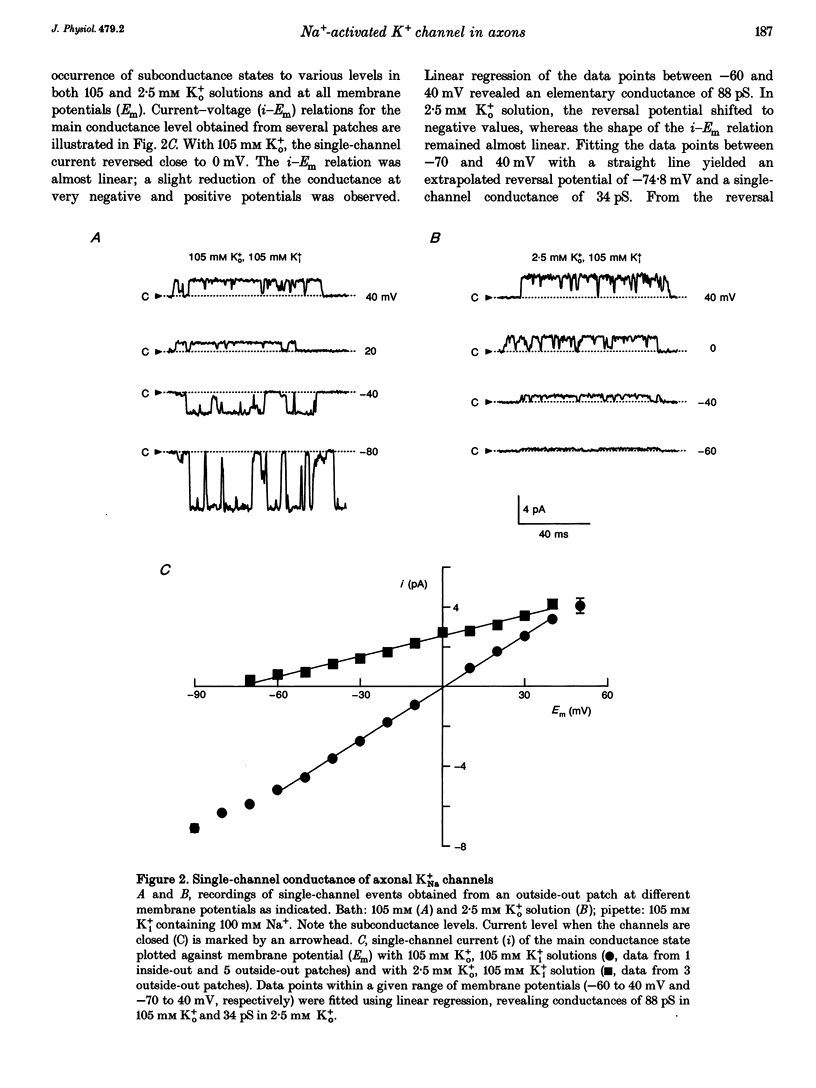
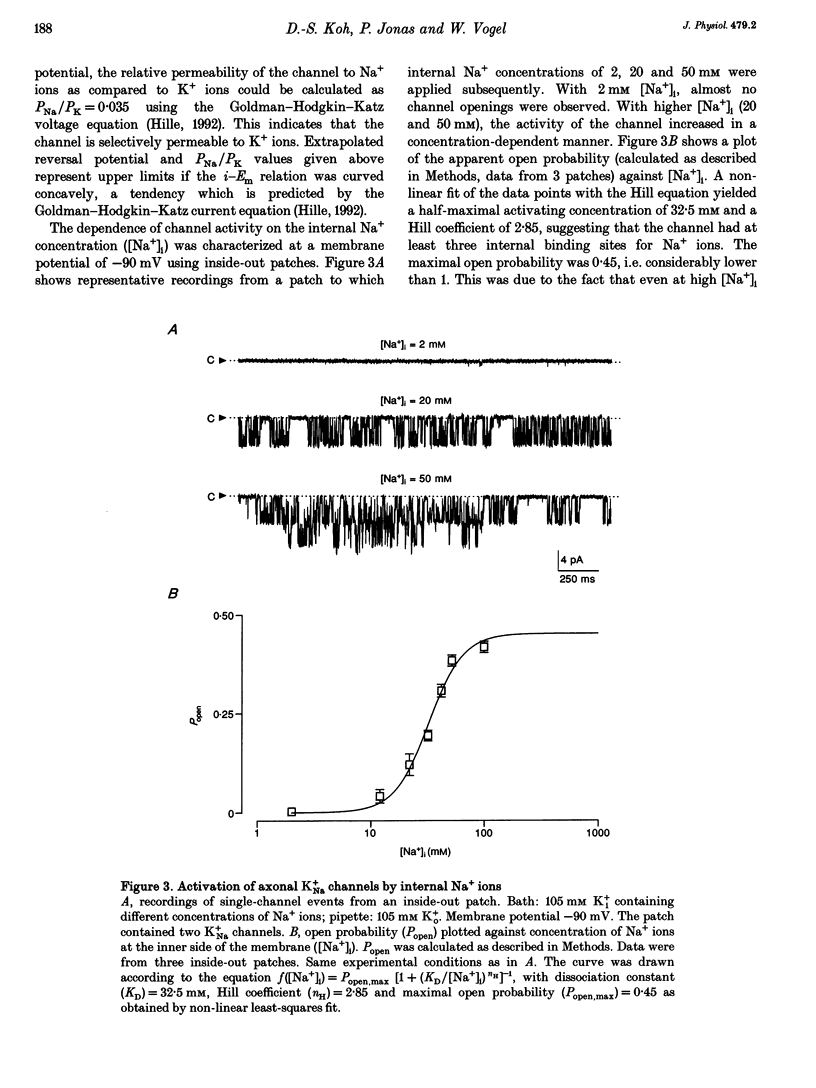
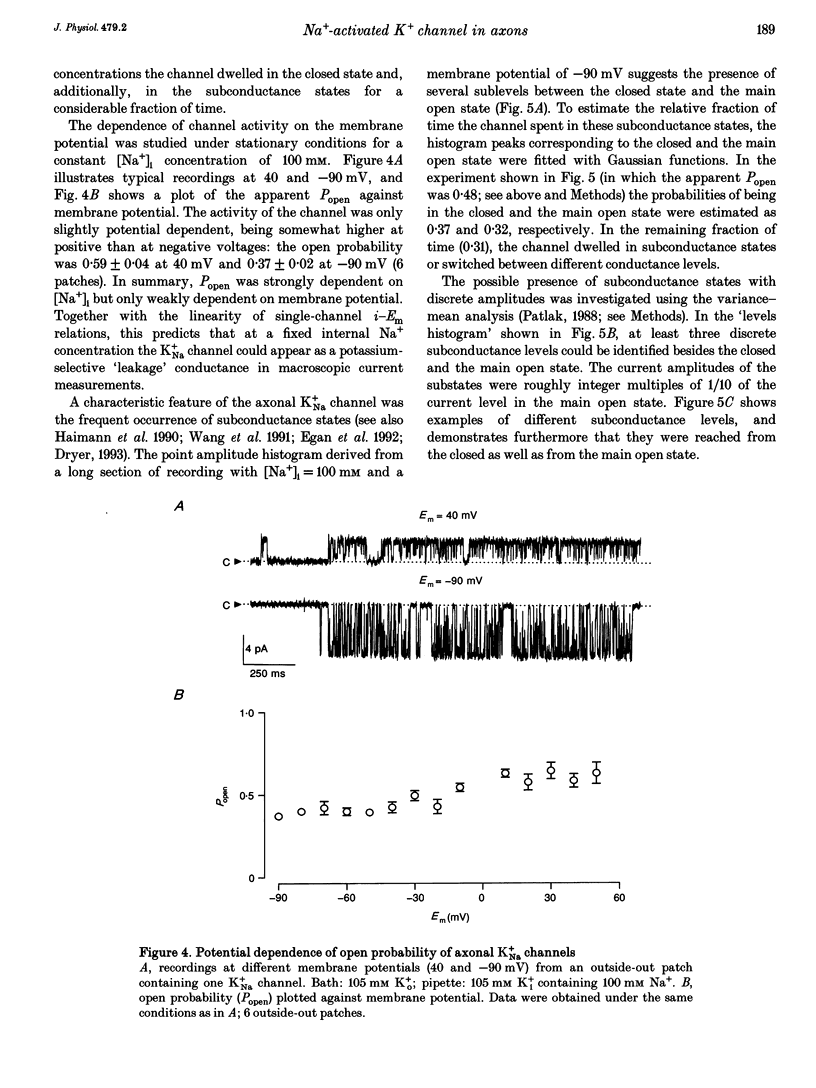
Abstract
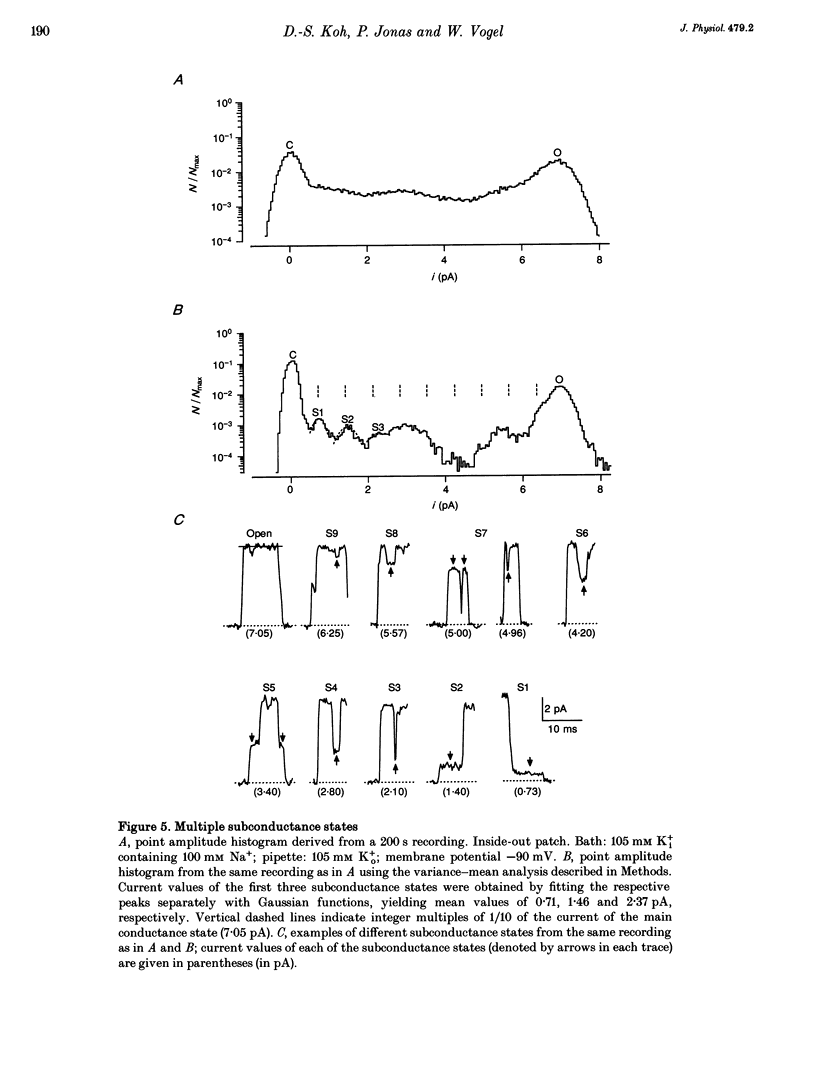
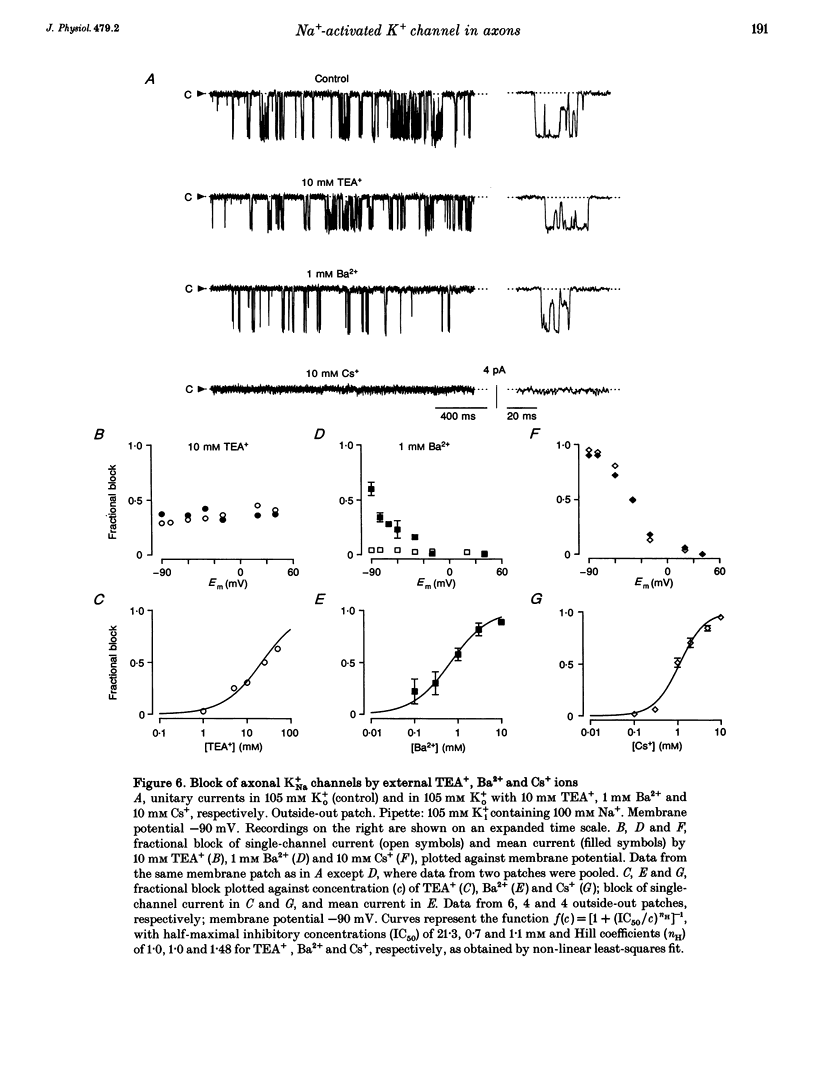
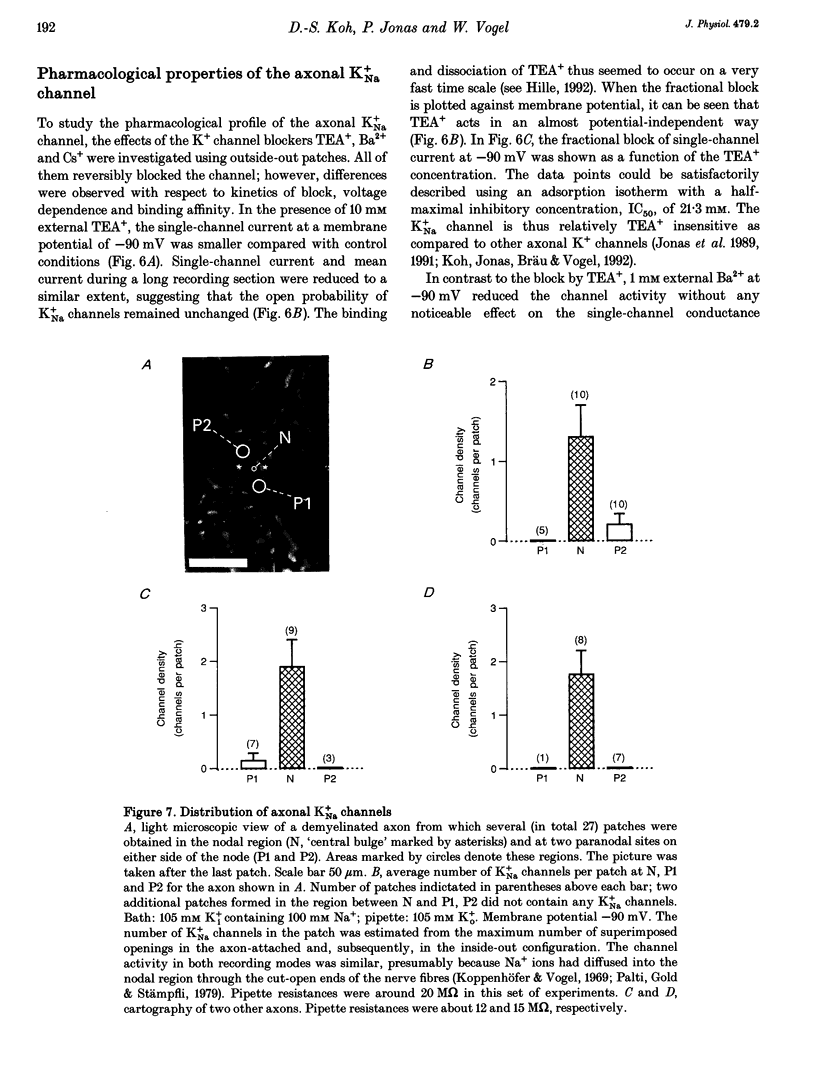
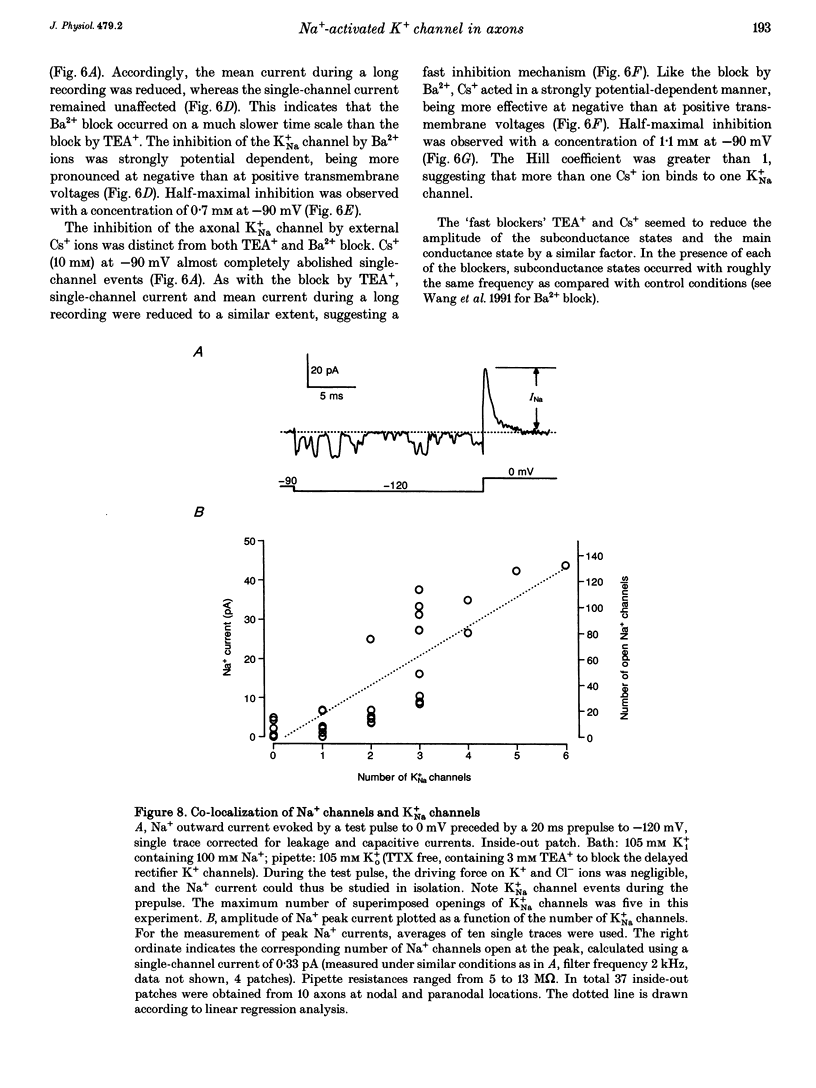
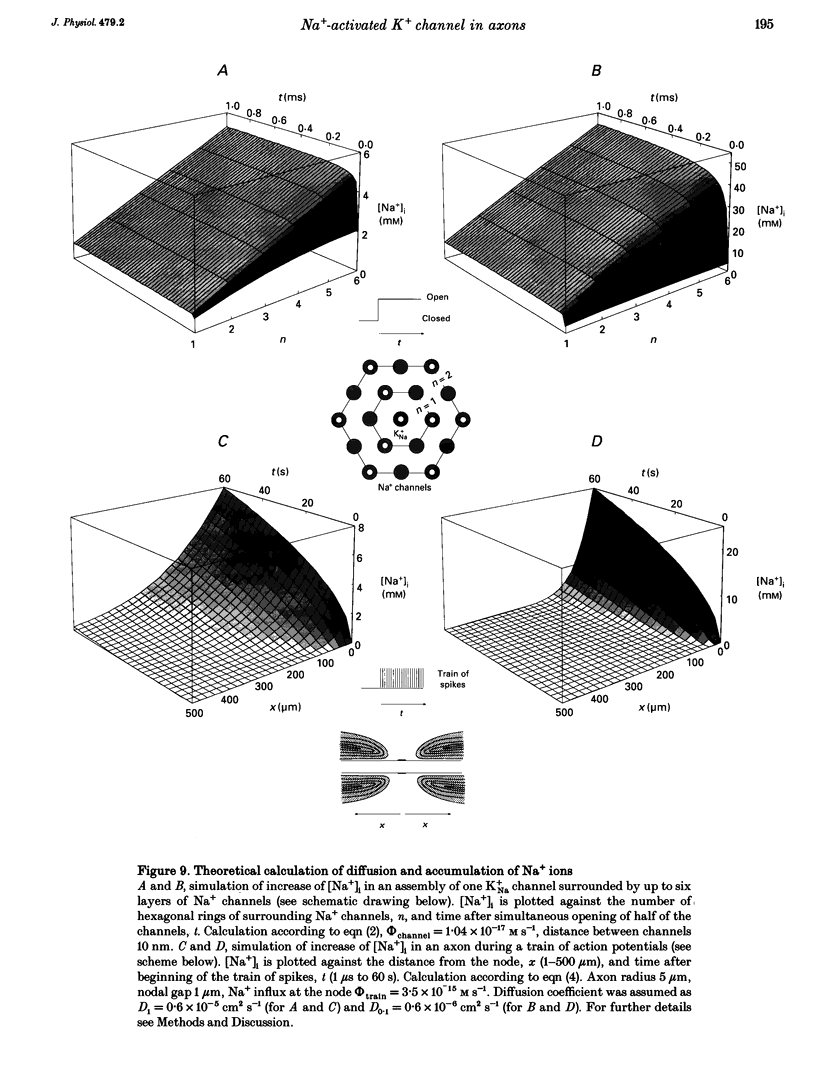
1. A potassium channel activated by internal Na+ ions (K+Na channel) was identified in peripheral myelinated axons of Xenopus laevis using the cell-attached and excised configurations of the patch clamp technique. 2. The single-channel conductance for the main open state was 88 pS with [K+]o = 105 mM and pS with [K+]o = 2.5 mM ([K+]i = 105 mM). The channel was selectively permeable to K+ over Na+ ions. A characteristic feature of the K+Na channel was the frequent occurrence of subconductance states. 3. The open probability of the channel was strongly dependent on the concentration of Na+ ions at the inner side of the membrane. The half-maximal activating Na+ concentration and the Hill coefficient were 33 mM and 2.9, respectively. The open probability of the channel showed only weak potential dependence. 4. The K+Na channel was relatively insensitive to external tetraethylammonium (TEA+) in comparison with voltage-dependent axonal K+ channels; the half-maximal inhibitory concentration (IC50) was 21.3 mM (at -90 mV). In contrast, the channel was blocked by low concentrations of external Ba2+ and Cs+ ions, with IC50 values of 0.7 and 1.1 mM, respectively (at -90 mV). The block by Ba2+ and Cs+ was more pronounced at negative than at positive membrane potentials. 5. A comparison of the number of K+Na channels in nodal and paranodal patches from the same axon revealed that the channel density was about 10-fold higher at the node of Ranvier than at the paranode. Moreover, a correlation between the number of K+Na channels and voltage-dependent Na+ channels in the same patches was found, suggesting co-localization of both channel types. 6. As weakly potential-dependent ('leakage') channels, axonal K+Na channels may be involved in setting the resting potential of vertebrate axons. Simulations of Na+ ion diffusion suggest two possible mechanisms of activation of K+Na channels: the local increase of Na+ concentration in a cluster of Na+ channels during a single action potential or the accumulation in the intracellular axonal compartment during a train of action potentials.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bernheim L., Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985 Oct 10;317(6037):540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- Bergman C. Increase of sodium concentration near the inner surface of the nodal membrane. Pflugers Arch. 1970;317(4):287–302. doi: 10.1007/BF00586578. [DOI] [PubMed] [Google Scholar]
- Berthold C. H., Rydmark M. Electrophysiology and morphology of myelinated nerve fibers. VI. Anatomy of the paranode-node-paranode region in the cat. Experientia. 1983 Sep 15;39(9):964–976. doi: 10.1007/BF01989761. [DOI] [PubMed] [Google Scholar]
- Bührle C. P., Sonnhof U. Intracellular ion activities and equilibrium potentials in motoneurones and glia cells of the frog spinal cord. Pflugers Arch. 1983 Feb;396(2):144–153. doi: 10.1007/BF00615519. [DOI] [PubMed] [Google Scholar]
- Dale N. A large, sustained Na(+)- and voltage-dependent K+ current in spinal neurons of the frog embryo. J Physiol. 1993 Mar;462:349–372. doi: 10.1113/jphysiol.1993.sp019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Fujii J. T., Martin A. R. A Na+-activated K+ current in cultured brain stem neurones from chicks. J Physiol. 1989 Mar;410:283–296. doi: 10.1113/jphysiol.1989.sp017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Na(+)-activated K+ channels and voltage-evoked ionic currents in brain stem and parasympathetic neurones of the chick. J Physiol. 1991 Apr;435:513–532. doi: 10.1113/jphysiol.1991.sp018522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E. Properties of single Na(+)-activated K+ channels in cultured central neurons of the chick embryo. Neurosci Lett. 1993 Jan 12;149(2):133–136. doi: 10.1016/0304-3940(93)90754-9. [DOI] [PubMed] [Google Scholar]
- Dubois J. M., Bergman C. Late sodium current in the node of Ranvier. Pflugers Arch. 1975;357(1-2):145–148. doi: 10.1007/BF00584552. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Dagan D., Kupper J., Levitan I. B. Properties and rundown of sodium-activated potassium channels in rat olfactory bulb neurons. J Neurosci. 1992 May;12(5):1964–1976. doi: 10.1523/JNEUROSCI.12-05-01964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HUXLEY A. F. THE ACTION POTENTIAL IN THE MYELINATED NERVE FIBER OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. J Physiol. 1964 Jun;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Dörge A., Beck F., Rick R. Intracellular electrolyte concentrations in rat sympathetic neurones measured with an electron microprobe. Pflugers Arch. 1984 Mar;400(3):274–279. doi: 10.1007/BF00581559. [DOI] [PubMed] [Google Scholar]
- Haimann C., Bernheim L., Bertrand D., Bader C. R. Potassium current activated by intracellular sodium in quail trigeminal ganglion neurons. J Gen Physiol. 1990 May;95(5):961–979. doi: 10.1085/jgp.95.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P. Salicylate: effects on ion transport and afterpotentials in frog sciatic nerve. Am J Physiol. 1965 Dec;209(6):1295–1303. doi: 10.1152/ajplegacy.1965.209.6.1295. [DOI] [PubMed] [Google Scholar]
- Jonas P., Bräu M. E., Hermsteiner M., Vogel W. Single-channel recording in myelinated nerve fibers reveals one type of Na channel but different K channels. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7238–7242. doi: 10.1073/pnas.86.18.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P., Koh D. S., Kampe K., Hermsteiner M., Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991 Mar;418(1-2):68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Koh D. S., Jonas P., Bräu M. E., Vogel W. A TEA-insensitive flickering potassium channel active around the resting potential in myelinated nerve. J Membr Biol. 1992 Nov;130(2):149–162. doi: 10.1007/BF00231893. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Vogel W. Wirkung von Tetrodotoxin und Tetraäthylammoniumchlorid an der Innenseite der Schnürringsmembran von Xenopus laevis. Pflugers Arch. 1969;313(4):361–380. doi: 10.1007/BF00593959. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M., Castiglia C. M., Saubermann A. J. Perturbation of axonal elemental composition and water content: implication for neurotoxic mechanisms. Neurotoxicology. 1992 Spring;13(1):123–137. [PubMed] [Google Scholar]
- Palti Y., Gold R., Stämpfli R. Diffusion of ions in myelinated nerve fibers. Biophys J. 1979 Jan;25(1):17–31. doi: 10.1016/S0006-3495(79)85275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov B. V., Kampe K., Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol. 1993 Jan;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfle G. M. Tetanic hyperpolarization of single medullated nerve fibers in sodium and lithium. Am J Physiol. 1976 Oct;231(4):1033–1038. doi: 10.1152/ajplegacy.1976.231.4.1033. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Wang Z., Kimitsuki T., Noma A. Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol. 1991 Feb;433:241–257. doi: 10.1113/jphysiol.1991.sp018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]