Abstract
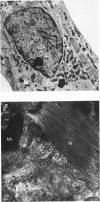
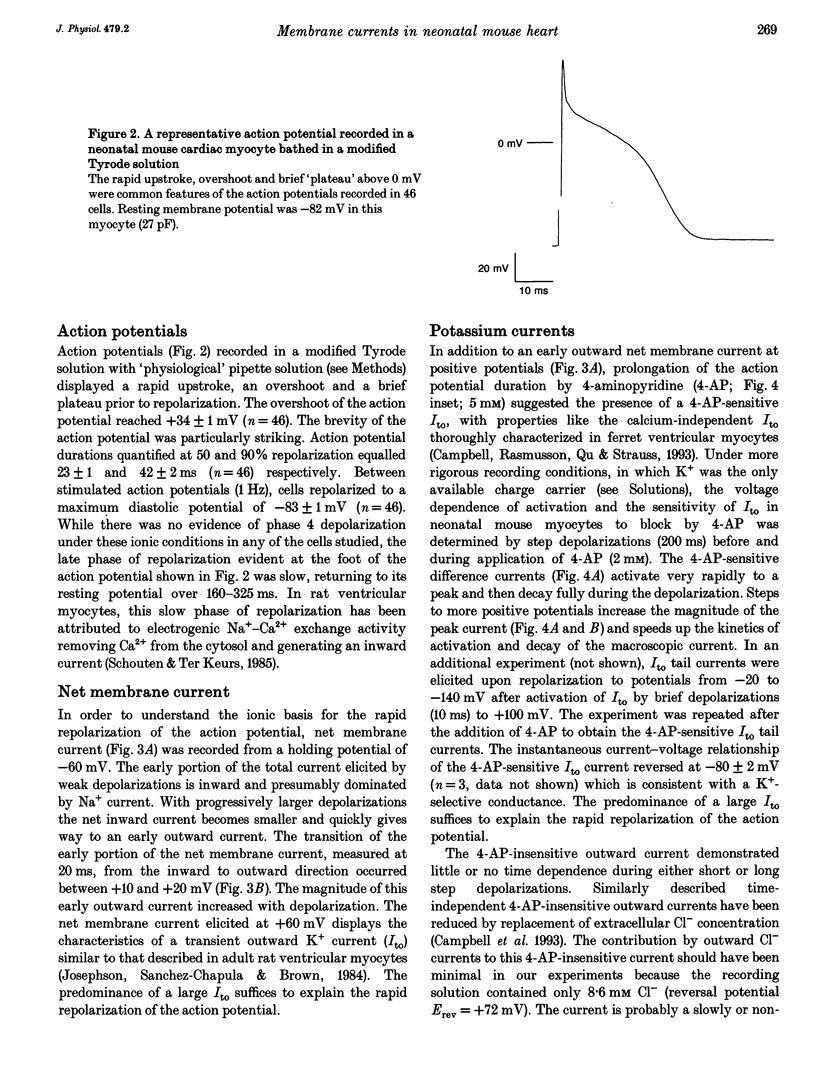
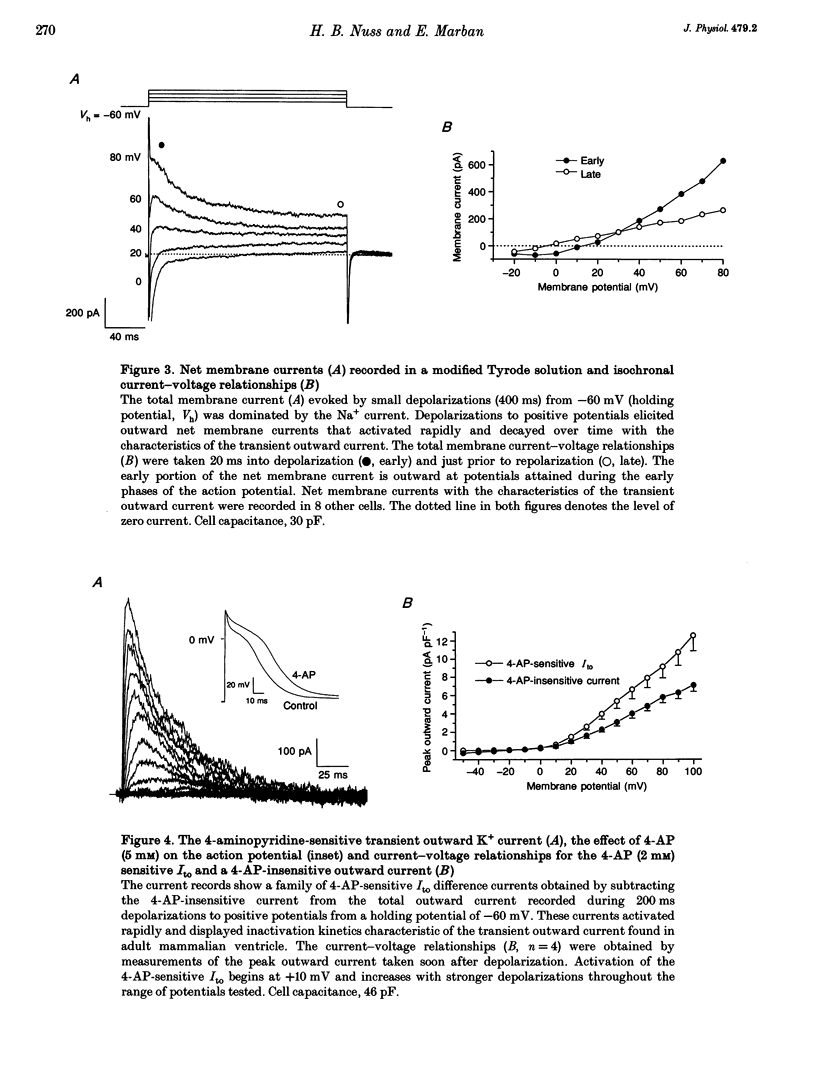
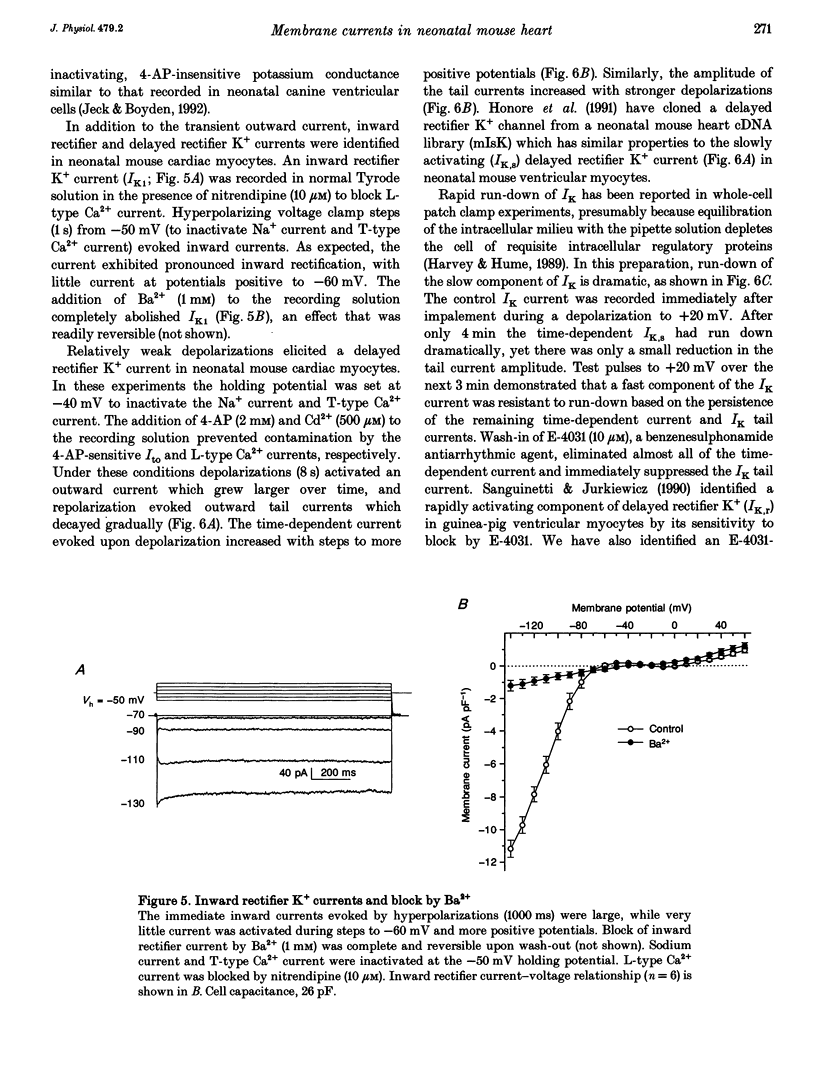
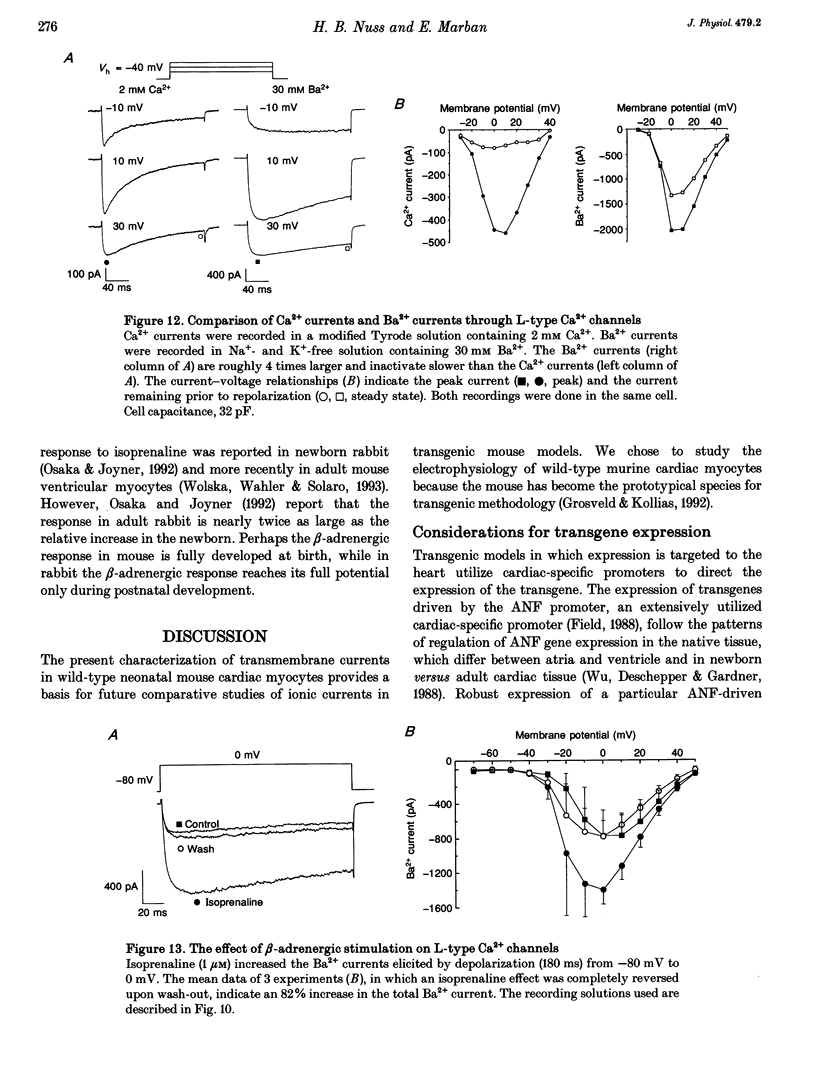
1. The increasing utility of transgenic mice in molecular studies of the cardiovascular system has motivated us to characterize the ionic currents in neonatal mouse ventricular myocytes. 2. Cell capacitance measurements (30 +/- 1 pF, n = 73) confirmed visual impressions that neonatal mouse ventricular myocytes in primary culture are considerably smaller than freshly isolated adult ventricular myocytes. With the use of electron microscopy, mitochondria and sarcoplasmic reticulum were found in close association with myofibrils, but transverse tubules were not observed. 3. Action potential durations, measured at 50 and 90% repolarization, were 23 +/- 1 and 42 +/- 2 ms respectively (n = 46). Application of 4-aminopyridine (4-AP; 5 mM) prolonged action potential duration at 50% repolarization by 26 +/- 5% (n = 3). The brevity of the action potential is explained by the rapid activation of a transient outward K+ current upon voltage-clamp depolarization to plateau potentials. 4. Potassium currents identified include an inward rectifier, a large 4-AP-sensitive transient outward, a slowly inactivating 4-AP-insensitive outward, a slowly activating delayed rectifier and a small rapidly activating E-4031 (10 microM)-sensitive delayed rectifier K+ current. 5. Sodium currents (-305 +/- 50 pA pF-1, n = 21) were recorded in 40 mM Na+ with Ni2+ (1 mM) to block Ca2+ currents and with K+ replaced by Cs+. The relative insensitivity of the Na+ current to block by tetrodotoxin (IC50 = 2.2 +/- 0.3 microM, n = 4) is distinctive of the cardiac Na+ channel isoform. 6. Nitrendipine-insensitive (10 microM) Ba2+ currents elicited during steps from -90 to -30 mV measured -25 +/- 5 pA pF-1 (n = 7, 30 mM Ba2+). Decay of these currents was complete during 180 ms depolarizations, even with Ba2+ as the charge carrier. These currents were not present when the holding potential was set at -50 mV. These data support the presence of a low threshold, T-type Ca2+ current. 7. The maximal nitrendipine-sensitive L-type Ca2+ current density was -10 +/- 2 pA pF-1 (n = 8) in 2 mM Ca2+ and -38 +/- 5 pA pF-1 (n = 9) in 30 mM Ba2+. Exposure to isoprenaline (1 microM) resulted in an 82% increase (n = 3) in the amplitude of the Ba2+ currents elicited at 0 mV. 8. Neonatal mouse cardiac myocytes in primary culture possess surprisingly large inward currents given the brevity of their action potentials.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba S., Creazzo T. L. Comparison of the number of dihydropyridine receptors with the number of functional L-type calcium channels in embryonic heart. Circ Res. 1993 Feb;72(2):396–402. doi: 10.1161/01.res.72.2.396. [DOI] [PubMed] [Google Scholar]
- Babinet C., Morello D., Renard J. P. Transgenic mice. Genome. 1989;31(2):938–949. doi: 10.1139/g89-165. [DOI] [PubMed] [Google Scholar]
- Balke C. W., Rose W. C., Marban E., Wier W. G. Macroscopic and unitary properties of physiological ion flux through T-type Ca2+ channels in guinea-pig heart cells. J Physiol. 1992 Oct;456:247–265. doi: 10.1113/jphysiol.1992.sp019335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benndorf K., Boldt W., Nilius B. Sodium current in single myocardial mouse cells. Pflugers Arch. 1985 May;404(2):190–196. doi: 10.1007/BF00585418. [DOI] [PubMed] [Google Scholar]
- Benndorf K. Patch clamp analysis of Na channel gating in mammalian myocardium: reconstruction of double pulse inactivation and voltage dependence of Na currents. Gen Physiol Biophys. 1988 Aug;7(4):353–377. [PubMed] [Google Scholar]
- Benndorf K. Three types of single K channels contribute to the transient outward current in myocardial mouse cells. Biomed Biochim Acta. 1988;47(4-5):401–416. [PubMed] [Google Scholar]
- Binah O., Arieli R., Beck R., Rosen M. R., Palti Y. Ventricular electrophysiological properties: is interspecies variability related to thyroid state? Am J Physiol. 1987 Jun;252(6 Pt 2):H1265–H1274. doi: 10.1152/ajpheart.1987.252.6.H1265. [DOI] [PubMed] [Google Scholar]
- Bossen E. H., Sommer J. R., Waugh R. A. Comparative stereology of the mouse and finch left ventricle. Tissue Cell. 1978;10(4):773–784. doi: 10.1016/0040-8166(78)90062-9. [DOI] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Qu Y., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993 Apr;101(4):571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Tohse N., Sperelakis N. Influence of sympathetic innervation on the membrane electrical properties of neonatal rat cardiomyocytes in culture. J Dev Physiol. 1991 Apr;15(4):237–246. [PubMed] [Google Scholar]
- Field L. J. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988 Feb 26;239(4843):1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of delayed rectifier K+ current in mammalian heart involves G proteins. Am J Physiol. 1989 Sep;257(3 Pt 2):H818–H823. doi: 10.1152/ajpheart.1989.257.3.H818. [DOI] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Heurteaux C., Ricard P., Lesage F., Lazdunski M., Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991 Oct;10(10):2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T., Allard M. F., Sreenan C. M., Doss L. K., Bishop S. P., Swain J. L. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol. 1990 Jul;10(7):3709–3716. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck C. D., Boyden P. A. Age-related appearance of outward currents may contribute to developmental differences in ventricular repolarization. Circ Res. 1992 Dec;71(6):1390–1403. doi: 10.1161/01.res.71.6.1390. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kubo Y., Baldwin T. J., Jan Y. N., Jan L. Y. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993 Mar 11;362(6416):127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. H., Yue D. T., Rose W. C., Marban E. Sodium channel inactivation from resting states in guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:629–650. doi: 10.1113/jphysiol.1991.sp018855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Na channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1987 Jul;52(1):95–100. doi: 10.1016/S0006-3495(87)83192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Boldt W., Benndorf K. Properties of aconitine-modified sodium channels in single cells of mouse ventricular myocardium. Gen Physiol Biophys. 1986 Oct;5(5):473–484. [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in calcium currents of rabbit ventricular cells. Circ Res. 1991 Mar;68(3):788–796. doi: 10.1161/01.res.68.3.788. [DOI] [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in the beta-adrenergic modulation of calcium currents in rabbit ventricular cells. Circ Res. 1992 Jan;70(1):104–115. doi: 10.1161/01.res.70.1.104. [DOI] [PubMed] [Google Scholar]
- Rampe D., Lacerda A. E. A new site for the activation of cardiac calcium channels defined by the nondihydropyridine FPL 64176. J Pharmacol Exp Ther. 1991 Dec;259(3):982–987. [PubMed] [Google Scholar]
- Rogers T. B., Gaa S. T., Allen I. S. Identification and characterization of functional angiotensin II receptors on cultured heart myocytes. J Pharmacol Exp Ther. 1986 Feb;236(2):438–444. [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin J., Kyle J. W., Chen M., Bell P., Cribbs L. L., Fozzard H. A., Rogart R. B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science. 1992 May 22;256(5060):1202–1205. doi: 10.1126/science.256.5060.1202. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. The slow repolarization phase of the action potential in rat heart. J Physiol. 1985 Mar;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A., Morton M., Gartside C. L., Hauschka S. D., Catterall W. A., Scheuer T. Tetrodotoxin-insensitive sodium channels in a cardiac cell line from a transgenic mouse. Am J Physiol. 1992 Mar;262(3 Pt 1):C724–C730. doi: 10.1152/ajpcell.1992.262.3.C724. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhelper M. E., Lanson N. A., Jr, Dresdner K. P., Delcarpio J. B., Wit A. L., Claycomb W. C., Field L. J. Proliferation in vivo and in culture of differentiated adult atrial cardiomyocytes from transgenic mice. Am J Physiol. 1990 Dec;259(6 Pt 2):H1826–H1834. doi: 10.1152/ajpheart.1990.259.6.H1826. [DOI] [PubMed] [Google Scholar]
- Tohse N., Masuda H., Sperelakis N. Novel isoform of Ca2+ channel in rat fetal cardiomyocytes. J Physiol. 1992;451:295–306. doi: 10.1113/jphysiol.1992.sp019165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. M., Terzic A. Alpha-adrenergic regulation of action potentials in isolated rat cardiomyocytes. Eur J Pharmacol. 1989 May 19;164(2):231–239. doi: 10.1016/0014-2999(89)90463-9. [DOI] [PubMed] [Google Scholar]
- Wu J. P., Deschepper C. F., Gardner D. G. Perinatal expression of the atrial natriuretic factor gene in rat cardiac tissue. Am J Physiol. 1988 Sep;255(3 Pt 1):E388–E396. doi: 10.1152/ajpendo.1988.255.3.E388. [DOI] [PubMed] [Google Scholar]