Summary
We present a man in his 60s with a dorsal thoracic arachnoid web spanning levels T6-T8. The patient presented with gait abnormalities, severe neuropathic lower back pain and mild urinary incontinence without sensory deficits. He underwent laminectomy with arachnoid web fenestration. At the 6-week postoperative follow-up appointment, he had increased muscle strength in his lower extremities and was able to walk without shuffling his feet, with a straight back and standing upright. This is a marked improvement from his previous hunched and shuffling gait. He has had partial resolution of his neuropathic low back pain. Arachnoid webs are often confused with other neurological disorders, most commonly idiopathic ventral cord herniation, which prolongs the time to surgical intervention. Eventual fenestration of our patient’s web led to significant improvement in gait and partial relief of his neuropathic low back pain.
Keywords: Neurological injury, Pain (neurology), Spinal cord
Background
Arachnoid webs are thought to be the remnants of collapsed arachnoid cysts or potentially the result of trauma, such as from repeated falls.1 2 They compress the spinal cord, resulting in myelopathic symptoms that may be permanent if they persist untreated. Furthermore, new theories suggest that webs disrupt the pressure gradient of the spinal cord, creating a suction effect at the region of the web, which obstructs the flow of cerebral spinal fluid.3 4 Arachnoid webs are notoriously subtle on imaging and, as they are a rare condition, often go undetected for a prolonged period during which patients experience progressive deterioration and profound reduction in quality of life. An MRI of a patient with suspected arachnoid web characteristically shows dorsal indentation of the spinal cord, primarily in the thoracic spine.5 A comprehensive review by Nisson et al supports that webs are preferentially present in the thoracic spine, with 100% of their 43 patients having webs in this region. This study additionally reports that webs more commonly affect males, with approximately two-thirds of cases presenting with a concomitant syrinx.5 6 Regardless of their aetiology, arachnoid webs can cause devastating neurological dysfunction and necessitate evaluation for neurosurgical intervention.
Case presentation
A man in his 60s with a history of atrial fibrillation status postablation and transurethral resection of the prostate procedure (TURP) was referred to the neurosurgery clinic for approximately 2 years of spastic paraparesis. He initially presented to his local neurologist with gradually worsening gait imbalance with frequent falls, severe thoracic and lumbar back pain, back and leg spasms and decreased functional endurance. On the physical examination, he had hyperreflexia in all four extremities with positive Babinski reflexes in his lower limbs bilaterally. All 12 of the cranial nerves were intact. There was no clonus or saddle anaesthesia appreciated, but there was a positive left-sided Hoffman reflex. His muscle strength was graded at 5/5 bilaterally in both the upper and lower extremities.
The patient described weakness that began in his left leg, which was quickly followed by right leg involvement with a complete absence of sensory symptoms. In less than 3 months, the patient’s gait imbalance went from necessitating a cane to using a walker.
Differential diagnosis
During this time, the patient reported only mild urinary changes, more likely attributed to his TURP procedure than as a part of cauda equina syndrome. Due to his shuffling gait, he received an initial diagnosis of Parkinson’s disease from his neurologist. When the levodopa/carbidopa (Sinemet) he was prescribed produced equivocal results, he was then referred to a neuromuscular neurologist at an academic centre due to concern for possible amyotrophic lateral sclerosis (ALS). ALS is typically a painless, progressive neuromuscular disorder with weakness that eventually involves all the muscles of the cervical, lumbosacral and bulbar regions with accompanying unintentional weight loss.7 The progression of ALS is unrelenting, and it would be unusual for it to remain confined to a single region of the body after 1.5 years. Electromyography testing demonstrated acute and chronic denervation changes in L3–S1 myotomes, although these findings were relatively mild and out of proportion to the severity of his clinical picture.
After several visits to the neuromuscular clinic, the patient’s disability had not progressed—an unusual scenario for ALS. In particular, he never displayed or developed any fasciculations or progressive muscle atrophy. This observation and atypical features, including severe back spasms, pain and mild urinary disturbances, prompted further work-up. Repeat spinal imaging, including a CT myelogram and a non-contrasted MRI of the thoracic spine, was ordered. When the imaging demonstrated a possible arachnoid cyst in the midthoracic spine (T7), which could potentially account for the patient’s presentation, the patient was then referred for neurosurgical evaluation. Arachnoid cysts, such as webs, are intradural and extramedullary lesions of the spinal meninges. Cysts are readily identifiable on MRI as distinct hyperintense pockets within the meninges of the spinal cord, as compared with the more subtle indentation into the spinal cord of the scalpel sign.
Myelopathy is not considered specific for a condition but rather is suggestive of spinal cord compression correlated to the vertebral level of dysfunction. The myelopathic features of arachnoid webs are remarkably similar to those seen in idiopathic ventral cord herniation, both presenting with upper motor neuron signs related to a distinct vertebral level. Idiopathic ventral cord herniation, such as arachnoid web, preferentially affects the thoracic spine. Ventral cord herniation, however, is a secondary feature of an anterior dural defect. MRI is the preferred modality for differentiating between these conditions. In lieu of a scalpel sign, cord herniation has a focal anterior protrusion at the level of the dural defect, causing local distortion of both the cord itself and the dura. In cases of minimally appreciable pathology on imaging, differentiating these conditions becomes more difficult and intraoperative ultrasound may be necessary. Other thoracic tumours, such as spinal meningioma, can also present with myelopathic symptoms. Spinal meningiomas have distinct borders on imaging, are generally isointense on MRI and have a classic ‘tail’ to help distinguish them from other spinal cord lesions.8 Given the MRI findings in this patient, arachnoid web was more likely than ventral cord herniation or meningioma of the spine. Surgical intervention is the definitive treatment for a symptomatic case of any of these three pathologies.
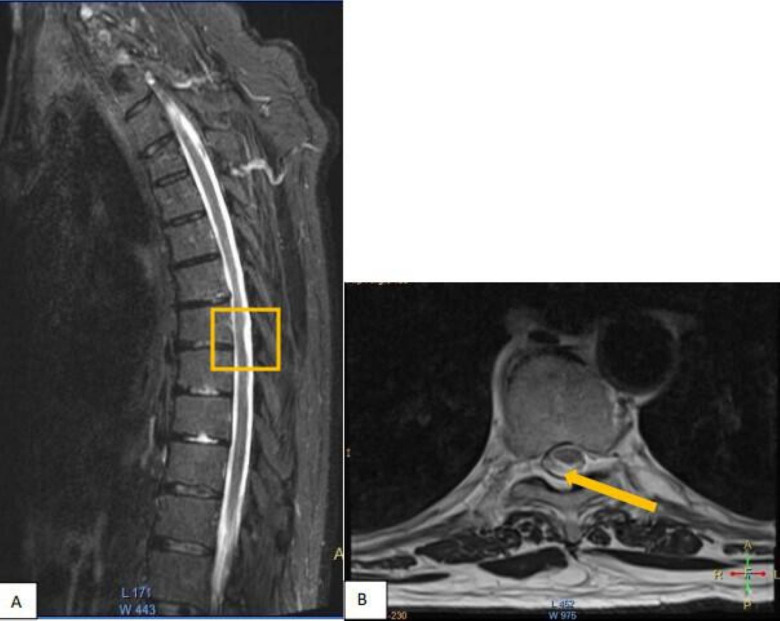
The arachnoid web was identified on MRI at vertebral levels 7 and 8 of the thoracic spine (figure 1A,B), indicated by a minimal dorsal indentation along the spinal cord, most suggestive of a positive scalpel sign. There was no associated syrinx in this case.
Figure 1. (A) Positive scalpel sign is shown on T2-weighted sagittal MRI scan at T7 indicated by the yellow box. (B) Axial MRI at the level of the arachnoid web T7.
Treatment
The initial 6-month period of conservative treatment consisted primarily of physical therapy and pharmacological management of symptoms with carbidopa/levodopa and pain management. Given his minimal improvement with this approach, lack of continued deterioration and suspicion from the neurologist that ALS or Parkinson’s was not the appropriate diagnosis, the patient underwent an MRI of the spine at this point and a very subtle scalpel sign was identified. A surgical approach was now reasonable, after consultation and evaluation by the neurosurgeon. The risks and benefits of surgery were discussed. The patient consented to surgical intervention with a laminectomy and intradural exploration with fenestration of the arachnoid adhesions or cysts if encountered.
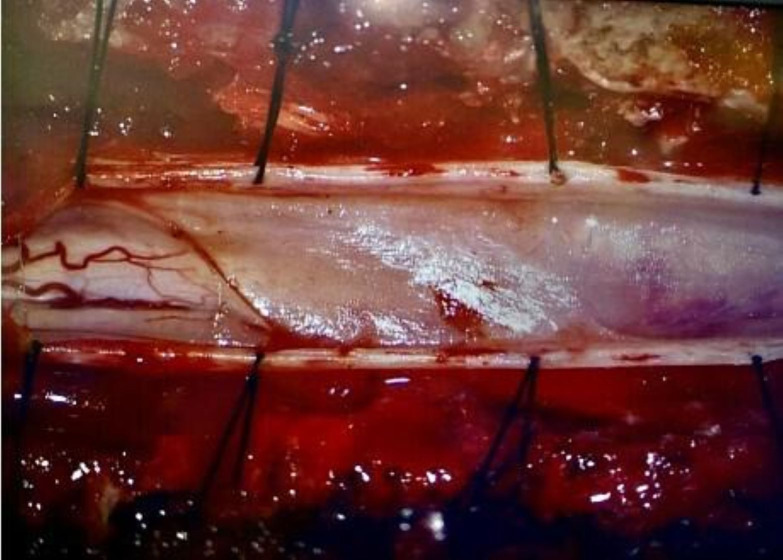
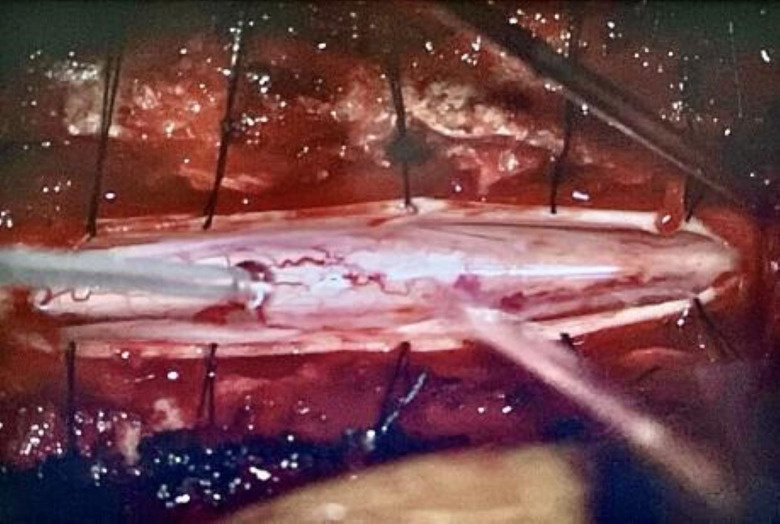
Intraoperatively, a high-speed drill was used to remove the posterior aspects of vertebrae T6–T8. The dura was opened, revealing thickened arachnoid webbing from T7 and T8. These adhesions resulted in an increased localised pressure over the spinal cord, as evidenced by the adhesions herniating through the dura on the initial opening. It was clear that the spinal cord involved in these adhesions was displaced ventrally in comparison with the area of normal appearing spinal cord above and below this level. Microdissection techniques were used to fenestrate the thickened arachnoid layer. Cystic components were found centrally and on the left side of the spinal cord (figure 2). Appropriate fenestration was completed, and the dura was reapproximated in a watertight fashion. After fenestration, there was a visible increase in cerebral spinal fluid flow across the affected region (figure 3). There were no surgical complications during this procedure.
Figure 2. Intraoperative photograph taken under the microscope. This was taken immediately after the opening of the dura and revealed a thickened arachnoid layer obscuring all vasculatures of the spinal cord. These adhesions displaced the spinal cord ventrally and occupied the entirety of the canal not filled by spinal cord. The adhesions disrupted the normal flow of cerebrospinal fluid. The adhesions were present from T7 and T8.
Figure 3. Intraoperative photograph under the microscope taken after the fenestration of the arachnoid webs. Normal spinal cord can now be visualised throughout this area of dural opening. Normal pulsations of cerebrospinal fluid across this segment were observed after fenestration and dissection.
Outcome and follow-up
At follow-up 5 months postsurgery, our patient is still managing issues with maintaining adequate balance. His neurologist has since diagnosed him with cervical myelopathy from the previous spinal cord compression by the arachnoid web. 5 months are still considered early in the recovery process following such an invasive surgery. He reports continuing to work with physical therapy to improve his strength gradually. He is now able to walk with his wife near their home with a walker, which he had stopped being able to do over the year prior to his surgery.
Discussion
Arachnoid web is a serious and rare pathology of the spinal cord causing myelopathy from the increased thickness of the arachnoid layer and interruption of cerebrospinal fluid flow around the cord. As of 2019, there were only 43 reported cases of this condition in the current literature.5
Presentation with both hyperreflexia and muscle atrophy of the lower limbs with gait disturbances, subtle findings on imaging and the relative rarity of arachnoid webs contributed to the difficulty in making an accurate clinical diagnosis for this patient.
In a 2022 retrospective study examining 17 patients with arachnoid webs, 100% of cases presented with scalpel signs, illustrating how critical imaging is in making this diagnosis.9 Although the presence of a scalpel sign is considered a primary diagnostic indicator for this condition,4 there are cases of minimal or no scalpel sign on imaging but an arachnoid web being found regardless.10 This suggests that improvements in imaging may allow for more diagnoses of webs in the future that exist without detectable scalpel signs. Continuing developments in MRI may further improve diagnostic capacity for arachnoid webs. Currently, MRI and CT myelography are variable in their capacity to successfully identify thickened arachnoid tissue directly and scalpel sign is more often used as an indirect suggestion of the presence of a web.11 CT myelography can indicate webs, but as webs typically produce incomplete CSF obstruction, these cannot be diagnostic in isolation.4 With increased resolution, imaging could more directly reveal arachnoid thickening. Constructive interface in steady state MRI—not widely available clinically—is very promising as it has ‘improved spatial resolution’ and can show in greater detail meningeal adhesions, differentiating these from arachnoid cysts or tumours when compared with currently used T2 MR images.12
Multiple previous case reports of arachnoid webs discuss significant sensory changes9 as a classic part of the patient presentation, particularly in the lower limbs. However, sensory changes cannot be considered diagnostic of arachnoid webs. As illustrated in table 1, 41% or less of patients will present with sensory perturbation. Additionally, the syrinx may be absent in as many as almost 60% of cases, according to the reviews, as shown in table 1. Although arachnoid webs are most typically found in the thoracic spine, case reports now describe them in the cervical spine as well, further muddying the waters on when to suspect a possible web.13
Table 1. Comparison of features from arachnoid web reviews. The characteristics of our patient’s presentation are compared with the frequency of occurrence of these traits across three separate reviews.
| Review study | Number of patients | Mean age in years | Male sex (%) | No syrinx (%) | No sensory loss (%) | Date range of study |
| Nisson et al5 | 43 | 52 (range 28–77 years) | 72 (31) | 43 (15) | 33 (14) | 1898–2018 |
| Voglis et al16 | 12 | 54.7 (SD 12.7) | 67 (8) | 33 (4) | 17 (2) | 2014–2020 |
| Delgardo et al9 | 17 | 50.5 (IQR 16) | 76 (13) | 59 (10) | 41 (7) | 2015–2019 |
Confusion in presentation leads to increased time until surgical intervention. Webs can cause irreversible spinal cord changes from prolonged compression, even if treated with surgery.13 Overwhelmingly, surgical treatment has resulted in improvement in patients’ back pain and lower limb muscle weakness. Thus, fenestration surgery is considered curative in 91% of cases.5 14 Significant gait improvement as soon as 2 weeks following fenestration of the arachnoid layer has been reported in at least one postoperative case.6
Arachnoid webs should be among the differential diagnoses for patients with symptoms resembling atypical Parkinson’s disease, ALS or other neurological conditions with new onset gait changes and myelopathic disturbances with or without sensory symptoms of the limbs. At this point in time, surgery remains the only curative option for patients with neurological symptoms. Arachnoid webs are not known to recur following surgical removal, although this may be due to the lack of long-term studies postsurgery.15 Further research investigating the 5-to-10-year postsurgical outcomes of these patients would provide insights into the long-term success of surgical intervention and rate of recurrence. Given current theories suggesting that webs may be the result of trauma or inflammation around the spinal cord, determining the rate of recurrence after invasive spinal cord surgery would prove useful in understanding the pathophysiology and complications of arachnoid webs.
Learning points.
Arachnoid webs may present with a variety of neurological deficits and should be on the differential for a patient with spastic paresis that fails to rapidly progress or improve with conservative approaches.
Surgery can resolve both motor and sensory symptoms in patients with clear neurological deficits.
MRI and high suspicion for this condition are essential to making a timely diagnosis; improved resolution in imaging will expedite diagnosis.
Delayed time to surgery for patients with symptomatic arachnoid webs results in irreparable myelopathic symptoms, including impaired gait, sensory loss and neuropathic pain.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Contributor Information
Kyly M Hiatt, Email: kyly.hiatt@burrell.edu.
John Cauchi, Email: jcauchi@salud.unm.edu.
Christopher Payne, Email: cpayne@sjrmc.net.
References
- 1.Arora V, Verma H, Kamal R, et al. Dorsal arachnoid web: the ‘scalpel’ sign—a case report and differential diagnosis. Egypt J Radiol Nucl Med. 2022;53 doi: 10.1186/s43055-022-00847-4. [DOI] [Google Scholar]
- 2.Buttiens A, Feyen B, Dekeyzer S. Dorsal Arachnoid Web: A Rare Cause of Myelopathy. J Belg Soc Radiol. 2021;105:88. doi: 10.5334/jbsr.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sridharan A, Heilman CB. TRANSVERSE DORSAL ARACHNOID WEB AND SYRINGOMYELIA. Neurosurgery. 2009;65:E216–7. doi: 10.1227/01.NEU.0000348007.84175.FA. [DOI] [PubMed] [Google Scholar]
- 4.Ben Ali H, Hamilton P, Zygmunt S, et al. Spinal arachnoid web-a review article. J Spine Surg. 2018;4:446–50. doi: 10.21037/jss.2018.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nisson PL, Hussain I, Härtl R, et al. Arachnoid web of the spine: a systematic literature review. J Neurosurg. 2019;31:175–84. doi: 10.3171/2019.1.SPINE181371. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Fresnedo A, Domingo RA, Clifton W, et al. Arachnoid Web Fenestration: Diagnostic and Surgical Nuances. World Neurosurg. 2021;150:92. doi: 10.1016/j.wneu.2021.03.100. [DOI] [PubMed] [Google Scholar]
- 7.Huynh W, Simon NG, Grosskreutz J, et al. Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clin Neurophysiol. 2016;127:2643–60. doi: 10.1016/j.clinph.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Gaillard F, Kearns C, Bell D. Radiopaedia.org; Spinal meningioma. [DOI] [Google Scholar]
- 9.Delgardo M, Higgins D, McCormick KL, et al. Clinical Characteristics, Outcomes, and Pathology Analysis in Patients With Dorsal Arachnoid Web. Neurosurgery. 2022;90:581–7. doi: 10.1227/neu.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 10.Nagashima Y, Nishimura Y, Ito H, et al. Atypical radiographic case of arachnoid web without scalpel sign. Surg Neurol Int. 2022;13:108. doi: 10.25259/SNI_179_2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reardon MA, Raghavan P, Carpenter-Bailey K, et al. Dorsal thoracic arachnoid web and the “scalpel sign”: a distinct clinical-radiologic entity. AJNR Am J Neuroradiol. 2013;34:1104–10. doi: 10.3174/ajnr.A3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Chen YA, Chow D, et al. Practical applications of CISS MRI in spine imaging. Eur J Radiol Open. 2019;6:231–42. doi: 10.1016/j.ejro.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto A, Fujimoto M, Aoki K, et al. A Dorsal Arachnoid Web of the Cervical Spine: A Case Report. NMC Case Rep J . 2021;8:281–6. doi: 10.2176/nmccrj.cr.2020-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieves-Ríos C, Layuno-Matos JG, Olivella G, et al. Thoracic spinal arachnoid web and syringomyelia with rostral expansion to the first cervical spinal cord level: Case report. Int J Surg Case Rep. 2022;96:107360. doi: 10.1016/j.ijscr.2022.107360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirai T, Taniyama T, Yoshii T, et al. Clinical Outcomes of Surgical Treatment for Arachnoid Web: A Case Series. Spine Surg Relat Res . 2019;3:43–8. doi: 10.22603/ssrr.2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voglis S, Romagna A, Germans MR, et al. Spinal arachnoid web—a distinct entity of focal arachnopathy with favorable long-term outcome after surgical resection: analysis of a multicenter patient population. Spine J. 2022;22:126–35. doi: 10.1016/j.spinee.2021.06.018. [DOI] [PubMed] [Google Scholar]