Abstract
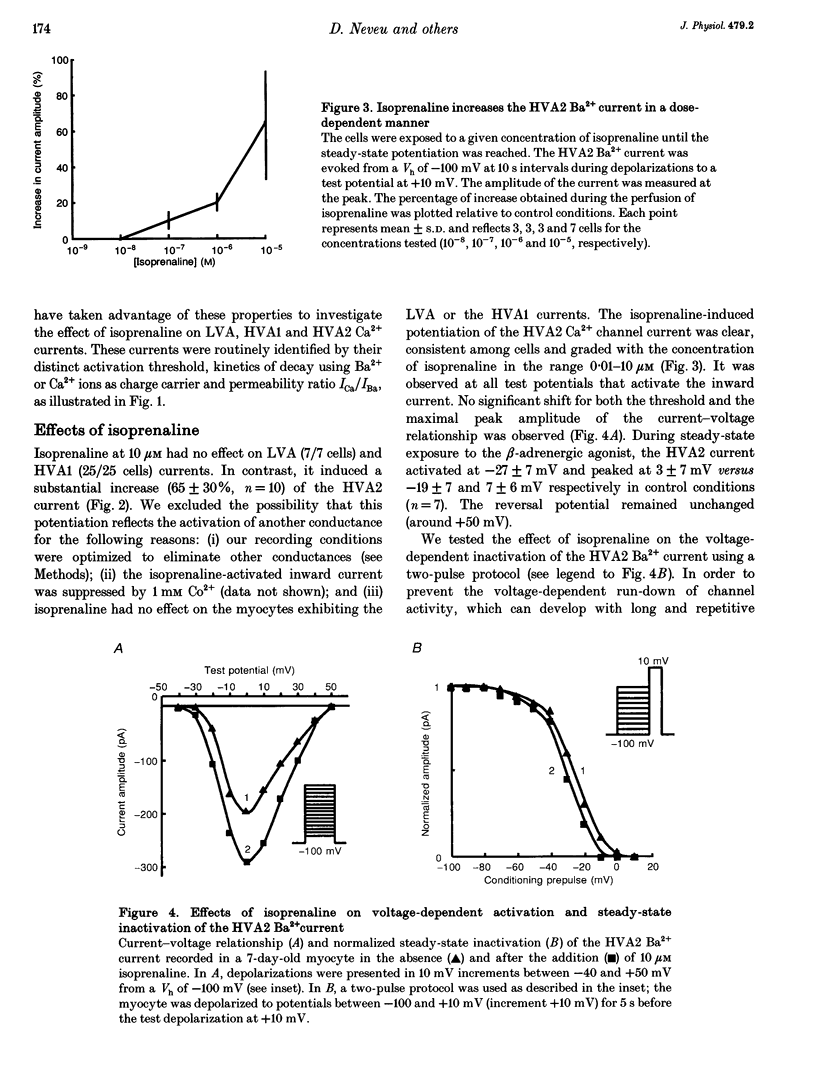
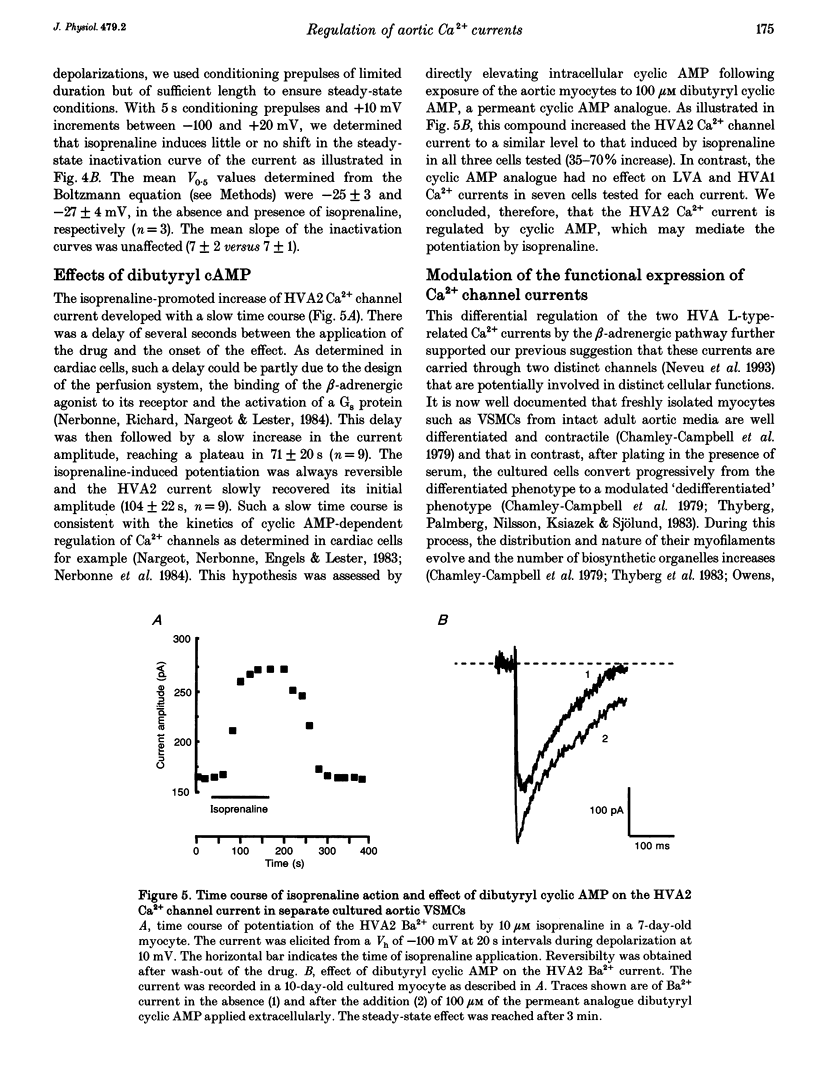
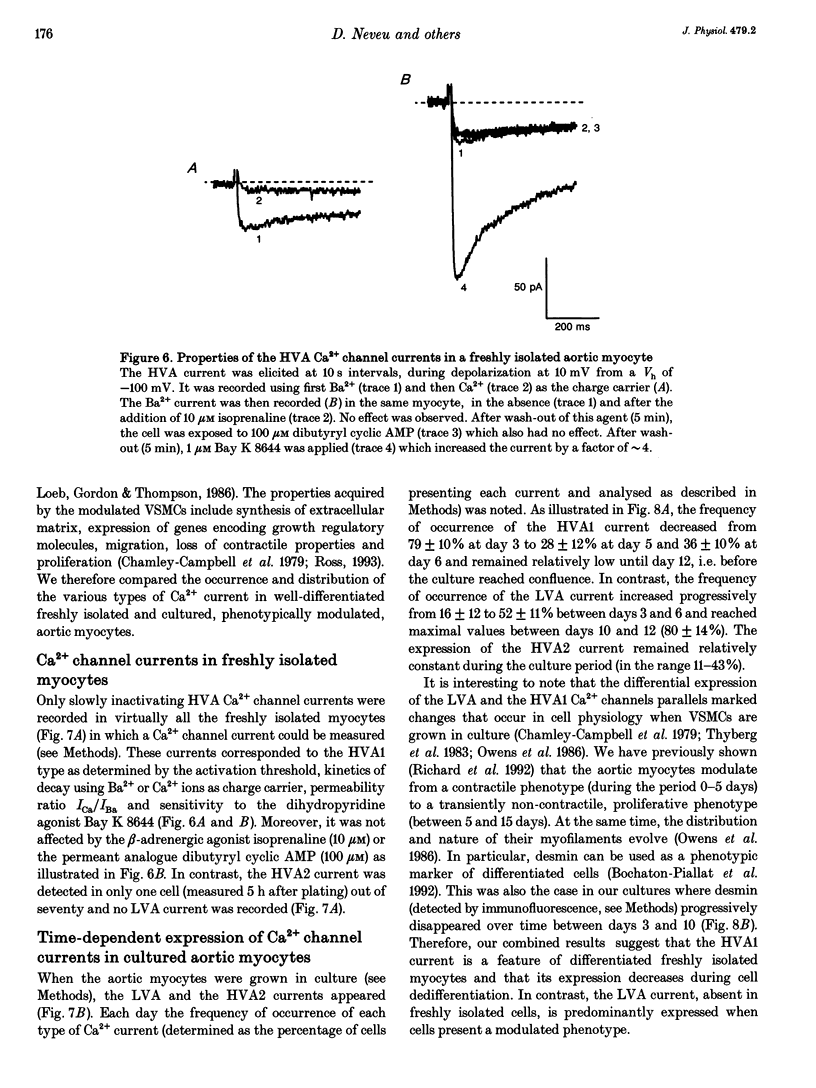
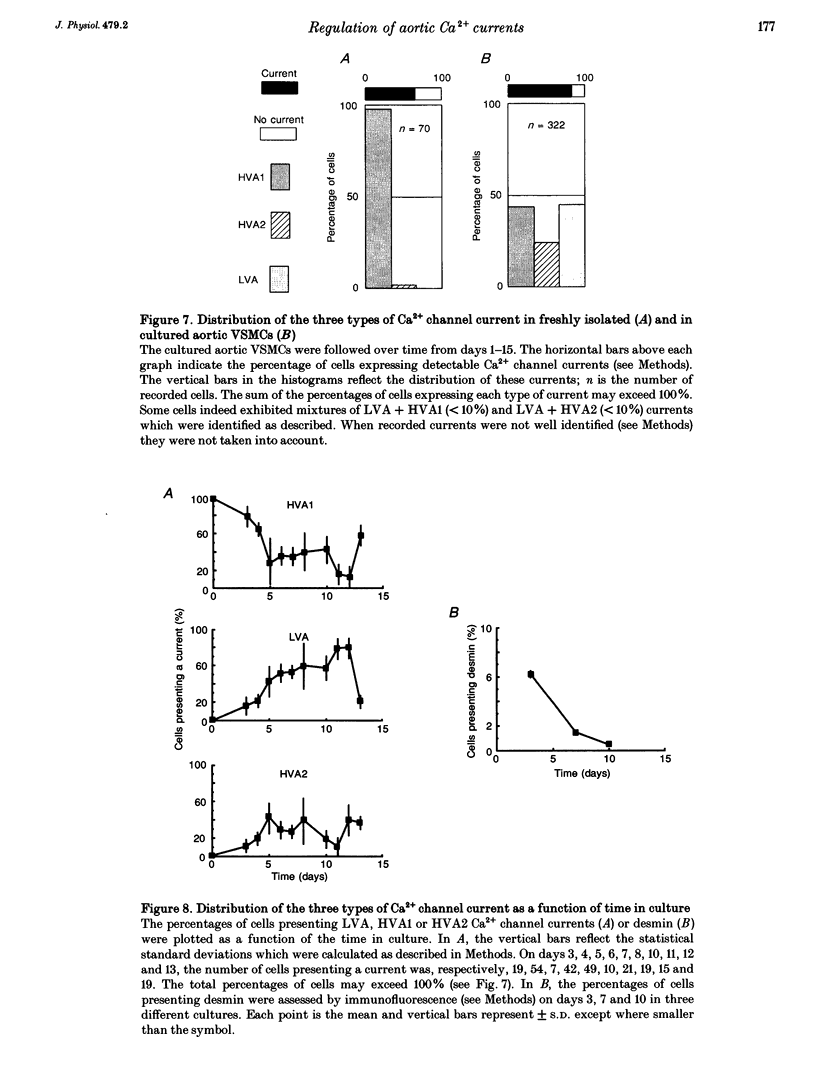
1. We studied the beta-adrenergic regulation of voltage-gated Ca2+ channel currents using the whole-cell patch-clamp technique (18-22 degrees C) in freshly isolated and in cultured (1-20 days) rat aortic vascular smooth muscle cells (VSMCs). These currents include a transient low-voltage-activated (LVA) current and two L-type-related high-voltage-activated currents (HVA1 and HVA2, respectively). 2. At 10 microM, the beta-adrenergic agonist, isoprenaline, increased the HVA2 current (65 +/- 30%, n = 10) but had no effect on LVA and HVA1 currents. This potentiation was dose dependent in the range 0.01-10 microM, developed with a slow time course and was mimicked by elevating intracellular cyclic AMP using the permeant analogue dibutyryl cyclic AMP (100 microM). 3. In the well-differentiated freshly isolated myocytes, only the HVA1 current was recorded. In cultured cells, a predominant frequency of occurrence of LVA and HVA1 currents was observed in modulated and differentiated myocytes, respectively. The occurrence of the HVA2 current was stable during culture but this current disappeared when the cells were confluent. It was retrieved when the confluent cells were dispersed and subcultured. 4. In conclusion, we present evidence for a differential beta-adrenergic regulation of three types of Ca2+ channel current in adult rat aortic VSMCs. The differential expression of these currents, associated with marked changes in cell phenotypes in vitro, suggests that they serve distinct physiological functions.
Full text
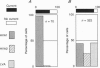
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Lindsay J. G. Hydroxyurea reversal of inhibition and use as a cell-synchronizing agent. J Biol Chem. 1967 Mar 25;242(6):1314–1317. [PubMed] [Google Scholar]
- Akaike N., Kanaide H., Kuga T., Nakamura M., Sadoshima J., Tomoike H. Low-voltage-activated calcium current in rat aorta smooth muscle cells in primary culture. J Physiol. 1989 Sep;416:141–160. doi: 10.1113/jphysiol.1989.sp017754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A., Pacaud P., Loirand G., Mironneau C., Mironneau J. Pharmacological block of Ca(2+)-activated Cl- current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1991 Dec;419(6):553–558. doi: 10.1007/BF00370294. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Peyrow M., Yamamoto T., Sculptoreanu A., Jacques D., Sperelakis N. Macroscopic Ca2+ -Na+ and K+ currents in single heart and aortic cells. Mol Cell Biochem. 1988 Mar-Apr;80(1-2):59–72. doi: 10.1007/BF00231004. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat M. L., Gabbiani F., Ropraz P., Gabbiani G. Cultured aortic smooth muscle cells from newborn and adult rats show distinct cytoskeletal features. Differentiation. 1992 Apr;49(3):175–185. doi: 10.1111/j.1432-0436.1992.tb00665.x. [DOI] [PubMed] [Google Scholar]
- Bodin P., Richard S., Travo C., Berta P., Stoclet J. C., Papin S., Travo P. Responses of subcultured rat aortic smooth muscle myocytes to vasoactive agents and KCl-induced depolarization. Am J Physiol. 1991 Jan;260(1 Pt 1):C151–C158. doi: 10.1152/ajpcell.1991.260.1.C151. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Pacaud P. Voltage-dependent calcium channels of smooth muscle. Jpn J Pharmacol. 1992;58 (Suppl 2):251P–257P. [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation. 1992 Sep;86(3):723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- Castellano A., Wei X., Birnbaumer L., Perez-Reyes E. Cloning and expression of a neuronal calcium channel beta subunit. J Biol Chem. 1993 Jun 15;268(17):12359–12366. [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Charnet P., Richard S., Gurney A. M., Ouadid H., Tiaho F., Nargeot J. Modulation of Ca currents in isolated frog atrial cells studied with photosensitive probes. Regulation by cAMP and Ca2+: a common pathway? J Mol Cell Cardiol. 1991 Mar;23(3):343–356. doi: 10.1016/0022-2828(91)90070-3. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991 Sep;76(5):677–693. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Declerck I., Casteels R. Effect of adrenergic agonists on Ca2+-channel currents in single vascular smooth muscle cells. Pflugers Arch. 1987 Jun;409(1-2):7–12. doi: 10.1007/BF00584744. [DOI] [PubMed] [Google Scholar]
- Fukumitsu T., Hayashi H., Tokuno H., Tomita T. Increase in calcium channel current by beta-adrenoceptor agonists in single smooth muscle cells isolated from porcine coronary artery. Br J Pharmacol. 1990 Jul;100(3):593–599. doi: 10.1111/j.1476-5381.1990.tb15852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Koch W. J., Ellinor P. T., Schwartz A. cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J Biol Chem. 1990 Oct 15;265(29):17786–17791. [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
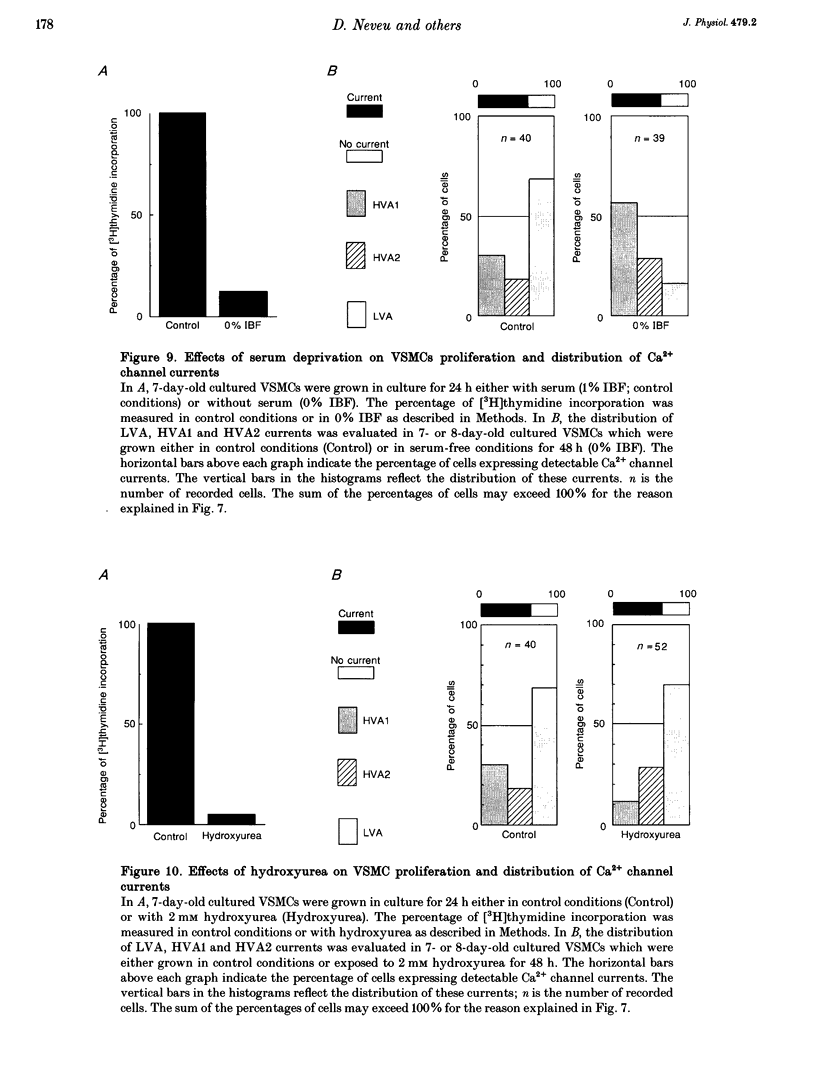
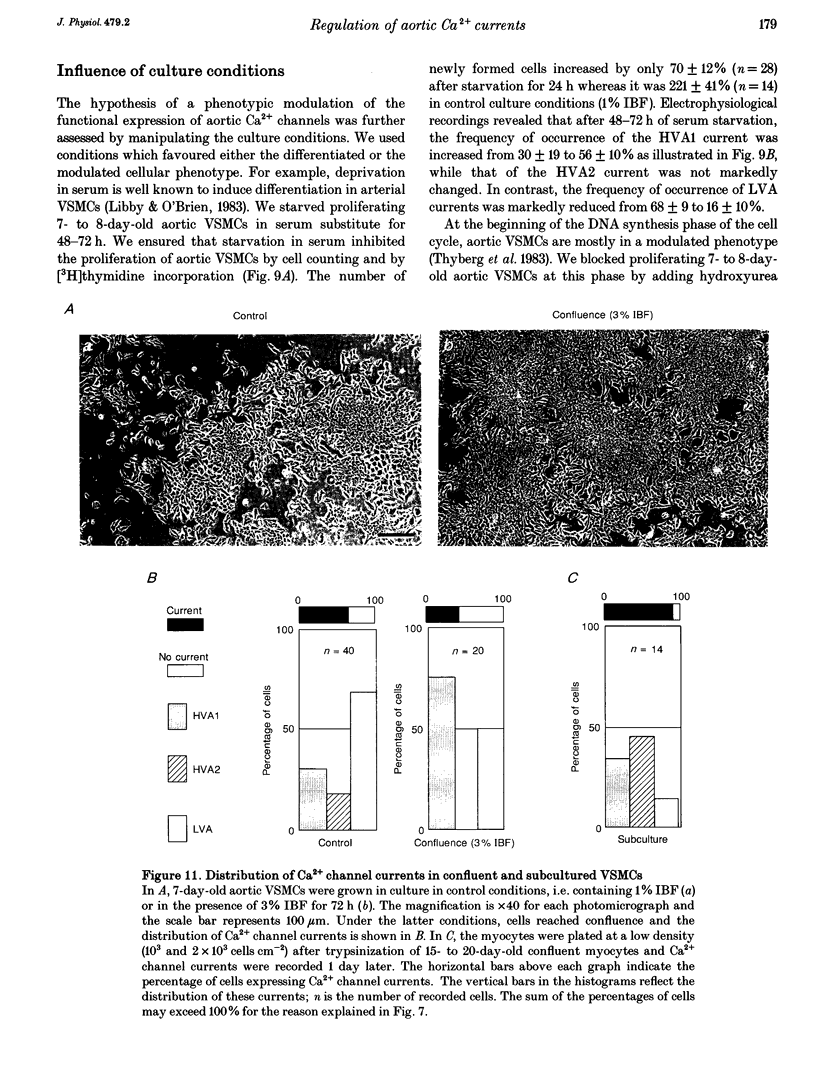
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Marks T. N., Dubyak G. R., Jones S. W. Calcium currents in the A7r5 smooth muscle-derived cell line. Pflugers Arch. 1990 Dec;417(4):433–439. doi: 10.1007/BF00370664. [DOI] [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne J. M., Richard S., Nargeot J., Lester H. A. New photoactivatable cyclic nucleotides produce intracellular jumps in cyclic AMP and cyclic GMP concentrations. Nature. 1984 Jul 5;310(5972):74–76. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- Neveu D., Nargeot J., Richard S. Two high-voltage-activated, dihydropyridine-sensitive Ca2+ channel currents with distinct electrophysiological and pharmacological properties in cultured rat aortic myocytes. Pflugers Arch. 1993 Jun;424(1):45–53. doi: 10.1007/BF00375101. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Richard S., Neveu D., Carnac G., Bodin P., Travo P., Nargeot J. Differential expression of voltage-gated Ca(2+)-currents in cultivated aortic myocytes. Biochim Biophys Acta. 1992 Nov 10;1160(1):95–104. doi: 10.1016/0167-4838(92)90042-c. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Reiner P. B. Ca2+ channels: diversity of form and function. Curr Opin Neurobiol. 1992 Jun;2(3):247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Soong T. W., Stea A., Hodson C. D., Dubel S. J., Vincent S. R., Snutch T. P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993 May 21;260(5111):1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. The effects of isoproterenol on intracellular calcium concentration. J Biol Chem. 1988 Jan 15;263(2):762–768. [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Tiaho F., Richard S., Lory P., Nerbonne J. M., Nargeot J. Cyclic-AMP-dependent phosphorylation modulates the stereospecific activation of cardiac Ca channels by Bay K 8644. Pflugers Arch. 1990 Sep;417(1):58–66. doi: 10.1007/BF00370769. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]