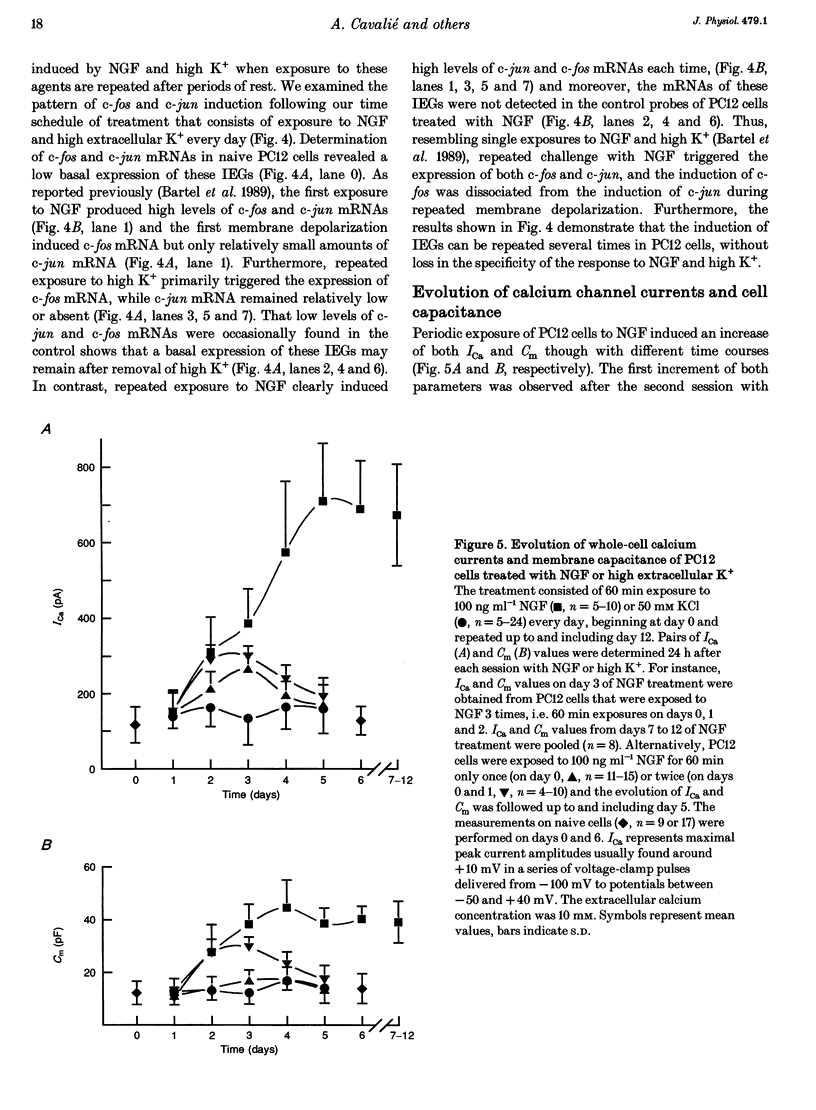
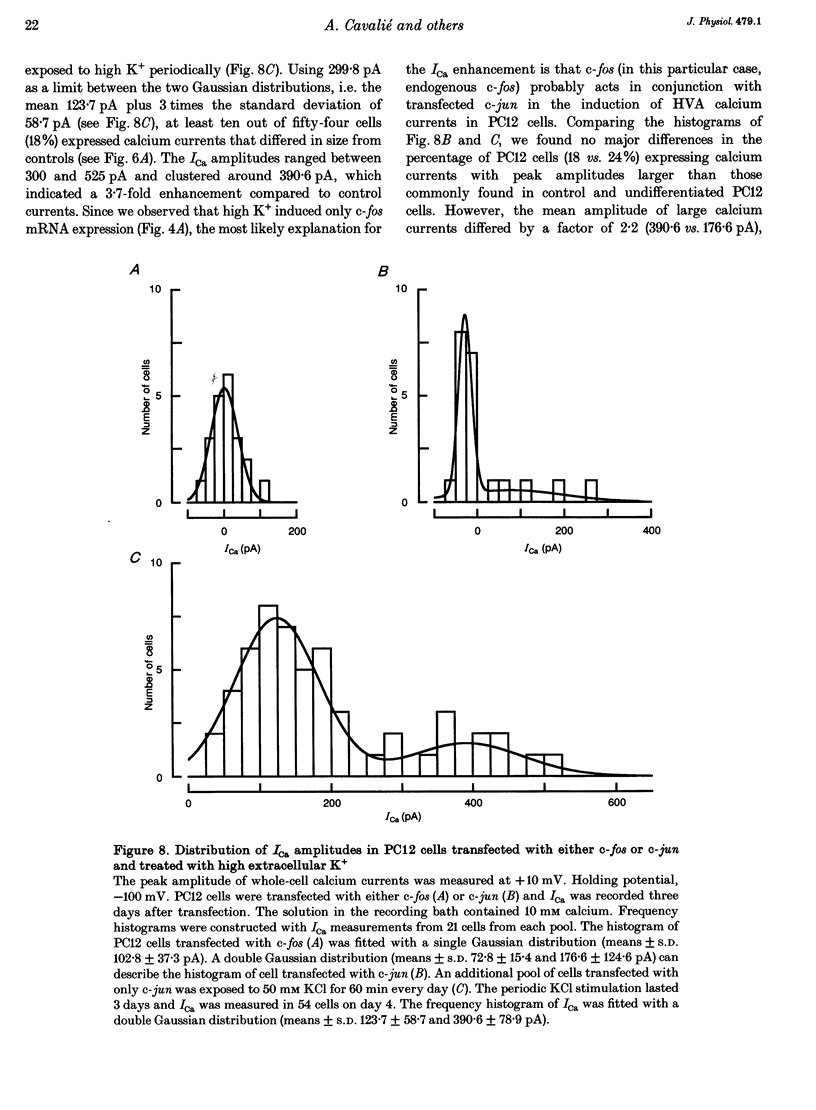
1. Northern blot analysis and cell transfection were used in conjunction with whole-cell current recordings to examine the involvement of the immediate early genes, c-fos and c-jun, in the expression of calcium channel currents. 2. Phaeochromocytoma cells (PC12 clone) were exposed to nerve growth factor (NGF) and to depolarizing concentrations of KCl for 60 min every day. Cells challenged with NGF developed extensive networks of neurites within 3 days. Cells depolarized periodically retained their undifferentiated morphology even after 5 days of treatment. 3. The maximal amplitude of high-voltage-activated calcium currents (ICa) increased from the control level of 117.8 +/- 48.3 (mean +/- S.D.) to 387.2 +/- 90.1 pA within 3 days of NGF treatment. omega-Conotoxin (5-10 microM) inhibited 24.6 +/- 8.5% of ICa in undifferentiated cells and 57.8 +/- 6.9% in NGF-treated cells. 4. The levels of c-fos and c-jun mRNAs increased transiently during each daily exposure to NGF. The level of c-fos mRNA also increased transiently during repeated KCl-induced depolarizations but c-jun mRNA remained low or absent. 5. Naive PC12 cells were transiently co-transfected with expression plasmids that contained the full length of c-fos and c-jun cDNA. After 2 days following transfection, the PC12 cells could be grouped according to the size of ICa. In 56% of cells, ICa was similar to control currents (106.1 +/- 37.4 pA). In the remaining 44% of cells, ICa showed a 2.2-fold enhancement with respect to control cells. Transfection of only c-fos had no effect on ICa but, in 24% of cells transfected with c-jun, ICa was 176.6 +/- 124.6 pA. Since periodic membrane depolarization induced c-fos but not c-jun mRNA, c-jun transfection was combined with a high-K+ treatment over 3 days. In 18% of treated cells, ICa was 3.7 times larger than control currents. Morphological differentiation was not observed in transfected cells. 6. In PC12 cells co-transfected with c-fos and c-jun or treated with high K+ after transfection of c-jun, omega-conotoxin (5-10 microM) inhibited 68.7 +/- 11.9% of ICa when the current amplitude was in the range of 200-600 pA. since similar concentrations of omega-conotoxin blocked 19.2 +/- 5.4% of ICa in control cells, the current increase induced by c-fos and c-jun was supported by up to 11-fold enhancement of the omega-conotoxin-sensitive component of ICa.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Hescheler J., Krautwurst D., Schultz G., Trautwein W. Calcium currents of neuroblastoma x glioma hybrid cells after cultivation with dibutyryl cyclic AMP and nickel. Pflugers Arch. 1990 Nov;417(3):329–335. doi: 10.1007/BF00371000. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Franklin J. L., Fickbohm D. J., Willard A. L. Long-term regulation of neuronal calcium currents by prolonged changes of membrane potential. J Neurosci. 1992 May;12(5):1726–1735. doi: 10.1523/JNEUROSCI.12-05-01726.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C., Sumikawa K., Lynch G. Levels of mRNA for a putative kainate receptor are affected by seizures. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7643–7647. doi: 10.1073/pnas.87.19.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber S. S., Hoshi T., Aldrich R. W. Regulation of ionic currents in pheochromocytoma cells by nerve growth factor and dexamethasone. J Neurosci. 1989 Nov;9(11):3976–3987. doi: 10.1523/JNEUROSCI.09-11-03976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García D. E., Cavalié A., Lux H. D. Enhancement of voltage-gated Ca2+ currents induced by daily stimulation of hippocampal neurons with glutamate. J Neurosci. 1994 Feb;14(2):545–553. doi: 10.1523/JNEUROSCI.14-02-00545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty D. D., Fanger G. R., Wagner J. A., Maue R. A. The activity of cAMP-dependent protein kinase is required at a posttranslational level for induction of voltage-dependent sodium channels by peptide growth factors in PC12 cells. J Cell Biol. 1992 Mar;116(6):1465–1473. doi: 10.1083/jcb.116.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K., Rohrer H., Lux H. D. Distribution of Ca2+ and Na+ conductances during neuronal differentiation of chick DRG precursor cells. J Neurosci. 1991 Nov;11(11):3371–3378. doi: 10.1523/JNEUROSCI.11-11-03371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Haas Carola A., Reddington Martin, Kreutzberg Georg W. Calcitonin Gene-related Peptide Stimulates the Induction of c-fos Gene Expression in Rat Astrocyte Cultures. Eur J Neurosci. 1991 Jul;3(7):708–712. doi: 10.1111/j.1460-9568.1991.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hengerer B., Lindholm D., Heumann R., Rüther U., Wagner E. F., Thoenen H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc Natl Acad Sci U S A. 1990 May;87(10):3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Murphy T. H., Worley P. F., Baraban J. M. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991 Oct;7(4):625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Passafaro M., Clementi F., Sher E. Metabolism of omega-conotoxin-sensitive voltage-operated calcium channels in human neuroblastoma cells: modulation by cell differentiation and anti-channel antibodies. J Neurosci. 1992 Sep;12(9):3372–3379. doi: 10.1523/JNEUROSCI.12-09-03372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Ross A. F., Green W. N., Hartman D. S., Claudio T. Efficiency of acetylcholine receptor subunit assembly and its regulation by cAMP. J Cell Biol. 1991 May;113(3):623–636. doi: 10.1083/jcb.113.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Streit J., Lux H. D. Voltage dependent calcium currents in PC12 growth cones and cells during NGF-induced cell growth. Pflugers Arch. 1987 May;408(6):634–641. doi: 10.1007/BF00581167. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaur M. L., Sheng M., Lowenstein D. H., Jan Y. N., Jan L. Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992 Jun;8(6):1055–1067. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Porzig H., Becker C., Reuter H. Differential expression by nerve growth factor of two types of Ca2+ channels in rat phaeochromocytoma cell lines. J Physiol. 1990 Jul;426:95–116. doi: 10.1113/jphysiol.1990.sp018128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. D., Gall C. M., McKelvy J. F. Enkephalin biosynthesis and enkephalin gene expression are increased in hippocampal mossy fibers following a unilateral lesion of the hilus. J Neurosci. 1987 Mar;7(3):753–759. doi: 10.1523/JNEUROSCI.07-03-00753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. E., Brust P. F., Feldman D. H., Patthi S., Simerson S., Maroufi A., McCue A. F., Veliçelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992 Jul 17;257(5068):389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Yaari Y., Hamon B., Lux H. D. Development of two types of calcium channels in cultured mammalian hippocampal neurons. Science. 1987 Feb 6;235(4789):680–682. doi: 10.1126/science.2433765. [DOI] [PubMed] [Google Scholar]
- Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




