Abstract
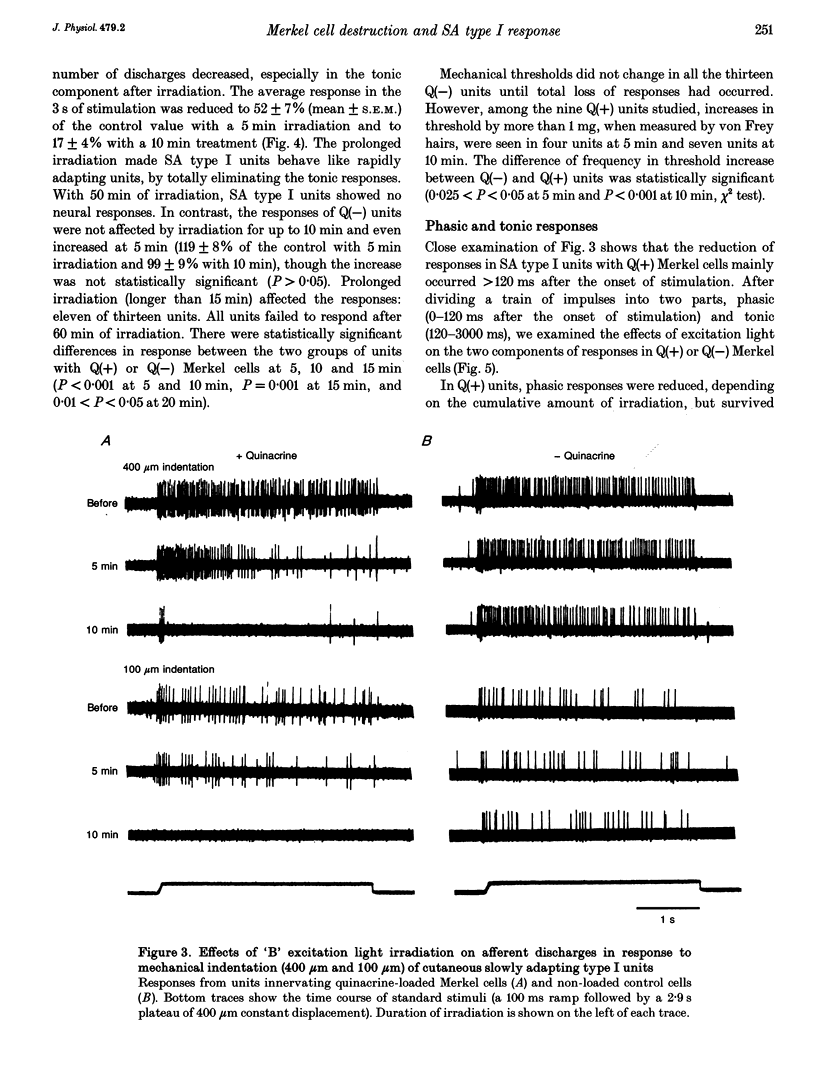
1. The fluorescent dye quinacrine which accumulates in Merkel cells in touch domes was administered to rats and the effects of excitation light irradiation on the mechanical responses of slowly adapting (SA) type I units innervating the touch domes were investigated. 2. Histological examination showed that after 10 min of irradiation degeneration was specifically localized to Merkel cells loaded with quinacrine. Nerve terminals associated with Merkel cells remained intact, even after treatment. 3. In SA type I units, responses to standard stimulation (a 100 ms ramp followed by a 2.9 s plateau of 400 microns constant displacement) decreased significantly after irradiation of the domes with quinacrine-excitation light through a 'B' filter ('B' light). With 5 min irradiation, the response decreased to 52 +/- 7% (n = 10, mean +/- S.E.M.) of the pretreated value, to 17 +/- 4% with a 10 min treatment and practically disappeared within 20 min. 4. In SA type I units with non-loaded Merkel cells, the response increased to 119 +/- 8% (n = 13) with 5 min irradiation and was 99 +/- 9% with the 10 min treatment. At around 15 min after the onset of irradiation there was a gradual decrease and within 60 min the response disappeared. 5. When responses were divided into phasic (0-120 ms after the onset of stimulation) and tonic (120-3000 ms) components, 'tonic' responses were more affected than 'phasic' ones in quinacrine-loaded SA type I units. 6. Stimulus-response curves shifted to the right and downwards in SA type I units with quinacrine-loaded Merkel cells after irradiation, but no significant change was seen in SA type I units without quinacrine. 7. Our observations are consistent with the hypothesis that Merkel cells are responsible for mechanoelectric transduction in SA type I units.
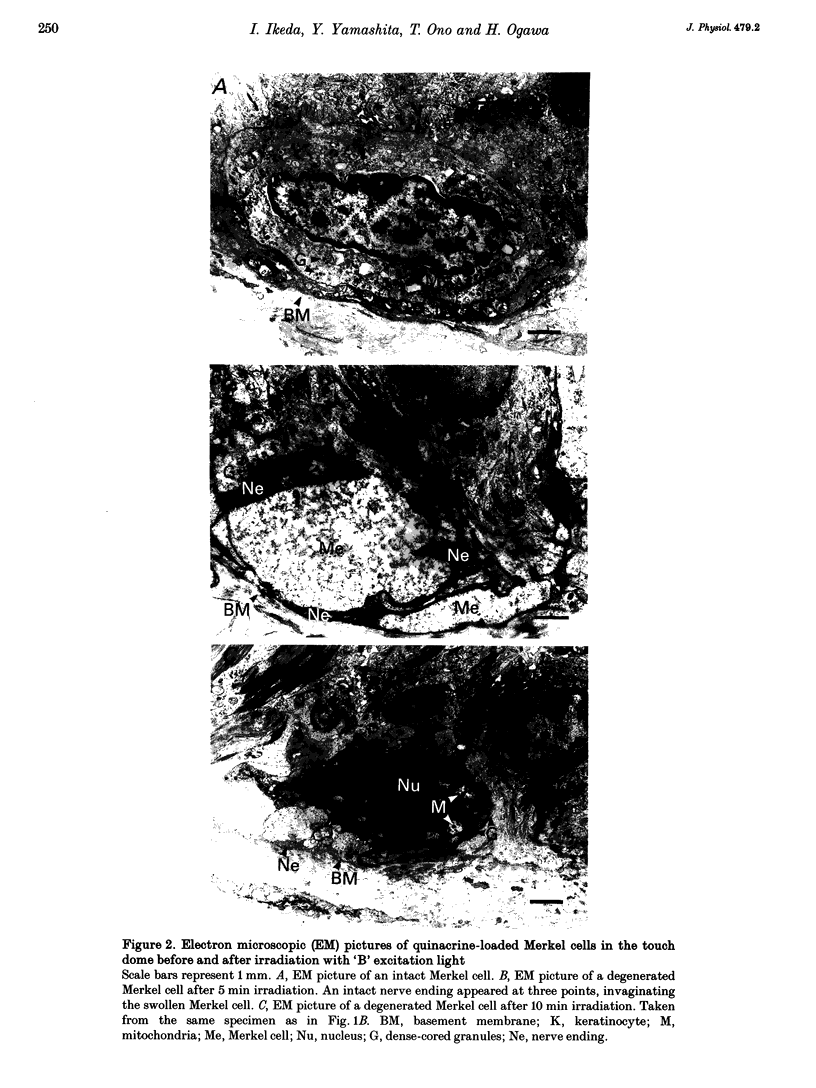
Full text
PDF


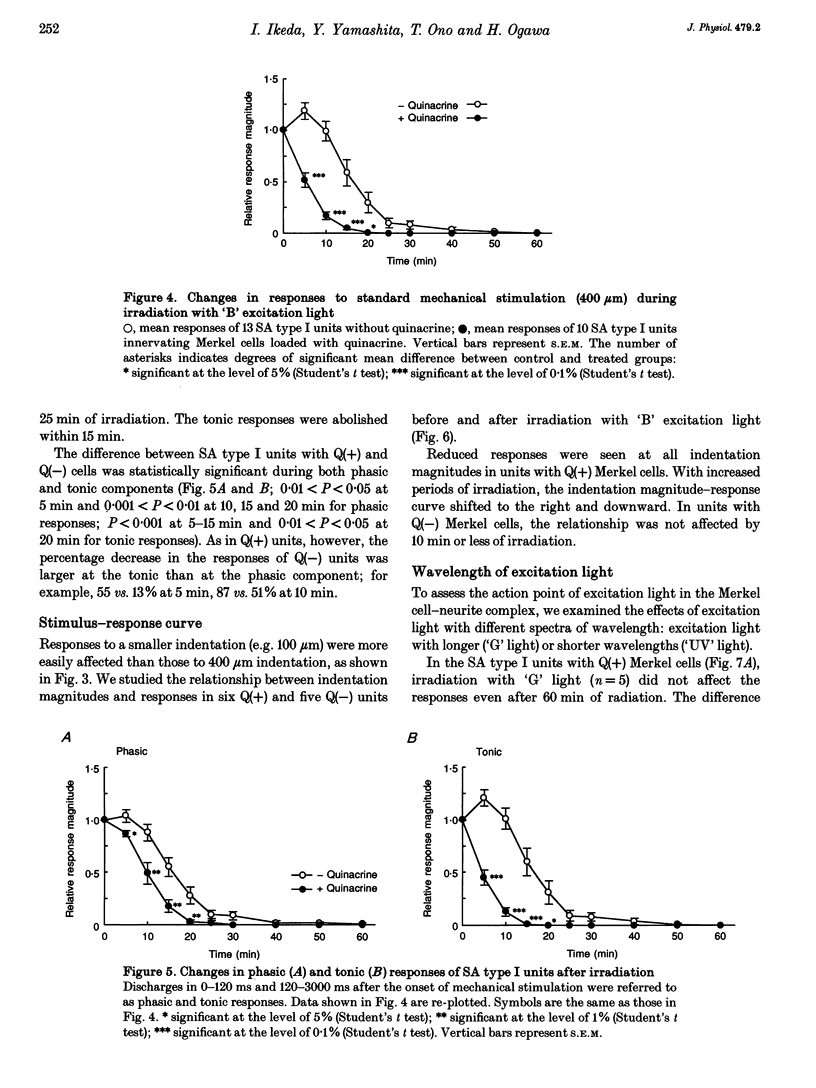



Images in this article
Selected References
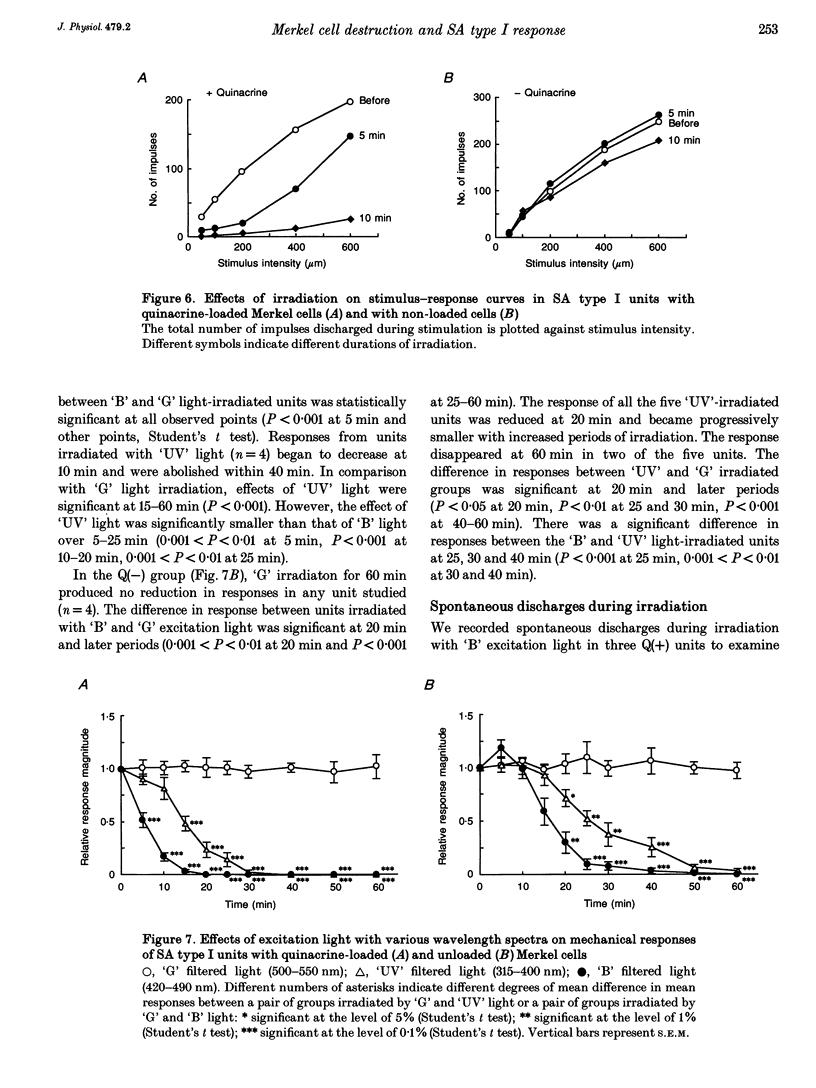
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K. I., Hamann W., Leung M. S. Acute effects of neomycin on slowly adapting type I and type II cutaneous mechanoreceptors in the anaesthetized cat and rat. J Physiol. 1990 Jun;425:527–544. doi: 10.1113/jphysiol.1990.sp018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe R., Whitear M. Quinacrine fluorescence of Merkel cells in Xenopus laevis. Cell Tissue Res. 1978 Jul 5;190(2):273–283. doi: 10.1007/BF00218175. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Jacobs E. R., Silver M. R. Potassium currents in rat type II alveolar epithelial cells. J Physiol. 1988 Jan;395:487–505. doi: 10.1113/jphysiol.1988.sp016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Mills L. R., Mearow K. M. Evidence that the Merkel cell is not the transducer in the mechanosensory Merkel cell-neurite complex. Prog Brain Res. 1988;74:51–56. doi: 10.1016/s0079-6123(08)62997-0. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Findlater G. S., Cooksey E. J., Anand A., Paintal A. S., Iggo A. The effects of hypoxia on slowly adapting type I (SAI) cutaneous mechanoreceptors in the cat and rat. Somatosens Res. 1987;5(1):1–17. doi: 10.3109/07367228709144615. [DOI] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Ferrario A., Wong S. Properties and applications of photodynamic therapy. Radiat Res. 1989 Oct;120(1):1–18. [PubMed] [Google Scholar]
- Gottschaldt K. M., Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981 Oct 9;214(4517):183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- Horch K. W., Whitehorn D., Burgess P. R. Impulse generation in type I cutaneous mechanoreceptors. J Neurophysiol. 1974 Mar;37(2):267–281. doi: 10.1152/jn.1974.37.2.267. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T. Cellular and subcellular mechanisms of photodynamic action: the 1O2 hypothesis as a driving force in recent research. Photochem Photobiol. 1978 Oct-Nov;28(4-5):493–508. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- Manyak M. J., Russo A., Smith P. D., Glatstein E. Photodynamic therapy. J Clin Oncol. 1988 Feb;6(2):380–391. doi: 10.1200/JCO.1988.6.2.380. [DOI] [PubMed] [Google Scholar]
- Mearow K. M., Diamond J. Merkel cells and the mechanosensitivity of normal and regenerating nerves in Xenopus skin. Neuroscience. 1988 Aug;26(2):695–708. doi: 10.1016/0306-4522(88)90175-3. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Pubols L. M., Pubols B. H. The Merkel rete papilla--a slowly adapting sensory receptor in mammalian glabrous skin. Brain Res. 1971 Jun 4;29(1):47–61. doi: 10.1016/0006-8993(71)90416-1. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., Mearow K. M., Holmes M., Visheau B., Diamond J. Merkel cell distribution in the epidermis as determined by quinacrine fluorescence. Cell Tissue Res. 1983;228(3):511–524. doi: 10.1007/BF00211472. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Morimoto K., Yamashita Y. Physiological characteristics of low threshold mechanoreceptor afferent units innervating frog skin. Q J Exp Physiol. 1981 Apr;66(2):105–116. doi: 10.1113/expphysiol.1981.sp002538. [DOI] [PubMed] [Google Scholar]
- Peak J. G., Peak M. J., MacCoss M. DNA breakage caused by 334-nm ultraviolet light is enhanced by naturally occurring nucleic acid components and nucleotide coenzymes. Photochem Photobiol. 1984 May;39(5):713–716. doi: 10.1111/j.1751-1097.1984.tb03914.x. [DOI] [PubMed] [Google Scholar]
- Peng Q., Moan J., Nesland J. M., Rimington C. Aluminum phthalocyanines with asymmetrical lower sulfonation and with symmetrical higher sulfonation: a comparison of localizing and photosensitizing mechanism in human tumor LOX xenografts. Int J Cancer. 1990 Oct 15;46(4):719–726. doi: 10.1002/ijc.2910460428. [DOI] [PubMed] [Google Scholar]
- Punnonen K., Jansén C. T., Puntala A., Ahotupa M. Effects of in vitro UVA irradiation and PUVA treatment on membrane fatty acids and activities of antioxidant enzymes in human keratinocytes. J Invest Dermatol. 1991 Feb;96(2):255–259. doi: 10.1111/1523-1747.ep12462271. [DOI] [PubMed] [Google Scholar]
- Scott S. A., Cooper E., Diamond J. Merkel cells as targets of the mechanosensory nerves in salamander skin. Proc R Soc Lond B Biol Sci. 1981 Mar 27;211(1185):455–470. doi: 10.1098/rspb.1981.0017. [DOI] [PubMed] [Google Scholar]
- Smith K. R., Jr The Haarscheibe. J Invest Dermatol. 1977 Jul;69(1):68–74. doi: 10.1111/1523-1747.ep12497883. [DOI] [PubMed] [Google Scholar]
- Theriault E., Otsuka M., Jessell T. Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res. 1979 Jul 6;170(1):209–213. doi: 10.1016/0006-8993(79)90957-0. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., DUGGAN D. E., VASTA B. M., BRODIE B. B. A spectrophotofluorometric study of organic compounds of pharmacological interest. J Pharmacol Exp Ther. 1957 May;120(1):26–32. [PubMed] [Google Scholar]
- Weber U., Hartschuh W., Feurle G. E., Weihe E. Met-enkephalin-like immuno- and bioreactivity in extracts from skin containing Merkel cells. Neurosci Lett. 1980 Nov;20(2):201–204. doi: 10.1016/0304-3940(80)90146-9. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Ogawa H. Slowly adapting cutaneous mechanoreceptor afferent units associated with Merkel cells in frogs and effects of direct currents. Somatosens Mot Res. 1991;8(1):87–95. doi: 10.3109/08990229109144732. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Ogawa H., Taniguchi K. Differential effects of manganese and magnesium on two types of slowly adapting cutaneous mechanoreceptor afferent units in frogs. Pflugers Arch. 1986 Feb;406(2):218–224. doi: 10.1007/BF00586686. [DOI] [PubMed] [Google Scholar]