Abstract
Background
Vital exhaustion, defined as excessive fatigue, demoralization, and irritability due to chronic stress, is independently associated with cardiovascular disease (CVD).
Objectives
The purpose of this study was to examine the association of vital exhaustion with biomarkers associated with CVD risk in the ARIC (Atherosclerosis Risk In Communities) study.
Methods
We examined the cross-sectional association of vital exhaustion (assessed using the Maastricht Vital Exhaustion Questionnaire [MVEQ]) with cardiac biomarker (high-sensitivity troponin T [hs-TnT], N-terminal pro–B-type natriuretic peptide [NT-proBNP]) and high-sensitivity C-reactive protein (hs-CRP) levels in 11,542 ARIC study participants without CVD at ARIC visit 2 using multivariable logistic and linear regression models. We then analyzed the association of vital exhaustion symptoms in the presence or absence of elevated biomarker levels with incident CVD events (coronary heart disease, ischemic stroke, or heart failure hospitalization) and all-cause mortality over a 10- and 20-year follow-up period using Cox proportional hazard models.
Results
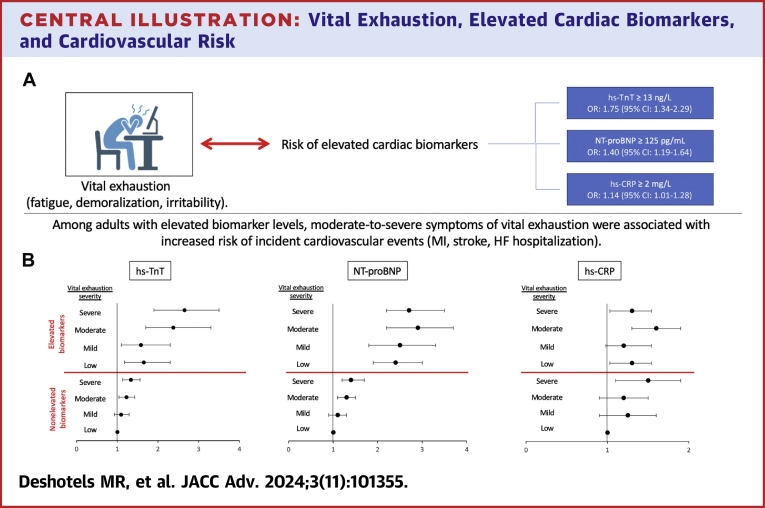
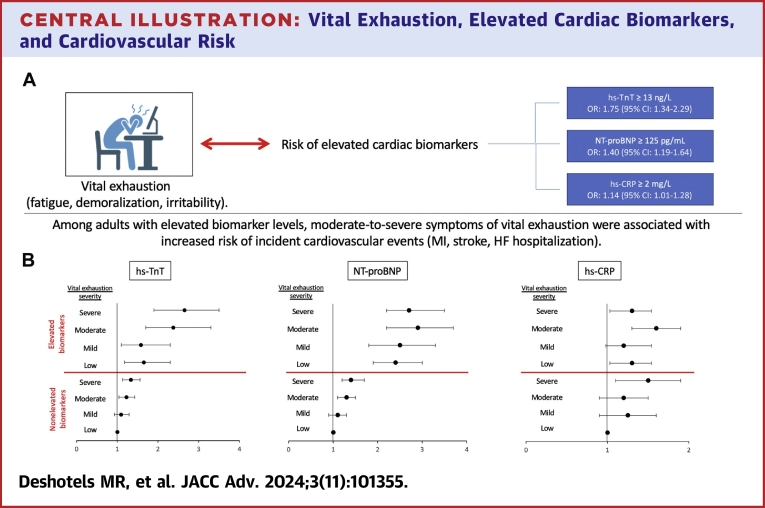
Compared with the lowest quartile of vital exhaustion (MVEQ ≤4), the highest quartile (MVEQ 16-42) was associated with elevated hs-TnT, NT-proBNP, and hs-CRP, with ORs of 1.75 (95% CI: 1.34-2.29), 1.40 (95% CI: 1.19-1.64), and 1.14 (95% CI: 1.01-1.28), respectively. The presence of both severe symptoms of vital exhaustion and elevated biomarker levels was associated with greater risk of CVD events and all-cause mortality.
Conclusions
In middle-aged adults without CVD, vital exhaustion was associated with elevated hs-TnT, NT-proBNP, and hs-CRP, independent of traditional CVD risk factors. Evaluation of vital exhaustion symptoms and cardiac biomarker levels can help identify individuals at increased risk for incident CVD events and all-cause mortality.
Key words: cardiac biomarkers, C-reactive protein (CRP), CVD (cardiovascular disease), NT-pro terminal BNP (NT-proBNP), troponin-T, vital exhaustion
Central Illustration
Research over the past few decades has demonstrated that mental health plays a role in cardiovascular health and disease.1 Vital exhaustion, defined as excessive fatigue, irritability, demoralization, and hopelessness, was first characterized by Appels et al in the 1980s as a chronic psychological malady resulting from a maladaptive response to chronic and uncontrolled stress.2 Symptoms of vital exhaustion, quantified using validated questionnaires, are associated with a significantly increased risk of incident cardiovascular disease (CVD) events including fatal and nonfatal myocardial infarction (MI).3,4 Vital exhaustion also increases the risk of heart failure hospitalization or death in adults without a history of congestive heart failure (CHF).5,6 Vital exhaustion has been associated with several risk factors for CVD including abnormal lipid metabolism,7 inflammation,8 impaired fibrinolysis,9 and low heart rate variability.10 However, no studies have examined whether vital exhaustion is associated with biomarkers of myocardial injury, wall stress, or inflammation.
Elevated cardiac biomarkers, such as cardiac troponin and N-terminal pro–B-type natriuretic peptide (NT-proBNP), have become essential diagnostic tools in the evaluation of coronary heart disease (CHD) and CHF. Even low elevations in circulating levels of cardiac biomarkers are predictive of incident cardiac events in adults without clinical CVD. For example, elevated cardiac troponin T using a high-sensitivity assay (high-sensitivity troponin T [hs-TnT]) is associated with incident CHD, incident CHF, and all-cause mortality in adults without prevalent CVD.11 Similarly, elevated NT-proBNP detected in adults without clinical CHF is predictive of incident heart failure hospitalization, CVD, or death.12,13 Additionally, high-sensitivity C-reactive protein (hs-CRP) is a sensitive but nonspecific marker of inflammation that is associated with incident CVD and all-cause mortality.14,15 Given the ability of these biomarkers to predict adverse CVD events, studying the association of cognitive stress with cardiac biomarkers can further characterize the relationship between mental health and CVD. Given that these biomarkers are representative of cardiac injury, hemodynamic stress, and inflammation, an association between vital exhaustion and cardiac biomarkers can potentially identify those with exhaustion who have the highest CVD risk.
We therefore analyzed the association between vital exhaustion and biomarkers of myocardial injury (hs-TnT), cardiac wall stress (NT-proBNP), and inflammation (hs-CRP) in adults without prevalent CVD in the ARIC (Atherosclerosis Risk In Communities) study. While previous studies have identified an association between vital exhaustion and CVD outcomes, we performed a novel analysis to determine if vital exhaustion is associated with myocardial stress and injury (as evidenced by elevated biomarkers) independent of traditional CVD risk factors.
Methods
The ARIC study is an ongoing longitudinal cohort study of U.S. adults from 4 communities (Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and suburbs of Minneapolis, Minnesota). The study protocol was approved by the Institutional Review Boards of all participating centers, and written consents were provided by all participants. A detailed description of the objectives and design of the study has been previously published.16
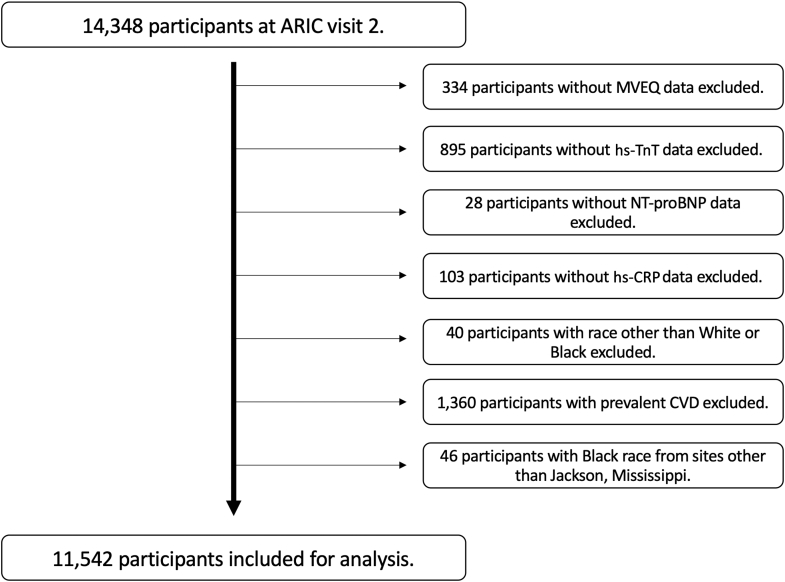
Baseline examinations of 15,792 men and women aged 45 to 64 years were obtained at ARIC visit 1 (1987-1989). The present study was performed using data from ARIC visit 2 (1990-1992), which was selected based on availability of psychometric questionnaires and biomarker measurements. We excluded participants with prevalent CVD, race other than White or Black and Black race from sites other than Jackson, MS (because of small numbers), missing biomarker data, or incomplete or missing questionnaires for vital exhaustion.
Symptoms of vital exhaustion were assessed using the Maastricht Vital Exhaustion Questionnaire (MVEQ) as part of the psychometric “Health and Life Profile” at visit 2. The MVEQ is a 21-item questionnaire that assesses the presence and severity of vital exhaustion. The Cronbach alpha for internal consistency was 0.89 as originally reported by Appels et al.2 Responses to the questionnaire are presented in a yes/no format and coded on a scale of 0 to 2 (yes = 2, not sure = 1, no = 0) (Supplemental Table 1). Two items, questions 9 and 14, are reverse coded (yes = 0, not sure = 1, no = 2). Responses are summed to obtain a vital exhaustion score ranging from 0 to 42, with higher scores representing a higher burden of exhaustion. For our analysis, MVEQ scores were modeled both as continuous variables and as categorical variables using approximate quartiles—1) 0 to 4 (low); 2) 5 to 8 (mild); 3) 9 to 15 (moderate); 4) 16 to 42 (severe)—based on previous studies.3,17 MVEQ data were only available at ARIC visit 2.
The cardiac biomarkers hs-TnT, NT-proBNP, and hs-CRP were measured in plasma collected from participants at ARIC visit 2 that was centrally stored at −80 °C. hs-TnT was measured in stored plasma samples at the University of Minnesota, using a higher-sensitivity sandwich immunoassay with a Roche Elecsys 2010 Analyzer (Roche Diagnostics). Measurements of NT-proBNP levels were conducted at the University of Minnesota in 2011 to 2013 using a Roche sandwich immunoassay in a Roche Elecsys 2010 analyzer (Roche Diagnostics). hs-CRP levels were measured from plasma in 2011 to 2013 using an immunoturbidimetric assay on the Roche Modular P chemistry analyzer (Roche Diagnostics). When analyzed as categorical variables, biomarkers were considered elevated if hs-TnT ≥13 ng/L, NT-proBNP ≥125 pg/mL, or hs-CRP ≥2 mg/L.
The cross-sectional associations among vital exhaustion, depressive symptoms, and biomarker levels were assessed using multivariable logistic or linear regression models. For linear models, continuous variables were assessed for normality and transformed if necessary. All models were adjusted for age, sex, race, education level, current smoking status, current drinking status, body mass index (BMI), systolic and diastolic blood pressure, hypertension, diabetes mellitus status, estimated glomerular filtration rate, total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and lipid-lowering medication use. For all biomarkers, we performed stratified analysis based on sex and antidepressant medication use to account for their potential effects on vital exhaustion symptoms. To account for variability in NT-proBNP levels based on obesity, results were stratified by participants with BMI ≥30 kg/m2 or BMI <30 kg/m2. Differences in means for normally distributed continuous variables were assessed using two-sample t-tests or 1-way analyses of variance; continuous variables without normal distribution were assessed using Kruskal–Wallis rank tests. Differences in proportions for categorical variables were assessed using chi-squared tests.
We also performed analysis comparing the risk of incident CVD events and all-cause mortality across MVEQ quartiles and the presence or absence of elevated biomarker levels. Based on prior observations that higher MVEQ scores were associated with increased CVD events,3,18 we hypothesized that adults with both high MVEQ scores and elevated biomarker levels would have greater risk of incident CVD events and all-cause mortality than those with either risk factor alone. To test this hypothesis, we created a composite variable based on severity of symptoms and biomarker elevation. We created 8 categories based on previously described MVEQ score quartiles (low, mild, moderate, or severe symptoms) plus the presence or absence of elevated biomarkers. Participants with minimal or low symptoms of vital exhaustion (MVEQ ≤4) and nonelevated biomarker levels (hs-TnT <13 ng/L, NT-proBNP <125 pg/mL, hs-CRP <2 mg/L) were used as the reference category. We used Cox proportional hazards regression analyses to estimate HRs and 95% CIs for the association between vital exhaustion (MVEQ scores at visit 2) and time to incident CVD events after adjustment for the covariates listed above. Tests for interaction between MVEQ score quartiles and elevated biomarker levels were performed using Wald tests in Cox proportional hazard regression models.
Incident CVD events and all-cause mortality were assessed during 10- and 20-year follow-up periods after each participant’s visit 2 evaluation. CVD events were defined as the composite of CHD (acute MI and fatal CHD), ischemic stroke, and heart failure. A detailed description of ascertainment of CVD outcomes has been previously described.11,19 Briefly, CHD incidents were defined as definite or probable hospitalized MI, definite CHD death, or unrecognized MI. Definite or probable hospitalized MI was based on evaluation of symptoms, electrocardiogram changes, and cardiac enzyme levels. Unrecognized MI was determined by follow-up examinations at subsequent ARIC study visits based on finding a major Q-wave or minor Q-wave with ischemic ST–T changes or an MI by Novacode criteria, confirmed by a side-by-side visual comparison of baseline and follow-up electrocardiograms.
Definite CHD death was determined by presenting symptoms, hospital information, medical history, and underlying cause of death from death certificates. Hospitalization for stroke was ascertained and validated if the discharge diagnosis contained codes indicative of cerebrovascular disease (International Classification of Diseases-9th Edition codes 430-438) and/or one of the following keywords was documented during admission: stroke, transient ischemic attack, cerebrovascular disease, cerebral infarction, cerebral embolus, paralysis, aphasia, diplopia, lacunar infarction, dysarthria, cerebral angiography, carotid, or endarterectomy. Medical records containing a diagnostic computed tomography or magnetic resonance imaging scan with cerebrovascular findings or admissions to the neurologic intensive care unit were also eligible for review.20 Incident heart failure was defined as a heart failure–associated hospitalization or death during the follow-up period after visit 2. Incident heart failure was diagnosed from hospitalization or death with an International Classification of Diseases-9th Edition code of 428. Review of clinical events and final diagnoses was performed by a Morbidity and Mortality Classification Committee. Deaths were ascertained by review of hospital discharge records, death certificates, informant interviews, or physician questionnaires for out-of-hospital deaths. Follow-up time was defined as the time between the baseline visit and the date of incident CVD event, death, or loss to follow-up. Participants who were lost to follow-up were censored.
Results
Cohort characteristics
ARIC visit 2 included 14,348 participants; after exclusions, a total of 11,542 participants were included for our analysis (Figure 1). The mean age of the cohort was 57.1 years, 57% of participants were female, and 24% of participants were Black (Table 1). Individuals with higher MVEQ scores were more likely to be female or Black and to have higher prevalence of current smoking, higher BMI, systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels. Higher prevalence of hypertension and diabetes and lower estimated glomerular filtration rate were associated with increasing MVEQ quartiles. Median NT-proBNP and hs-CRP levels were higher with increasing MVEQ quartiles (P < 0.001 for both), whereas no significant differences were seen for median hs-TnT levels (P = 0.17).
Figure 1.
Patient Selection Flow Chart
ARIC = Atherosclerosis Risk In Communities; CVD = cardiovascular disease; hs-TnT = high-sensitivity troponin-T; hs-CRP = high-sensitivity C-reactive protein; NT-proBNP = N-terminal pro–B-type natriuretic peptide; MVEQ = Maastricht Vital Exhaustion Questionnaire.
Table 1.
Baseline Characteristics of Participants Without a History of Cardiovascular Disease by Vital Exhaustion Quartiles
| MVEQ Score Quartile |
P Value | ||||
|---|---|---|---|---|---|
| 0-4 (Low) (n = 3,871, 33.5%) | 5-8 (Mild) (n = 2,319, 20.1%) | 9-15 (Moderate) (n = 2,720, 23.6%) | 16-42 (Severe) (n = 2,632, 22.8%) | ||
| Age, y | 56.8 ± 5.6 | 57.2 ± 5.7 | 57.3 ± 5.7 | 57.3 ± 5.8 | 0.21 |
| Female | 1,622 (41.9) | 1,298 (55.9) | 1,754 (64.5) | 1,911 (72.6) | <0.001 |
| Black race | 700 (18.1) | 524 (22.6) | 721 (26.5) | 803 (30.5) | <0.001 |
| Education level | <0.001 | ||||
| Less than high school | 492 (12.8) | 383 (16.6) | 551 (20.3) | 826 (31.5) | |
| High school | 1,516 (39.2) | 1,003 (43.2) | 1,184 (43.7) | 1,156 (43.9) | |
| At least some college | 1,856 (48.0) | 931 (40.2) | 979 (36.0) | 646 (24.5) | |
| Current smoker | 681 (17.7) | 469 (20.2) | 645 (23.7) | 684 (26.0) | <0.001 |
| Current drinker | 2,505 (64.7) | 1,397 (60.2) | 1,521 (55.9) | 1,284 (48.8) | <0.001 |
| BMI, kg/m2 | 27.3 ± 4.6 | 27.7 ± 5.1 | 27.9 ± 5.4 | 28.8 ± 6.1 | <0.001 |
| Systolic BP, mm Hg | 120.2 ± 17.5 | 121.7 ± 18.2 | 120.9 ± 18.6 | 121.2 ± 19.7 | <0.001 |
| Diastolic BP, mm Hg | 72.5 ± 9.8 | 72.6 ± 10.1 | 71.7 ± 10.4 | 71.7 ± 10.4 | 0.001 |
| Total cholesterol, mg/dL | 207.4 ± 37.1 | 209.3 ± 39.2 | 209.7 ± 38.3 | 211 ± 41.1 | <0.001 |
| LDL-C, mg/dL | 132.9 ± 34.5 | 132.9 ± 37.0 | 132.2 ± 36.6 | 133.2 ± 38.3 | <0.001 |
| HDL-C, mg/dL | 48.7 ± 16.2 | 50.7 ± 17.3 | 51.2 ± 17.2 | 51.5 ± 17.1 | <0.001 |
| Hypertension | 1,111 (28.7) | 747 (32.1) | 907 (33.3) | 981 (37.2) | <0.001 |
| Diabetes mellitus | 411 (10.6) | 275 (11.8) | 375 (13.8) | 449 (17.0) | <0.001 |
| eGFR, mL/min/1.73 m2 | 96.2 ± 13.9 | 96.6 ± 14.6 | 97.4 ± 15.4 | 97.9 ± 16.7 | <0.001 |
| LLT within past 2 weeks | 203 (5.2) | 109 (4.7) | 145 (5.3) | 142 (5.4) | 0.67 |
| hs-TnT, ng/L | 3 (1.5, 6) | 1.5 (1.5, 6) | 1.5 (1.5, 6) | 1.5 (1.5, 6) | 0.17 |
| hs-TnT ≥3 ng/L | 1,948 (50.1) | 1,123 (48.2) | 1,265 (46.2) | 1,219 (46.1) | 0.003 |
| hs-TnT ≥13 ng/L | 152 (3.9) | 110 (4.9) | 122 (4.7) | 160 (6.4) | 0.001 |
| NT-proBNP, pg/mL | 43.0 (23.2, 77.7) | 49.4 (26.2, 86.7) | 51.2 (27.8, 92.8) | 56.6 (30.2, 102.6) | <0.001 |
| NT-proBNP ≥125 pg/mL | 430 (11.1) | 323 (13.9) | 402 (14.7) | 483 (18.3) | <0.001 |
| hs-CRP, mg/L | 1.8 (0.89-3.6) | 2.04 (0.98, 4.29) | 2.36 (1.09, 4.95) | 2.7 (1.2, 5.9) | <0.001 |
| hs-CRP, ≥2 mg/L | 1,777 (45.8) | 1,179 (50.6) | 1,503 (55.0) | 1,617 (61.2) | <0.001 |
Values are mean ± SD, n (%), or median (25th, 75th percentile).
BMI = body mass index; BP = blood pressure; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; hs-CRP = high-sensitivity C-reactive protein; hs-TnT = high-sensitivity troponin-T; LDL-C = low-density lipoprotein cholesterol; LLT = lipid-lowering therapy; MVEQ = Maastricht Vital Exhaustion Questionnaire; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Association between vital exhaustion and cardiac biomarkers
When MVEQ scores and biomarkers were modeled as categorical variables, the highest MVEQ quartile was associated with significantly higher odds for elevated hs-TnT, NT-proBNP, and hs-CRP after adjustment for covariates, with ORs of 1.75 (95% CI: 1.34-2.29, P < 0.001), 1.40 (95% CI: 1.19-1.64, P < 0.001), and 1.14 (1.01-1.28, P = 0.035), respectively (Central Illustration, Table 2). No significant differences were observed when stratifying by sex or antidepressant medication use for any of the 3 biomarkers (Supplemental Table 2). When MVEQ scores and biomarker levels were modeled as continuous variables, each 1-U increase in MVEQ score was associated with a small but statistically significant elevation in all 3 log-transformed biomarkers, with beta-coefficients of 0.003 for hs-TnT, 0.006 for NT-proBNP, and 0.003 for hs-CRP (all P < 0.01) (Supplemental Table 3).
Central Illustration.
Vital Exhaustion, Elevated Cardiac Biomarkers, and Cardiovascular Risk
(A) Severe symptoms of vital exhaustion were associated with increased Risk of elevated high-sensitivity troponin T (hs-TnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and high-sensitivity c-reactive protein (hs-CRP). ORs and 95% CIs were calculated using multivariable logistic regression to account for variables listed below. (B) Forest plots depict HRs that represent the association of vital exhaustion severity in the setting of nonelevated or elevated biomarker levels with incident cardiovascular events including myocardial infarction (MI), stroke, or heart failure (HF) hospitalization over a 10-year follow-up period. Adults with nonelevated biomarker levels and low symptoms of vital exhaustion were used as the reference categories. HRs were calculated using cox proportional hazard models. Error bars represent 95% CIs. All statistical models were adjusted for age, sex, race, education level, current smoking status, current drinking status, body mass index, systolic and diastolic blood pressure, hypertension, diabetes status, estimated glomerular filtration rate, total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and lipid-lowering medication use.
Table 2.
Adjusted ORs (95% CIs) of Elevated Biomarkers by MVEQ Quartiles
| MVEQ Quartile |
||||
|---|---|---|---|---|
| 0-4 (Low) [Reference] | 5-8 (Mild) | 9-15 (Moderate) | 16-42 (Severe) | |
| hs-TnT ≥13 ng/L | 1.00 | 1.25 (0.95-1.64) | 1.28 (0.98-1.68) | 1.75 (1.34-2.29) |
| NT-proBNP ≥125 pg/mL | 1.00 | 1.09 (0.92-1.28) | 1.09 (0.93-1.28) | 1.40 (1.19-1.64) |
| hs-CRP ≥2 mg/L | 1.00 | 1.02 (0.91-1.15) | 1.06 (0.95-1.18) | 1.14 (1.01-1.28) |
ORs were adjusted for age, sex, race, education level, current smoking status, current drinking status, body mass index, systolic and diastolic blood pressure, hypertension, diabetes status, estimated glomerular filtration rate, total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and lipid-lowering medication use.
Abbreviations as in Table 1.
Association between vital exhaustion and CVD outcomes and all-cause mortality
During a 10-year follow-up period after visit 2, there were 1,417 CVD events (13.5 events per 1,000 person-years) and 884 deaths (8.1 events per 1,000 person-years). Severe symptoms of vital exhaustion (MVEQ ≥16) were independently associated with an increased risk of CVD events in adults with nonelevated biomarker levels irrespective of individual biomarkers (Central Illustration, Table 3). Elevated biomarker levels were associated with an increased risk of CVD events even in the absence of vital exhaustion symptoms (MVEQ score ≤4). Among adults with elevated hs-TnT levels, higher quartiles of MVEQ scores demonstrated a linear increase in risk of CVD events. Higher MVEQ quartiles were also associated with greater risk of CVD events in adults with elevated NT-proBNP and hs-CRP levels, but the increase in risk was not linear. The greatest risk of CVD events was seen in adults with elevated NT-proBNP levels and moderate or severe symptoms of vital exhaustion (HRs: 2.89 and 2.73, respectively).
Table 3.
Association of Vital Exhaustion and Elevated Biomarkers With Incident Cardiovascular Disease Events During 10-Y Follow-Up
| Nonelevated Biomarker Levels |
Elevated Biomarker Levels |
P Value for Interactiona | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low MVEQ [Reference] | Mild MVEQ | Moderate MVEQ | Severe MVEQ | Low MVEQ | Mild MVEQ | Moderate MVEQ | Severe MVEQ | ||
| hs-TnT | 1.00 | 1.09 (0.93-1.29, P = 0.26) | 1.22 (1.04-1.43 (P = 0.01) | 1.33 (1.13-1.56, P < 0.01) | 1.65 (1.18-2.3, P < 0.01) | 1.58 (1.07-2.33, P = 0.02) | 2.37 (1.72-3.27, P < 0.01) | 2.65 (1.99-3.52, P < 0.01) | 0.52 |
| NT- proBNP |
1.00 | 1.10 (0.92-1.32, P = 0.28) | 1.27 (1.08-1.51, P < 0.01) | 1.44 (1.21-1.71, P < 0.01) | 2.37 (1.86-3.03, P < 0.01) | 2.49 (1.88-3.31, P < 0.01) | 2.89 (2.24-3.72, P < 0.01) | 2.73 (2.16-3.48, P < 0.01) | 0.59 |
| hs-CRP | 1.00 | 1.25 (0.99-1.59, P = 0.06) | 1.20 (0.95-1.52, P = 0.13) | 1.48 (1.16-1.89, P < 0.01) | 1.26 (1.03-1.54, P = 0.02) | 1.23 (0.98-1.54, P = 0.07) | 1.61 (1.31-1.99, P < 0.01) | 1.26 (1.03-1.54, P = 0.02) | 0.27 |
Values are HR (95% CI, P value). HRs were adjusted for age, sex, race, education level, current smoking status, current drinking status, body mass index, systolic and diastolic blood pressure, hypertension, diabetes status, estimated glomerular filtration rate, total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and lipid-lowering medication use. Cardiovascular disease events included acute myocardial infarction, coronary revascularization, ischemic stroke, and heart failure hospitalization. Symptoms of vital exhaustion were categorized as follows: 1) no symptoms (MVEQ score 0-4); 2) mild symptoms (MVEQ score 5-8); 3) moderate symptoms (MVEQ score 9-15); and severe symptoms (MVEQ score ≥16). Nonelevated biomarker levels indicate plasma levels below the following thresholds: hs-TnT <13 ng/L; NT-proBNP <125 pg/mL; hs-CRP <2 mg/L. Elevated biomarker levels indicate plasma levels above the following thresholds: hs-TnT ≥13 ng/L; NT-proBNP ≥125 pg/mL; hs-CRP ≥2 mg/L.
Abbreviations as in Table 1.
aP value for interaction between MVEQ quartiles and presence of elevated biomarker levels.
Higher levels of vital exhaustion were also associated with increasing risk of 10-year all-cause mortality in adults without elevated biomarkers (Table 4). In adults with elevated NT-proBNP, increasing MVEQ quartiles were associated with a linear increase in risk of all-cause mortality, whereas a nonlinear increase in risk was seen in adults with elevated hs-TnT or hs-CRP. Those with elevated NT-proBNP and severe symptoms of vital exhaustion demonstrated the greatest risk of all-cause mortality. Vital exhaustion and elevated biomarker levels were associated with a higher risk of all-cause mortality relative to incident CVD events.
Table 4.
Association of Vital Exhaustion and Elevated Biomarkers With All-Cause Mortality During 10-Y Follow-Up
| Nonelevated Biomarker Levels |
Elevated Biomarker Levels |
P Value for Interactiona | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low MVEQ [Reference] | Mild MVEQ | Moderate MVEQ | Severe MVEQ | Low MVEQ | Mild MVEQ | Moderate MVEQ | Severe MVEQ | ||
| hs-TnT | 1.00 | 1.18 (0.94-1.47, P = 0.14) | 1.36 (1.11-1.67, P < 0.01) | 1.57 (1.27-1.93, P < 0.01) | 2.09 (1.37-3.21, P < 0.01) | 2.76 (1.82-4.20, P < 0.01) | 2.51 (1.68-3.76, P < 0.01) | 3.81 (2.76-5.25, P < 0.01) | 0.72 |
| NT-proBNP | 1.00 | 1.23 (0.97-1.57, P = 0.07) | 1.32 (1.05-1.66, P = 0.02) | 1.54 (1.23-1.94, P < 0.01) | 2.21 (1.61-3.04, P < 0.01) | 2.52 (1.79-3.55, P < 0.01) | 3.22 (2.39-4.35, P < 0.01) | 4.04 (3.08-5.30, P < 0.01) | 0.70 |
| hs-CRP | 1.00 | 1.23 (0.89-1.71, P = 0.20) | 1.34 (0.98-1.83, P = 0.06) | 1.75 (1.29-2.39, P < 0.01) | 1.42 (1.08-1.88, P = 0.01) | 1.70 (1.27-2.27, P < 0.01) | 1.94 (1.47-2.56, P < 0.01) | 2.32 (1.77-3.06, P < 0.01) | 0.97 |
Values are HR (95% CI, P value). HRs were adjusted for age, sex, race, education level, current smoking status, current drinking status, body mass index, systolic and diastolic blood pressure, hypertension, diabetes status, estimated glomerular filtration rate, total cholesterol level, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and lipid-lowering medication use. Symptoms of vital exhaustion were categorized as follows: 1) no symptoms (MVEQ score 0-4); 2) mild symptoms (MVEQ score 5-8); 3) moderate symptoms (MVEQ score 9-15); and severe symptoms (MVEQ score ≥16). Nonelevated biomarker levels indicate plasma levels below the following thresholds: hs-TnT <13 ng/L; NT-proBNP <125 pg/mL; hs-CRP <2 mg/L. Elevated biomarker levels indicate plasma levels above the following thresholds: hs-TnT ≥13 ng/L; NTproBNP ≥125 pg/mL; hs-CRP ≥2 mg/L.
Abbreviations as in Table 1.
P for interaction between MVEQ quartiles and presence of elevated biomarker levels.
During a 20-year follow-up period after visit 2, there were 3,023 CVD events (16.5 events per 1,000 person-years) and 2,967 deaths (14.6 events per 1,000 person-years). Overall, a similar trend was seen at 10- and 20-year follow-up. However, vital exhaustion and elevated biomarker levels were associated with a greater risk of incident CVD events than of all-cause mortality (Supplemental Tables 4 and 5) over the 20-year follow-up. There was no statistically significant interaction between elevated biomarker levels and MVEQ quartiles.
Discussion
Our study demonstrates several important findings regarding vital exhaustion and cardiac injury/stress as evidenced by elevated biomarkers. Symptoms of vital exhaustion in middle age were more common in women, Black adults, and individuals who were actively smoking or had lower levels of education, higher BMI, and greater prevalence of hypertension and diabetes mellitus. The presence of severe symptoms of vital exhaustion (MVEQ score ≥16) was independently associated with elevated hs-TnT, NT-proBNP, and hs-CRP after adjusting for demographics, socioeconomic factors, and prevalent chronic medical conditions. The association between vital exhaustion and CVD risk factors has been reported in previous studies.4 Although several studies have demonstrated that vital exhaustion increases the risk for incident CVD,3,4,6 this is the first study demonstrating an independent association between vital exhaustion and increased cardiac biomarker levels in adults without established CVD. This suggests that the physiologic stress of vital exhaustion (excessive fatigue, demoralization, sleep disturbance) can potentially result in cardiovascular injury regardless of a person’s traditional cardiac risk factor burden.
Having severe symptoms of vital exhaustion was associated with an increased risk of incident CVD events and all-cause mortality in adults without elevated hs-TnT, NT-proBNP, or hs-CRP. The 10-year risk of CVD events and all-cause mortality was higher in adults with both vital exhaustion and elevated biomarker levels. In particular, the presence of severe vital exhaustion and elevated NT-proBNP levels was associated with nearly 3-fold greater risk of CVD events (HR: 2.73) and 4-fold greater risk of all-cause mortality (HR: 4.0). In the presence of elevated biomarker levels, increasing vital exhaustion categories were associated with higher risk of CVD events. Taken together, testing for biomarker levels in adults with vital exhaustion can help identify individuals at higher risk of CVD events or all-cause mortality potentially resulting from chronic stress. Conversely, adults with known elevated cardiac biomarker levels can be screened for the degree of vital exhaustion present for further risk stratification. This is a particularly important finding given that vital exhaustion is a less commonly known form of mental stress. Therefore, symptoms of vital exhaustion may warrant greater clinical awareness given the heightened risk for myocardial injury, stress, and adverse cardiovascular outcomes.
Vital exhaustion, characterized by excessive fatigue, demoralization, and irritability, is a manifestation of chronic psychological distress and predicts incident CVD and increased mortality, independent of traditional cardiovascular risk factors.3,4 However, those with higher levels of vital exhaustion had a higher burden of cardiovascular risk factors. One proposed mechanism by which chronic psychological stress might contribute to heart disease is by accelerating the progression of atherosclerosis. Chronic stress can lead to maladaptive behavioral changes including tobacco use, substance abuse, sleep disturbance, poor exercise, and compromised adherence to medical therapy and clinician follow-up.1 Furthermore, chronic stress can potentially result in overactivation of the hypothalamic-pituitary axis and autonomic nervous system, leading to increased circulating catecholamines, increased cortisol levels, hypertension, insulin resistance, and inflammation.8, 9, 10 The results of this study are suggestive of both direct and indirect mechanisms of cardiac injury as higher levels of vital exhaustion were associated with a higher prevalence of traditional risk factors but also associated with elevated cardiac biomarkers levels independent of these risk factors.
The strengths of this study include the size of the cohort studied, assessment of vital exhaustion using a well-validated questionnaire, and the comprehensive longitudinal characterization and phenotyping of participants in the ARIC study.
Study limitations
Measurement of vital exhaustion was based on self-reported questionnaires, and these symptoms are inherently complicated and dynamic. Vital exhaustion was treated as a surrogate for mental health. Although there is an abundance of data demonstrating an association between vital exhaustion and adverse cardiac events, its use in clinical practice is limited compared to traditional screening tools for depression. Symptoms of vital exhaustion have been correlated with that of depression, but this study cannot draw any conclusions related to clinical depression.21 We were unable to assess longitudinal changes in self-reported vital exhaustion over time. Furthermore, these data were collected from over 20 years ago, and this time factor may limit generalizability in a contemporary population. Lastly, the potential of residual confounding must be considered in the interpretation of this study.
Conclusions
In conclusion, symptoms of vital exhaustion were independently associated with elevated hs-TnT, NT-proBNP, and hs-CRP levels in middle-aged adults without CVD. The presence of severe symptoms of vital exhaustion was associated with significantly increased risk for adverse CVD outcomes and all-cause mortality, and the risk was even greater in the presence of elevated biomarker levels. Further research is warranted to investigate the mechanism linking psychological stress, myocardial injury, and CVD, and whether interventions to improve mental health translate to better cardiovascular outcomes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Severe symptoms of vital exhaustion (fatigue, irritability, demoralization) are associated with elevated biomarkers of cardiac injury and inflammation (hs-TnT, N-terminal pro-B-type natriuretic peptide, and hs-CRP) independent of traditional cardiovascular risk factors.
TRANSLATIONAL OUTLOOK: The mechanism and directionality of vital exhaustion and elevated cardiac biomarkers is unknown. Further studies on this association can facilitate our understanding of the link between mental health and CVD.
Funding support and author disclosures
The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. The funders had no role in the design and conduct of the study; analysis and interpretation of the data; preparation, review, approval, and decision to submit the manuscript for publication. Dr Nambi has received research grant from Abbott Labs (relevant to this article); holds stock options in Insera Therapeutics; and is a site co-investigator for a study sponsored by Ionis Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Grant support was provided by the National Institutes of Health and not the Department of Veterans Affairs. The views presented are those of the authors and do not necessarily represent those of the Department of Veterans Affairs.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Levine G.N., Cohen B.E., Commodore-Mensah Y., et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American heart association. Circulation. 2021;143(10):e763–e783. doi: 10.1161/CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 2.Appels A., Hoppener P., Mulder P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17(1):15–24. doi: 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- 3.Williams J.E., Mosley T.H., Jr., Kop W.J., Couper D.J., Welch V.L., Rosamond W.D. Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2010;105(12):1661–1665. doi: 10.1016/j.amjcard.2010.01.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frestad D., Prescott E. Vital exhaustion and coronary heart disease risk: a systematic review and meta-analysis. Psychosom Med. 2017;79(3):260–272. doi: 10.1097/PSY.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 5.Rod N.H., Andersen I., Prescott E. Psychosocial risk factors and heart failure hospitalization: a prospective cohort study. Am J Epidemiol. 2011;174(6):672–680. doi: 10.1093/aje/kwr144. [DOI] [PubMed] [Google Scholar]
- 6.Cene C.W., Loehr L., Lin F.C., et al. Social isolation, vital exhaustion, and incident heart failure: findings from the Atherosclerosis Risk in Communities Study. Eur J Heart Fail. 2012;14(7):748–753. doi: 10.1093/eurjhf/hfs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koertge J.C., Ahnve S., Schenck-Gustafsson K., Orth-Gomer K., Wamala S.P. Vital exhaustion in relation to lifestyle and lipid profile in healthy women. Int J Behav Med. 2003;10(1):44–55. doi: 10.1207/s15327558ijbm1001_04. [DOI] [PubMed] [Google Scholar]
- 8.Wirtz P.H., von Kanel R., Schnorpfeil P., Ehlert U., Frey K., Fischer J.E. Reduced glucocorticoid sensitivity of monocyte interleukin-6 production in male industrial employees who are vitally exhausted. Psychosom Med. 2003;65(4):672–678. doi: 10.1097/01.psy.0000062529.39901.c7. [DOI] [PubMed] [Google Scholar]
- 9.von Kanel R., Frey K., Fischer J. Independent relation of vital exhaustion and inflammation to fibrinolysis in apparently healthy subjects. Scand Cardiovasc J. 2004;38(1):28–32. doi: 10.1080/14017430310015884. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T., Sugiyama Y., Sumi Y., et al. Effects of vital exhaustion on cardiac autononomic nervous functions assessed by heart rate variability at rest in middle-aged male workers. Int J Behav Med. 2002;9(1):68–75. doi: 10.1207/s15327558ijbm0901_05. [DOI] [PubMed] [Google Scholar]
- 11.Saunders J.T., Nambi V., de Lemos J.A., et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nambi V., Liu X., Chambless L.E., et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the Atherosclerosis Risk in Communities study. Clin Chem. 2013;59(12):1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marz W., Tiran B., Seelhorst U., et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007;53(6):1075–1083. doi: 10.1373/clinchem.2006.075929. [DOI] [PubMed] [Google Scholar]
- 14.Oluleye O.W., Folsom A.R., Nambi V., Lutsey P.L., Ballantyne C.M., ARIC Study Investigators Troponin T, B-type natriuretic peptide, C-reactive protein, and cause- specific mortality. Ann Epidemiol. 2013;23(2):66–73. doi: 10.1016/j.annepidem.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker P.M. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 16.Wright J.D., Folsom A.R., Coresh J., et al. The ARIC (Atherosclerosis risk in communities) study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939–2959. doi: 10.1016/j.jacc.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg P.K., Claxton J.S., Soliman E.Z., et al. Associations of anger, vital exhaustion, anti- depressant use, and poor social ties with incident atrial fibrillation: the Atherosclerosis Risk in Communities Study. Eur J Prev Cardiol. 2021;28(6):633–640. doi: 10.1177/2047487319897163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott E., Holst C., Gronbaek M., Schnohr P., Jensen G., Barefoot J. Vital exhaustion as a risk factor for ischaemic heart disease and all-cause mortality in a community sample. A prospective study of 4084 men and 5479 women in the Copenhagen City Heart Study. Int J Epidemiol. 2003;32(6):990–997. doi: 10.1093/ije/dyg235. [DOI] [PubMed] [Google Scholar]
- 19.White A.D., Folsom A.R., Chambless L.E., et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 20.Koton S., Sang Y., Schneider A.L.C., Rosamond W.D., Gottesman R.F., Coresh J. Trends in stroke incidence rates in older US adults: an update from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. JAMA Neurol. 2020;77(1):109–113. doi: 10.1001/jamaneurol.2019.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp M.S., Falger P.R., Appels A., Szedmák S. Depressive symptomatology and vital exhaustion are differentially related to behavioral risk factors for coronary artery disease. Psychosom Med. 1998;60(6):752–758. doi: 10.1097/00006842-199811000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.